Back to Journals » Drug Design, Development and Therapy » Volume 13
Enzyme Inhibitory, Antioxidant And Antibacterial Potentials Of Synthetic Symmetrical And Unsymmetrical Thioureas
Authors Naz S , Zahoor M , Umar MN, Ali B , Ullah R, Shahat AA , Mahmood HM, Sahibzada MUK
Received 30 July 2019
Accepted for publication 10 September 2019
Published 7 October 2019 Volume 2019:13 Pages 3485—3495
DOI https://doi.org/10.2147/DDDT.S225311
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Sumaira Naz,1 Muhammad Zahoor,1 Muhammad Naveed Umar,1 Barkat Ali,1,2 Riaz Ullah,3 Abdelaaty A Shahat,3,4 Hafiz Majid Mahmood,5 Muhammad Umar Khayam Sahibzada6
1Department of Chemistry, University of Malakand Chakdara, Dir Lower, Kpk 18800, Pakistan; 2Department of Chemistry, GC University Faisalabad, Faisalabad, Punjab, Pakistan; 3Medicinal, Aromatic and Poisonous Plants Research Center (MAPRC), College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia; 4Phytochemistry Department, National Research Centre, Giza, Egypt; 5Department of Pharmacology, College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia; 6Department of Pharmacy, Sarhad University of Science and Information Technology, Peshawar, Kpk 25000, Pakistan
Correspondence: Muhammad Umar Khayam Sahibzada
Department of Pharmacy, Sarhad University of Science and Information Technology, Peshawar, KPK 25000, Pakistan
Email [email protected]
Muhammad Zahoor
Department of Chemistry, University of Malakand Chakdara, Dir Lower, KPK 18800, Pakistan
Email [email protected]
Background: In this study, 2 symmetrical and 3 unsymmetrical thioureas were synthesized to evaluate their antioxidant, antibacterial, antidiabetic, and anticholinesterase potentials.
Methods: The symmetrical thioureas were synthesized in aqueous media in the presence of sunlight, using amines and CS2 as starting material. The unsymmetrical thioureas were synthesized using amines as a nucleophile to attack the phenyl isothiocyanate (electrophile). The structures of synthesized compounds were confirmed through H1 NMR. The antioxidant potential was determined using DPPH and ABTS assays. The inhibition of glucose-6-phosphatase, alpha amylase, and alpha glucosidase by synthesized compounds was used as an indication of antidiabetic potential. Anticholinesterase potential was determined from the inhibition of acetylcholinesterase and butyrylcholinesterase by the synthesized compounds.
Results: The highest inhibition of glucose-6-phosphatase was shown by compound V (03.12 mg of phosphate released). Alpha amylase was most potently inhibited by compound IV with IC50 value of 62 μg/mL while alpha glucosidase by compound III with IC50 value of 75 μg/mL. The enzymes, acetylcholinesterase, and butyrylcholinesterase were potently inhibited by compound III with IC50 of 63 μg/mL and 80 μg/mL respectively. Against DPPH free radical, compound IV was more potent (IC50 = 64 μg/mL) while ABTS was more potently scavenged by compound I with IC50 of 66 μg/mL. The antibacterial spectrum of synthesized compounds was determined against Gram-positive bacteria (Staphylococcus aureus) and Gram-negative bacteria (Agrobacterium tumefaction and Proteus vulgaris). Compound I and compound II showed maximum activity against A. tumefaction with MIC values of 4.02 and 4.04 μg/mL respectively. Against P. vulgaris, compound V was more active (MIC = 8.94 μg/mL) while against S. aureus, compound IV was more potent with MIC of 4.03 μg/mL.
Conclusion: From the results, it was concluded that these compounds could be used as antibacterial, antioxidant, and antidiabetic agents. However, further in vivo studies are needed to determine the toxicological effect of these compounds in living bodies. The compounds also have potential to treat neurodegenerative diseases.
