Back to Journals » Infection and Drug Resistance » Volume 16
Epidemiological Characteristics, Antifungal Susceptibility, Risk Factors, and Outcomes of Candida Bloodstream Infection: A Ten-Year Surveillance in a Teaching Hospital in China
Authors Qiao Y, Tao Z, Hao F, Huang Y, Sun H, Guo P
Received 21 March 2023
Accepted for publication 5 July 2023
Published 21 July 2023 Volume 2023:16 Pages 4769—4778
DOI https://doi.org/10.2147/IDR.S411283
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yan Qiao,1,* Zhaoyu Tao,2,* Feiran Hao,3 Yongqiang Huang,2 Hong Sun,2 Pu Guo2
1Department of Infectious Disease, The First Affiliated Hospital of Bengbu Medical College, Bengbu, Anhui, 233004, People’s Republic of China; 2Department of Clinical Laboratory Science, The First Affiliated Hospital of Bengbu Medical College, Bengbu, 233004, People’s Republic of China; 3Department of Clinical Medicine, School of Basic Medicine, Qiqihar Medical college, Hei Longjiang Qiqihar, 161006, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Pu Guo; Hong Sun, Department of Clinical Laboratory Science, The First Affiliated Hospital of Bengbu Medical College, 287 Zhihuai Road, Bengbu, 233004, People’s Republic of China, Email [email protected]; [email protected]
Background: Candida is one of the most important pathogens of hospital-acquired bloodstream infections. Its morbidity and mortality are still high, which is a serious global public problem.
Purpose: To investigate the strain distribution, drug susceptibility, clinical characteristics of patients, and risk factors affecting the prognosis of Candida bloodstream infection (BSI).
Materials and Methods: We retrospectively collected the clinical data, infection-related indicators, prognosis, strain prevalence and drug susceptibility of 163 patients with Candida BSI in a teaching hospital from January 2012 to December 2022. Univariate and multivariate logistic regression were used to analyze the risk factors affecting the prognosis.
Results: In 163 cases of Candida BSI, Candida albicans accounted for 48.47%, and Candida non-albicans accounted for 51.53%. A total of 163 patients with Candida BSI were mainly distributed in intensive care unit (ICU) and emergency department, accounting for 40.49% and 14.72%, respectively. The resistance rate of Candida albicans to fluconazole, itraconazole and voriconazole was less than 10%, and the sensitivity rate of Candida tropicalis to fluconazole, itraconazole and voriconazole was less than 80%. The mortality rate of 163 patients with Candida BSI was 33.13%, with Candida non-albicans higher than that of Candida albicans (p = 0.04). Multivariate analysis showed that hemodialysis (OR = 0.199, 95% CI: 0.059– 0.673, P = 0.009), arteriovenous catheters (OR = 0.344, 95% CI: 0.130– 0.913, P = 0.032), elevated neutrophil count (OR = 0.409, 95% CI: 0.194– 0.862, P = 0.019) and APACHE II score (OR = 0.848, 95% CI: 0.789~0.911, P < 0.001) were independent risk factors for death in patients with candidemia.
Conclusion: The blood flow infection rate of Candida non-albicans is increasing, and the mortality rate and resistance to antifungal drugs are higher than that of Candida albicans. Hemodialysis, arteriovenous catheters, elevated neutrophil count and APACHE II score were associated with death in patients with Candida BSI.
Keywords: Candida, bloodstream infection, clinical features, prognosis
Introduction
Candida exists widely in nature, is one of the conditioned pathogens of human body, and is also the most common pathogen causing fungal bloodstream infections.1,2 Candida-caused bloodstream infections significantly prolong the length of hospital stay and cost, increase the mortality rate, and pose a serious threat to the health of patients.3 Studies have found that there are differences in Candida species and epidemiological characteristics of bloodstream infections caused by different regions. Different from the results of studies in European countries,4 the incidence of Candida non-albicans is on the rise in some parts of Asia,5–7 and the resistance rate to antifungal drugs is also on the rise, resulting in poor efficacy and increased mortality. In addition, different species of Candida also have different prognoses in patients with bloodstream infection. Al-Dorzi et al,8 in monitoring 174 patients with fungal bloodstream infections at a Saudi hospital ICU, found higher mortality for C. albicans compared with C. non-albicans. However, in a study in a Thai hospital, Candida tropicalis caused a higher mortality.9 This regional difference suggests that we need to conduct necessary studies on Candida BSI in local hospitals.