Keywords: picolylamine, symmetrical thioureas, enzyme inhibition, anti-diabetic, antioxidant, Alzheimer’s disease, antibacterial
Introduction
Thioureas are an important class of compounds that are used as intermediates/precursors in synthesis of many synthetic drugs.1,2 A number of substituted derivatives of thioureas can be synthesized by simple condensation of different primary and secondary amines (aliphatic and/or aromatic) with iso-thiocyanate or its derivatives.3 They can also be prepared by reaction of carbon disulphide and amines (with or without catalyst).4 N,N′-disubstituted thioureas, whether aliphatic, aromatic or heterocyclic can be used as building blocks in the synthesis of heterocyclic compounds (having a variety of applications in different fields). In organic synthesis, they played a very important role as catalysts in important organic reactions (Mannich and Biginelli reactions) and as chelating agents.5 The diverse applications of these compounds in industry, agriculture, and medicine made them unique. Due to the presence of substituted sites in their structures, they have been used in drug design and synthesis.6 Literature on thiourea derivatives shows that they possess biological activities like anti-bacterial,7 anti-fungal, anti-cancer, analgesic, and antimalarial.8–13 They have also been used as inhibitors of certain enzymes like acetylcholinesterase, butyrylcholinesterase, carbonic anhydrase etc.14–17
Oxidative stress, diabetes mellitus, neurodegenerative diseases, and antibacterial resistance are the burning issues of this era. Free radicals are continuously produced during normal metabolism and nearly 1/4th of the inhaled oxygen is converted into free radicals, which have deteriorating effects on biologically important molecules like protein and DNA. Although human bodies are equipped with efficient systems to cope with these free radicals, for the last two decades, humans' dependence on synthetically processed food has complicated the issue and the amount of free radicals now produced is high as compared to the body's free radical scavenging capacities. Therefore, there is a need for certain potent compounds to be taken additionally, to help the body in scavenging the free radicals. As mentioned earlier, thioureas have two substation sites that could be useful in such type of research.18–21
In neurodegenerative diseases, there is maximum hydrolysis of acetylcholine, the substance responsible for the transmission of nerve impulses at synoptic gaps between the two nerves. A practical approach applied to relieve the symptoms of neurodegenerative diseases is the use of inhibitors of acetylcholinesterase and butyrylcholinesterase (the enzymes responsible for hydrolyses of neurotransmitter acetylcholine).22 An example of neurodegenerative disease is Alzheimer’s disease (AD) where an uncontrolled hydrolysis of the mentioned neurotransmitter occurs, rendering the neurotransmission difficult or almost impossible. If acetylcholinesterase is inhibited, the hydrolysis of acetylcholine can be controlled which can be helpful in the symptomatic relief of dementia or AD.18 Butyrylcholinesterase normally has a less significant role in hydrolysis of acetylcholine, but in the diseased condition, when acetylcholinesterase is inhibited by medicines, its therapeutic importance increases as it could hydrolyze the available acetylcholine, minimizing the effect of the medicines, thus exaggerates the disease and its inhibition is then desired.19,23
Diabetes mellitus is a metabolic disorder characterized by hyperglycemia, impairment of carbohydrate, lipid and protein metabolism, which is either due to insufficient secretion of insulin or insulin resistance. It is considered to be one of the chronic diseases after cancer and cardiovascular diseases. Among the different strategies used to control hyperglycemia, inhibition of the key enzymes of glucose metabolism like alpha amylase, alpha glucosidase and glucose-6-phosphatase is considered to be the most effective strategy.20 α-Amylase cleaves polysaccharides at α-1,4 glycosidic linkages and the products (oligo and disaccharides) are further hydrolyzed to free glucose by intestinal α-glucosidase. Controlling/lowering activity of these two enzymes slows down the digestion of carbohydrates, absorption of glucose hence lowering the overall blood glucose level.24,25 Glucose-6-phosphatase catalyses the last step of both glycogenolysis (breakdown of glycogen) and gluconeogenesis (synthesis of glucose from non-carbohydrate sources) pathways, as a result glucose level is maintained during fasting condition. Inhibition of this enzyme is also considered to be a good therapeutic target in disorders of carbohydrate metabolism and would be helpful in minimizing the severity of fasting hyperglycemia occurring in diabetic patients.26
Keeping in mind the therapeutic uses of thioureas, an attempt was made to synthesize novel symmetric and unsymmetric derivatives of thiourea and use them as remedy for oxidative stress, diabetes mellitus, and neurodegenerative disorders. The synthesized derivatives were also evaluated for their antibacterial potential against Gram positive and Gram negative bacterial strains.