Recent studies have shown that risk factors for Candida BSI vary with time and region. Currently reported risk factors include old age, more underlying diseases, surgical operations, history of use of broad-spectrum antibiotics, APACHE II score ≥20, and history of invasive procedures (central venous catheters, mechanical ventilation, etc.).10,11 Due to lack of characteristic clinical manifestations, early diagnosis and antifungal therapy are difficult for patients with candidemia. The factors related to infection and prognosis of patients are not well defined. Therefore, it is essential to improve patient survival by investigating local epidemiology, monitoring antifungal susceptibility, and identifying risk factors for morbidity and mortality.
By reviewing the clinical data of patients with Candida BSI in our hospital in the past 10 years, this study analyzed the epidemiological characteristics, antifungal drug sensitivity and prognostic factors related to patients with Candida BSI, so as to provide evidence for early identification and treatment of patients with candidemia, so as to improve the clinical outcome of patients.
Materials and Methods
Research Object
A total of 163 patients with Candida BSI admitted to the First Affiliated Hospital of Bengbu Medical College from January 1, 2012 to December 31, 2022 were included. The hospital is a tertiary teaching hospital with 3800 beds. Inclusion criteria: The patient was diagnosed with bloodstream infection, and Candida was cultured at least once in the blood culture samples submitted for examination. The same patient was cultured with the same Candida multiple times, and the results of the first culture were adopted. Exclude patients with incomplete medical records. Previous treatment was defined as: (1) surgery within the past 3 months; (2) broad-spectrum antimicrobial use for more than 5 days within 30 days of the first pathogen detection; (3) antifungal use within 3 months after diagnosis; (4) immunosuppressive use for 3 weeks within 2 months after diagnosis. This study was approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical College (No.: 2022208). The study was conducted in accordance with the Declaration of Helsinki.
Clinical and Epidemiological Data
For each case, we recorded demographics (age, sex, department, length of stay) and analyzed the APACHE II score of the patients. The laboratory tests (neutrophil count, hemoglobin, platelet count, PCT, CRP, 1, 3-β-D glucan, etc.) during the occurrence of Candida bloodstream infection and the risk factors of candidemia (including invasive procedures (urinary catheter, stomach tube, body cavity drainage tube, arteriovenous catheters, mechanical ventilation, hemodialysis), ICU admission, underlying diseases, use of broad-spectrum antibiotics, etc.). We discussed the epidemiology of this cohort, including the department distribution of candidemia and susceptibility to antifungal drugs. Next, patients were assessed for infection and prognostic risk factors.
Microbiological Tests
Blood samples were collected under sterile conditions, species identification was performed using VITEK 2 microbial identification instrument and VITEK MS (France Merieux Company), drug sensitivity test was performed using ATB FUNGUS 3 yeast reagent strip (France Merieux company), operation was strictly in accordance with the instructions of the kit. Candida isolates were classified as susceptible (S), intermediate (I), and resistant (R) based on the minimum inhibitory concentrations (MIC) of clinical breakpoints (CBPs) of antifungal agents. CBPs for fluconazole and voriconazole were determined according to the Clinical and Laboratory Standards Institute (CLSI M60), and CBPs for itraconazole susceptibility were determined according to the European Committee for Antimicrobial Susceptibility Testing (EUCAST 10.0) and epidemiological cut-off values (ECVs). The definition of CBPs for amphotericin B was based on EUCAST 10.0 criteria. 5-Flucytosine currently lacks CBPs in CLSI or EUCAST.12,13
Statistical Analysis
SPSS25.0 statistical analysis was used. The measurement data of normal distribution were expressed by (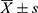 ) and t-test was used. The non-normal distribution was expressed as median and quartile, and Mann–Whitney U-test was used. Counting data were compared between groups using Chi-square test or Fisher’s exact probability method. Statistically significant indicators in univariate analysis were included in multivariate logistic regression analysis. P < 0.05 was considered statistically significant.
) and t-test was used. The non-normal distribution was expressed as median and quartile, and Mann–Whitney U-test was used. Counting data were compared between groups using Chi-square test or Fisher’s exact probability method. Statistically significant indicators in univariate analysis were included in multivariate logistic regression analysis. P < 0.05 was considered statistically significant.
Results
Species and Department Distribution
A total of 257 Candida strains were isolated from 163 blood samples of patients with Candida bloodstream infection. Among them, 143 cases were positive in one culture, and 20 cases were positive in two or more cultures. A total of 109 cases were positive in one vial, and 74 cases were positive in two vials. After removing duplicate strains from the same patient, a total of 163 strains were obtained. Candida albicans accounted for 48.47% (79/163), and Candida non-albicans accounted for 51.53% (84/163). Among the 163 strains of fungal BSI, 66 cases (40.49%) were from intensive care, 24 cases (14.72%) were from emergency department, and 21 cases (12.88%) were from hematology department (Figures 1 and 2).
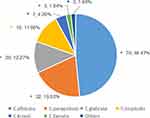 |
Figure 1 Constituent ratio of 163 cases of fungal bloodstream infection in strains (%). |
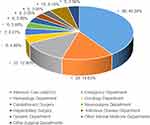 |
Figure 2 Constituent ratio of 163 cases of fungal bloodstream infection in departments (%). |
Clinical Characteristics
Among 163 patients with Candida BSI, 94 cases (57.67%) were male, with an average age of 61.25 ± 18.11 years old, and the infection rate of elderly patients ≥60 years old was 56.44%. Most patients (96.93%, 158/163) had underlying diseases, and the common underlying diseases were lung infection (61.35%), heart disease (31.90%), hypertension (31.90%), solid-organ cancer (23.31%), diabetes (22.09%), hematological malignancies (15.34%), etc. Among them, 133 patients (81.60%) had two or more underlying diseases. Among the patients with candidiasis, 103 cases (63.19%) were treated with catheter, 85 cases (52.15%) with mechanical ventilation, 74 cases (45.40%) with ICU admission, 69 cases (42.33%) with arteriovenous catheterization, 61 cases (37.42%) with stomach tube, and 58 cases (35.58%) with body cavity drainage tube. We further compare and analyze the clinical characteristics of patients with Candida albicans and Candida non-albicans bloodstream infection. The differences between C. albicans and C. non-albicans group in length of stay, prognosis, ICU admission, previous surgery, hemodialysis, invasive operation (urinary catheter, arteriovenous catheters, stomach tube, body cavity drainage tube, mechanical ventilation), use of immunosuppressive agents, pulmonary infection, combined with other fungal infection, solid malignant tumor, hematological malignancy, elevated neutrophil count, and decreased platelet count are statistically significant (P < 0.05). See Table 1.
 |
Table 1 Clinical Characteristics of 163 Patients with Candida BSI |
Drug Susceptibility
All 163 strains were tested for antifungal susceptibility in vitro. The sensitivity of Candida tropicalis to fluconazole, itraconazole and voriconazole was 70%–80%. In Table 2, except for the natural resistance of Candida krusei to fluconazole, the resistance rate of Candida tropicalis to azole drugs was the highest (4/19, 21.05%).
 |
Table 2 Drug Susceptibility of 157 Strains of Candida to 4 Antifungal Drugs |
Characterization of Deceased Patients
Among 163 patients with Candida BSI, 54 died, with a mortality rate of 33.13% (54/163). In Figure 3, the mortality rate of patients with Candida albicans BSI was 25.32% (20/79), and that of patients without Candida albicans infection was 40.38% (34/84). The rates of ICU admission, hemodialysis, invasive procedures (urinary catheter, arteriovenous catheter, stomach tube, body cavity drainage tube, mechanical ventilation), hematological malignancy, elevated neutrophil count, decreased hemoglobin and APACHE II score were all higher in death patients than in survival group (P < 0.05). Multivariate analysis was performed for statistically significant factors, and the results showed that hemodialysis (OR = 0.199, 95% CI: 0.059–0.673, P = 0.009), arteriovenous catheters (OR = 0.344, 95% CI: 0.130–0.913, P = 0.032), elevated neutrophil count (OR = 0.409, 95% CI: 0.194–0.862, P = 0.019) and APACHE II score (OR = 0.848, 95% CI: 0.789~0.911, P < 0.001) were independent risk factors for death in patients with candidemia (Table 3 and Table 4).