Experimental
Materials
All the chemicals, reagents, and solvents used were of analytical grade and were used without any further purification. They were purchased from Sigma Aldrich Co, St Louis, MO, USA. The reaction progress was monitored using thin layer chromatography (TLC). The structures of synthesized compounds were confirmed by 1H-NMR in CDCl3 using NMR-Bruker apparatus.
Methods
Cinchona alkaloids are a group of naturally occurring compounds with promising biological activities due to the presence of primary and tertiary nitrogens in their structures. Keeping in mind the importance of these alkaloids, the simplest commercially available analog 2-picolyl amine was taken and a simpler symmetrical thiourea presented as compound II was synthesized using CS2 (1 equiv) in water and in the presence of sunlight. After confirmation that 2-picolyl amine has the capability of forming thiourea, the starting material 2-picolyl amine was derivatized to dibenzyl picolyl amine (Precursor P). Compound I and III were then synthesized using dibenzyl picolyl amine as starting material by two different methods described in the following section. Compound IV was prepared from 2,4-dimethyl aniline and phenyl isothiocyanate. Phenyl isothiocyanate is a commercially available compound, while 2,4-dimethyl aniline was prepared from 2, 4-dimethyl nitrobenzene by reduction of nitro group into amines by heterogenous catalyst under high pressure in the presence of hydrogen. Compound V was prepared from phenyl isothiocyanate and 2-fluoro aniline. The 2-fluoro aniline was prepared by reduction of the nitro group as described previously.
Preparation Of Dibenzyl Picolyl Amine (P)
The precursor dibenzyl picolyl amine was synthesized starting from acetylpyridine as more readily available starting material (Figure 1). The sodium hydride (NaH) was used to abstract proton from acetyl pyridine to make enolate which then attack on benzyl bromide (added dropwise). As a result, first substitution takes place. With reaction progress under same condition, a second substitution takes place. The reaction product was then treated with hydroxyl amine hydrochloride. As a result, oxime was obtained which was then reduced into amine. The amine was purified further by column chromatography using ethyl acetate and hexane as solvent system.27 The product was obtained as yellowish viscous oil with 68% yield.
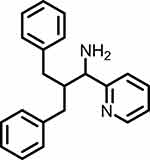 |
Figure 1 Chemical structure of 2-Benzyl-3-phenyl-1-(pyridin-2-yl)propan-1-amine (Precursor P). |
Synthesis Of Symmetrical Thioureas (Compounds I And II)
Picolylamine/Picolylamine derivatives (1.0 mmol, 2.0 equiv) were mixed with carbon disulphide (0.5 mmol, 1.0 equiv) in 10 mL EtOH/H2O (1:1) and placed in sunlight for 6–8 hrs. After 8 hrs, white precipitates were formed which were confirmed by NMR as symmetrical thioureas. The reaction mixture was kept at room temperature for 12 hrs until crystal formation.
Characterization Of Compound I
Brown crystals (yield 79%).
1HNMR (300 MHz, CDCl3-d) δ ppm: 4.5–4.7 (m, 8H), 5.2 (m, 2H), 5.4 (m, 2H), 6.8 (m, 4H), 6.9–7.1 (m, 8H), 7.2 (m, 8H), 7.5 (m, 2H), 7.7 (m, 2H), 8.1 (m, 2H), 8.5 (m, 2H), 8.9 (s, 2H). The chemical structure of compound I is given in Figure 2.
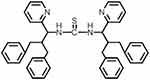 |
Figure 2 1,3-bis(2-benzyl-3-phenyl-1-(pyridine-2-yl)propyl)thiourea (Compound I). |
Characterization Of Compound II
Needle-like crystals (81% yield).
1HNMR (300 MHz, CDCl3-d) δ ppm 3.2 (d, 4H), 6.4 (d, 4H), 6.5 (d, 2H), 7.3 (m, 4H), 8.2 (s, 2H). The structural formula of compound II has been presented in Figure 3.