 |
Table 3 Univariate Analysis of Characteristics of 54 Dead Patients with Candida BSI |
 |
Table 4 Multivariate Logistic Analysis of 54 Dead Patients with Candida BSI |
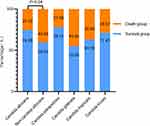 |
Figure 3 Death rate of patients with candida bloodstream infection. Note: P, significance. |
Discussions
Candida is a common pathogen causing human invasive mycosis. In recent years, with the extensive clinical application of invasive medical procedures, new broad-spectrum antibacterial drugs and transplantation and other advanced medical technologies, the incidence of Candida BSI is on the rise, and there are epidemiological differences in bloodstream infections caused by Candida in different regions.14 Koehler15 et al conducted a meta-analysis of candidemia in European countries and found that there were considerable differences among different central strains, with Candida non-albicans strains generally dominating. The results of this study show that Candidia non-albicans accounted for more than half of the Candida bloodstream infections in our hospital, which were similar to domestic reports, but showed different results reported for a university teaching hospital in Saudi Arabia by Tariq et al.8,16 This may be related to the geographical environment and climate of different regions, the underlying diseases of patients and the medical measures taken, so as to cause different Candida infections. Candida parapsilosis infection is mostly associated with indwelling deep venous catheter and parenteral nutrition. Patients with hematological malignancies are susceptible to Candida tropicalis, and exposure to antifungal agents is associated with Candida glabrata infection.17–19
Current studies have found that the main causes of Candida BSI are low immunity and impaired mucosal barrier in patients.20,21 Chen Xia22 et al reported that the incidence of Candida BSI in ICU patients over 60 years old was the highest, accounting for 47.5%. Our data show that the infection rate of Candida bloodstream infection in patients ≥60 years old was 55.83%, and the infection rate gradually increased with increasing age, which was similar to that report. The intensive care unit, oncology department and hematology department of our hospital were the departments with high incidence of candidemia. The patients in ICU were critically ill and had low immunity. Most patients underwent urinary catheter, stomach tube, arteriovenous catheters, mechanical ventilation, and other invasive procedures. Candida BSI in patients from departments of oncology and hematology may be related to immune dysfunction caused by tumor cell infiltration, radiotherapy and chemotherapy.23 In this study, 80% of patients with Candida BSI were complicated with two or more underlying diseases, mainly pulmonary infection, cardiovascular disease, hypertension and diabetes. More than 50% of the patients had a history of invasive procedures such as catheterization and arteriovenous catheterization. Invasive procedures destroy mucosal integrity. Catheter colonization and biofilm formation may be the important reasons for Candida entering the bloodstream and leading to bloodstream infection. At the same time, complicated with a variety of underlying diseases will further decrease the patient’s immune function, prolong the length of hospital stay, and increase the chance of hospital-acquired Candida infection. Further analysis found that patients with Candida non-albicans candidemia had a longer hospital stay and higher ICU admission rate than those with Candida albicans candidemia. In addition, it has a greater proportion of malignant tumors, pulmonary infections, other fungi, hemodialysis, invasive procedures (urinary catheter, stomach tube, body cavity drainage tube, arteriovenous catheter and mechanical ventilation) and elevated neutrophil count. Therefore, for clinically high-risk patients, the use of invasive procedures should be minimized, and patients with already invasive procedures should be removed early. In addition, the extensive use of immunosuppressants can lead to immunodeficiency or impaired immune function, which may increase the risk of Candida infection in patients. Therefore, the dose of drugs for such patients should be moderate in clinical practice. Clinicians should pay attention to patients at high risk of Candida BSI, and also pay attention to the evaluation of Candida species that may be infected.