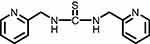 |
Figure 3 1,3-bis(1-(pyridin-2-ylmethyl)thiourea (Compound II). |
Synthesis Of Unsymmetrical Thioureas (Compound III-V)
The required amines were mixed with phenyl iso-thiocyanate (both at the ratio of 1.0 equiv at a concentration of 1.0 mmol, 1.0) in 6 mL anhydrous acetone. The reaction mixture was then refluxed for 8–10 hrs at 52–56°C. Progress of the reaction was checked through TLC with EtOAc/n-hexane solution (3:7). The reaction mixture was then cooled using crushed ice. Precipitates formed were then filtered. Filtered precipitates were washed with water, dried and recrystallized using ethanol.
Characterization Of Compound III
Yellow crystals (yield 87%).
1 HNMR (300 MHz, CDCl3-d) δ ppm: 2.32 (m, 1H), 2.59 (m, 2H), 2.77 (m, 2H), 3.08 (s, 1H), 7.04–7.28 (m, 15H), 7.37 (m, 2H), 7.65 (m, 2H), 7.82 (s, H, –NH), 8.47 (s, H, –NH). Compound III is presented in Figure 4.
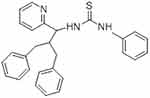 |
Figure 4 1-(2-benzyl-3-phenyl-1-(pyridine-2-yl) propyl)-3-phenylthiourea (Compound III). |
Characterization Of Compound IV
Yield: 93%.
1HNMR (300 MHz, CDCl3-d) δ ppm 2.30 (s, 3H), 2.34 (s, 3H), 7.05 (d, 1H), 7.11 (d, 1H), 7.20 (s, 1H), 7.25 (m, 1H), 7.35–7.42 (m, 4H), 7.97 (s, 2H). Compound IV is presented in Figure 5.
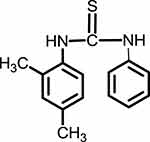 |
Figure 5 1-(2,4-dimethylphenyl)-3-phenylthiourea (Compound IV). |
Characterization Of Compound V
Yield: 96%.
1HNMR (300 MHz, CDCl3-d) δ ppm 7.0–7.49 (m, 9H), 7.8 (s, 1H, –NH), 7.9 (s, 1H, –NH). The chemical structure of compound V is given in Figure 6.
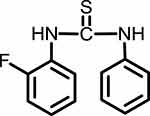 |
Figure 6 1-(2-fluorophenyl)-3-phenylthiourea (Compound V). |
Anticholinesterase Assay
The acetylcholinesterase inhibitory potential of the synthesized compounds was examined using Ellman’s assay.28 Acetylcholinesterase and butyrl choliesterase hydrolyses their respective substrates acetylthiocholine iodide and butyrylthiocholine iodide. The resulting product reacts with 5-thio-2-nitrobenzoate anion formed from DTNB resulting in the formation of yellow color product. The color changes were measured spectrophotometrically which were then converted into enzyme activity and percent inhibition.
Briefly, 205 µL compound dilutions (1000–125 µL/mL) were mixed with 5 µL of AChE (0.03 U/mL)/BChE (0.01 U/mL) and 5 µL DTNB and incubated in water bath at 30°C for 15 min. Then, 5 µL acetyl choline iodide/butyryl choline iodide (substrate). As a result, formation of yellow color anion (5-Thio-2-nitro benzoate) took place. The color change was measured after 4 mins at 412 NM using a double beam spectrophotometer (Thermo electron-corporation; USA). A blank solution was considered as a control. Galanthamine was used as positive control. Activity and inhibition of the selective enzymes were calculated by following relations:
(1) (2) (3)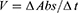
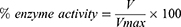
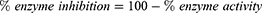
Where, V is the inhibitor dependent rate of reaction while, Vmax is the inhibitor independent rate of reaction.
Antidiabetic Potential Of The Synthesized Compounds
The antidiabetic potential of synthesized compounds was checked by monitoring their inhibitory effects on three important enzymes of carbohydrate metabolism viz alpha amylase, alpha glucosidase, and glucose-6-phosphatase.