The insidious onset of Candida infection and lack of characteristic clinical manifestations lead to difficult early diagnosis and poor prognosis in patients with candidemia. It has been reported in the literature24,25 that approximately 1.5 million people die from fungal infections each year, and Candida BSI accounts for half of these deaths, with mortality rates that can reach 40% or higher. Therefore, it is necessary to control risk factors to reduce the mortality of hospitalized patients. Studies have shown that patients with underlying diseases such as diabetes mellitus, cardiovascular disease, tumor, history of broad-spectrum antibiotic use, APACHE II score ≥20, history of invasive operations (central venous catheterization, mechanical ventilation, etc.), length of ICU stay, surgery, and parenteral nutrition are associated with the death of patients with Candida BSI.26,27 The results of univariate analysis between the death group and the survival group in this study showed that ICU admission, hemodialysis, invasive operation (urinary catheter, arteriovenous catheter, stomach tube, body cavity drainage tube, mechanical ventilation), hematological malignancy, elevated neutrophil count, decreased hemoglobin and APACHE II score were related to the prognosis of patients with candidemia, and there were statistically significant differences between the two groups (P < 0.05). Multivariate logistic regression analysis showed that hemodialysis, arteriovenous catheterization, increased neutrophil count and APACHE II score were independent risk factors for death in patients with Candida BSI. It is suggested that timely and appropriate treatment and nursing measures should be taken to improve the survival rate of these patients. In various studies, the prognosis of different Candida species is different, which may be related to the differences in virulence of different strains. The analysis of this study found that there was a statistically significant difference in the prognosis of patients with Candida albicans and Candida non-albicans in our hospital (P < 0.05). Patients with Candida non-albicans bacteremia had a higher mortality rate, and the mortality rate of Candida glabrata, Candida tropicalis and Candida krusei BSI was higher, which was consistent with recent reports.28,29
Candida showed high sensitivity to most antifungal drugs, and only one of the 163 Candida strains in this study showed that amphotericin B was non-wild type. Candida albicans has a small number of strains resistant to triazole antifungal agents. It is noteworthy that the resistance of Candida non-albicans to triazole antifungal drugs has increased significantly, among which more than 15% of strains of Candida tropicalis are resistant to drug, which is significantly higher than that of other Candida, and similar to literature reports.30,31 This may be due to the low adverse reactions, reasonable price and wide penetration into tissues of triazole drugs, fluconazole has become the first choice of clinicians for preventive or empirical medication of Candida patients, and the extensive clinical use of this type of antifungal drug has induced drug resistance.27 Therefore, clinicians need to consider the factors of Candida non-albicans infection and formulate a more reasonable treatment plan when conducting empirical antifungal therapy for patients.
This study was based on the clinical data of 163 patients with Candida BSI admitted to the First Affiliated Hospital of Bengbu Medical College in the past 10 years. The study has limitations in some respects. Firstly, we used time-of-flight mass spectrometry to identify clinical isolates of Candida. Due to the limitations of the bacterial bank, there may be a bias in the identification of strains. Secondly, with the increase of resistance to azole antifungal drugs, more antifungal drugs have attracted clinical attention. Echinocandins antifungal drugs interfere with the synthesis of β-1, 3-glucose in the fungal cell wall, leading to changes in the permeability of the fungal cell wall and cell lysis and death. Because of its high susceptibility to Candida and low toxicity to humans, it has been recommended as the first-line treatment for candidemia by the Infectious Diseases Society of America and the European Society of Clinical Microbiology and Infectious Diseases guidelines. However, sensitivity to echinocandins was not tested in this study due to the limitations of drug susceptibility testing kits. Finally, this study is only a single-center retrospective study in our hospital, which may affect the control factors of the variables and lead to bias in the results. It will be better to verify the results of this study through the analysis of a large number of multi-center data in the region, so as to reflect the relevant situation of Candida BSI in the region.
Conclusion
Our study found that the bloodstream infection rate of Candida non-albicans is increasing, and the high rate of drug resistance and mortality is more worthy of attention. For people with risk factors, laboratory tests should be performed in time to make an early diagnosis. More attention should be paid to middle-aged and elderly patients with multiple underlying diseases and critically ill patients admitted to ICU, and the risk factors of death should be eliminated as early as possible to reduce the risk of death of patients.
Ethical Approval
In this study, strains isolated from patient samples were used for research without adverse reactions and risks to the subjects, and the research data should be kept confidential for the information of the subjects, including cases and biological samples. The study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Bengbu Medical College. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgment
This work is supported by the Natural Science Research Project of Universities in Anhui Province (KJ2021A0761).
Disclosure
The authors declare that there is no conflict of interest in this work.
References
1. Posteraro B, De Carolis E, Criscuolo M., et al. Candidaemia in haematological malignancy patients from a SEIFEM study: epidemiological patterns according to antifungal prophylaxis. Mycoses. 2020;63:900–910. doi:10.1111/myc.13130
2. Barac A, Cevik M, Colovic N, et al. Investigation of a healthcare-associated Candida tropicalis candidiasis cluster in a haematology unit and a systematic review of nosocomial outbreaks. Mycoses. 2020;63:326–333. doi:10.1111/myc.13048
3. Lourdes R, Beatriz B, Luz H, et al. A multi-centric Study of Candida bloodstream infection in Lima-Callao, Peru: species distribution, antifungal resistance and clinical outcomes. PLoS One. 2017;12(4):e0175172.
4. Schroeder M, Weber T, Denker T, et al. Epidemiology, clinical characteristics, and outcome of candidemia in critically ill patients in Germany: a single-center retrospective 10-year analysis. Ann Intensive Care. 2020;10:142. doi:10.1186/s13613-020-00755-8
5. Zheng YJ, Xie T, Wu L, et al. Epidemiology, species distribution, and outcome of nosocomial Candida spp. bloodstream infection in Shanghai: an 11-year retrospective analysis in a tertiary care hospital. Ann Clin Microbiol Antimicrob. 2021;20:34. doi:10.1186/s12941-021-00441-y
6. Xiao M, Chen SC, Kong F, et al. Distribution and antifungal susceptibility of Candida species causing candidemia in China: an Update from the CHIF-NET study. J Infect Dis. 2020;221:S139–S147. doi:10.1093/infdis/jiz573
7. Zakhem AE, Istambouli R, Alkozah M, et al. Predominance of Candida glabrata among non-albicans Candida species in a 16-year study of candidemia at a tertiary care center in Lebanon. Pathogens. 2021;10:82. doi:10.3390/pathogens10010082
8. Al-Dorzi HM, Sakkijha H, Khan R, et al. Invasive candidiasis in critically ill patients: a prospective cohort study in two tertiary care centers. Intensive Care Med. 2022;35(6):542–553. doi:10.1177/0885066618767835
9. Tariq SA, Wala AA, Norah AA, et al. A seven-year surveillance of Candida bloodstream infection at a university hospital in KSA. J Taibah Univ Med Sci. 2021;16(2):184–190.
10. Kato H, Yoshimura Y, Suido Y, et al. Mortality and risk factor analysis for Candida blood stream infection: a multicenter study. J Infect Chemother. 2019;25(5):341–345. doi:10.1016/j.jiac.2019.01.002
11. Santolaya ME, Thompson L, Benadof D, et al. A prospective, multi-center study of Candida bloodstream infections in Chile. PLoS One. 2019;14(3):e0212924. doi:10.1371/journal.pone.0212924
12. Clinical and Laboratory Standards Institute. Performance Standards for Antifungal Susceptibility Testing of Yeasts, M60.
13. Arendrup MC, Friberg N, Mares M, et al. How to interpret MICs of antifungal compounds according to the revised clinical breakpoints v. 10.0 European committee on antimicrobial susceptibility testing (EUCAST). Clin Microbiol Infect. 2020;26:1464–1472. doi:10.1016/j.cmi.2020.06.007
14. Enoch DA, Yang H, Aliyu SH, et al. The Changing epidemiology of invasive fungal infections. Methods Mol Biol. 2017;1508:17–65.