The stock solutions of the compounds were prepared in 10% DMSO and phosphate buffer (pH 6.9) having a concentration of 20 mm. Different dilutions (1000–125 mg/mL) were prepared for each compound. About 200 µL of these dilutions was mixed with 200 µL of porcine α-amylase (2 U/mL) and pre-incubated for 10 mins at 30°C. Added to the mixture, was 200 µL of starch solution (1% w/v, made in 20 mM phosphate buffer, pH 6.9) and it was further incubated for 3 mins at 30°C. The reaction was stopped by adding 1 mL of 96 mM 3,5dinitrosalicylic acid (DNS). Again, the reaction mixture was incubated for 10 mins in boiling water bath (85–90°C) and then cooled at room temperature. Acarbose was used as positive control. Absorbance was monitored at 540 nm and percent inhibition was calculated using the following equation:
(4)
The α-glucosidase inhibition was carried out by mixing 100 µL of enzyme stock solution (0.5 unit/mL) with 600 µL stock phosphate buffer (pH 6.9) and 50 µL of each dilution prepared in the previous step and incubated at 37°C for 15 mins. To start the enzymatic reaction, 100 μL p-nitro-phenyl-α-D-glucopyranoside (5 mM) was added to the mixture which reached completion after 15 mins incubation at 37°C. To stop the reaction, 400 μL sodium carbonate (0.2 M) solution was added to the mixture. The absorbance of resulting mixture was measured at 405 nm. Percent inhibition of glucosidase was calculated using Formula 4. Acarbose was used as positive control and the same dilution was prepared by serial dilution.
The activity of the glucose-6-phosphatase was measured, which is based on the release of inorganic phosphate when the substrate glucose-6-phosphate is hydrolyzed by the enzyme. About 1 mL of 1 mM of the enzyme was taken, to which 1.5 mL tris (hydroxy methyl) amino methane buffer of pH 6.7 and 2.5 mL of 0.01 mM glucose-6-phosphate were added. The reaction mixture was incubated at 33°C for 1 hr. After the incubation, 1 mL 10% trichloroacetic acid was added to stop the reaction, and inorganic phosphate released during the reaction was measured by method of Fiske and Subbarow.29
Free Radical Scavenging Activities Of Synthesized Compounds
The free radical scavenging activities of synthesized compounds were determined following standard DPPH and ABTS protocols.10 DPPH stock solution was prepared by dissolving 20 mg of DPPH in 100 mL methanol. Blank (control) solution was prepared by taking 3 mL from the DPPH solution and its absorbance was adjusted to 0.75 at 515 nm. For the development of free radicals in stock solution, it was covered and kept in the dark for about 24 hrs. Stock solution (in 5 mL of methanol) of each compound was prepared and a series of dilutions (1000, 500, 250, 125, 62.5 µg/mL) were then prepared from it, using dilution formula. About 2 mL from each dilution was mixed with 2 mL DPPH and allowed to react for 15 mins in the dark. The percent inhibition by DPPH was calculated with the following equation:
(5)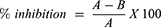
Where; A = absorbance of pure DPPH in oxidized form.
B = absorbance of sample taken after 15 mins of reaction with DPPH.
Ascorbic acid (5 mg/5 mL) was taken as a standard and its different solutions (1000, 500, 250, 125 µg/mL) were prepared.
The ABTS free radical scavenging was carried out using standard protocol of Re et al.30 About 7 mM of ABTS and 2.45 mM of potassium per sulphate were dissolved, each in 100 mL of methanol and were then mixed together. To develop free radicals of ABTS, the reaction mixture was kept in the dark for 12 hrs. Absorbance of blank control (3 mL) was adjusted to 0.75 at 745 nm by diluting it with 50% methanol. About 300 μL of each sample was taken, mixed with 3 mL of ABTS solution, and incubated for 15 mins at 25°C. Absorbance of the incubated mixture was measured at 745nm. The same procedure was done to prepare various dilutions of ascorbic acid (positive control). Percent free radical inhibition was calculated using Equation (5).
Anti-Bacterial Activities
The anti-bacterial activities of the synthesized compounds were determined against Staphylococcus aureus (Gram-positive bacteria), Agrobacterium tumefaction, and Proteus vulgaris (Gram-negative bacteria) bacterial strain. Agar well diffusion method was used following the standard protocol.31
Statistical Analysis
All the experiments were performed in three replicates and the results have been presented as mean ± SEM.