15. Koehler P, Stecher M, Cornely OA, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect. 2019;25(10):1200–1212. doi:10.1016/j.cmi.2019.04.024
16. Xiao M, Sun ZY, Kang M, et al. Five-year national surveillance of invasive candidiasis: species distribution and azole susceptibility from the China Hospital Invasive Fungal Surveillance Net (CHIF-NET) study. J Clin Microbiol. 2018;56(7):e00577–18. doi:10.1128/JCM.00577-18
17. Eschenauer GA, Carver PL, Patel TS, et al. Survival in patients with Candida glabrata bloodstream infection is associated with fluconazole dose. Antimicrob Agents Chemother. 2018;62(6):e02566–17. doi:10.1128/AAC.02566-17
18. Arias S, Denis O, Montesinos I, et al. Epidemiology and mortality of candidemia both related and unrelated to the central venous catheter: a retrospective cohort study. Eur J Clin Microbiol Infect Dis. 2017;36(3):501–507. doi:10.1007/s10096-016-2825-3
19. Zhang L, Yu SY, Ning YT, et al. Multicenter retrospective study on antifungals susceptibility and molecular epidemiology of Candida parapsilosis isolated from bloodstream infections in China. Chin J Mycol. 2021;16(5):289–295.
20. Chen YZ, Xia Y. Laboratory characteristics of candidemia in terms of immune status of patients. Chin J Infect Chemother. 2020;20(1):465–469.
21. Vaquero-Herrero MP, Ragozzino S, Iriart X, et al. Candida bloodstream infection in patients with systemic autoimmune diseases. Med Mal Infect. 2020;50(4):372–376. doi:10.1016/j.medmal.2020.01.014
22. Chen X, Yang YW, Li YM, et al. Clinical characteristics and risk factors for death in patients with Candida bloodstream infection in Intensive Care Unit. J Cent South Univ. 2021;46(7):719–724.
23. Cao HW, Zhao XH, Lu SY, et al. Relationship between development of an infection after chemotherapy and immune function and intestinal microecology in patients with a hematologic malignancy. Pathog Biol. 2017;12(5):456–459.
24. Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi. 2017;3:57. doi:10.3390/jof3040057
25. Gunsalus KT, Tornberg-Belanger SN, Matthan NR, Lichtenstein AH, Kumamoto CA. Manipulation of host diet to reduce gastrointestinal colonization by the opportunistic pathogen Candida albicans. MSphere. 2015;1(1):e00020–15. doi:10.1128/mSphere.00020-15
26. Huang TT, Xia WY, Xu YQ, et al. A salt controlled scalable approach for formation of polyelectrolyte complex fiber†. Chin J Infect Chemother. 2020;20(5):465–469. doi:10.1002/cjoc.201900496
27. Ye NF, Liu Z, Tang W, et al. Systematic characterization of epidemiology, antifungal susceptibility, risk factors and outcomes of candidaemia: a six-year Chinese study. Infect Drug Resist. 2022;15:4887–4898. doi:10.2147/IDR.S378629
28. Chaiyapong N, Piriyaporn C, Amiroh W, et al. Risk factors and outcomes of non-albicans Candida bloodstream infection in patients with candidemia at siriraj hospital-Thailand’s largest national tertiary referral hospital. J Fungi. 2021;7(4):269.
29. Jin F, Xia WY, Ni F, et al. Epidemiology, risk factors, and prognosis of Candida albicans and non-albicans candidemia. J Nanjing Med Univ. 2022;42(3):401–405.
30. Jing R, Hou X, Xiao M, et al. A retrospective analysis of invasive yeast strains in China Hospital Invasive Fungal Surveillance Net from 2010 to 2014: changing pattern of species distribution and azoles susceptibility. Chin J Infect Chemother. 2020;20(2):175–180.
31. Liu WJ, Sun HL, Zhang XJ. Analysis the pathogen distribution and Drug susceptibility of yeast bloodstream infection in Peking Union Medical College Hospital from 2014 to 2018. Chin J Mycol. 2019;14(6):357–361.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