Results And Discussion
Effect Of Synthesized Compounds On Acetylcholinesterase And Butyrylcholinesterase
The effectiveness of the synthesized compounds as inhibitors of the selected cholinesterases was tested. The activities of both the enzymes were suppressed to a lesser or greater extent. The results are presented in Tables 1 and 2 respectively, for acetylcholinesterase and butyrylcholinesterase. As is clear from Table 1, compound III was more potent against acetylcholinesterase with IC50 value of 63 µg/mL, followed by compound II (IC50 = 82 µg/mL). Compound V was moderately active with IC50 value of 182 µg/mL while compound VI and I were merely active (IC50 = 503 and 1002 µg/mL respectively).
 |
Table 1 Effect Of The Compounds On The Activity Of Acetylcholinesterase Enzyme |
 |
Table 2 Effect Of The Compounds On The Activity Of Butrylcholinesterase Enzyme |
The butyrylcholinesterase inhibitory activities of the synthesized compounds are presented in Table 2. Almost the same trend observed for acetylcholinesterase was also observed here with little variations. Compound III was more active with IC50 value of 80 µg/mL, followed by compound II with IC50 = 82 µg/mL. Comparatively improved activities were observed for compound I, IV, and V with IC50 values of 200, 150, and 100 µg/mL respectively.
Based on the results obtained it can be concluded that compound III and II have some therapeutic values in the treatment of neurodegenerative diseases. However, in vivo studies regarding their toxicology are needed.
Effect Of Synthesized Compounds On The Activity Of Alpha Amylase
Alpha amylase is a key enzyme of carbohydrate metabolism that makes glucose available for the absorption in the small intestine from starch sources. Inhibition of this enzyme would be helpful in lessening the burden of blood glucose in diabetic patients. Table 3 shows the inhibitory potential of the compounds on alpha amylase. Compound IV with IC50 value of 62 µg/mL was the most potent, followed by compound I (IC50 = 84 µg/mL). Compound II, III, and V were also quite potent, but less than IV and I with IC50 values of 135, 115, and 100 µg/mL respectively. All these compounds have inhibitory effects of alpha amylase which is clear from their IC50 values.
 |
Table 3 Effect Of The Compounds On The Activity Of Alpha Amylase Enzyme |
Effect Of Synthesized Compounds On The Activity Of Alpha Glucosidase
Glucosidase is another important enzyme for carbohydrate metabolism and it helps in glucose release from starch sources. In diabetic patients, its inhibition relieves the blood glucose burden. Compound III and II were more effective inhibitors of alpha glucosidase with IC50 values of 75 and 80 µg/mL respectively. Compound IV appeared moderately active with IC50 value of 180 µg/mL. Compound I and V were merely active with IC50 values of 500 and 1000 µg/mL respectively (Table 4).
Effect Of Synthesized Compounds On The Activity Of Glucose-6-Phosphatase
Glucose-6-phosphatase is another important enzyme of carbohydrate metabolism that plays a very important role in glycogenolytic and gluconeogenic pathways. Its inhibition is needed in case of diabetes mellitus and enhancement of its activity is desired in patients suffering from glycogen storage disease. The inhibition of this enzyme is interpreted from the mg of inorganic phosphate released during the reaction. The smallest amount of phosphate released in a given reaction indicates high inhibition. Compound V and II were the most potent inhibitors of this enzyme as the amount of phosphate released was comparatively less than that of other compounds (Table 5). Compound I, III, and V were also quite potent when compared to control experiments. As stated earlier, enhancers of this enzyme are also required in case of glycogen storage disease patients, however, even though all of these compounds were inhibitors of this enzyme, none of them exhibited the enhanced role.
Free Radical Scavenging Activities Of The Compounds
Free radicals are the chemically reactive substances with singlet electron. Free radicals are continuously produced in the human body and are promptly scavenged by the body's defense system. However, when their production increases, supplementation of antioxidants are needed from outside, otherwise they damage the biologically important molecules (DNA and protein). All these synthesized compounds showed scavenging activities against the commercially available free radicals DPPH and ABTS, which is clear from Table 6. Compound IV was a more potent inhibitor of DPPH free radical (IC50 = 64 µg/mL) followed by compound I (IC50 = 67 µg/mL). Compound V and III were also quite effective in scavenging the DPPH radical and exhibited IC50 values of 70 and 79 µg/mL respectively. The least effective compound was compound II.
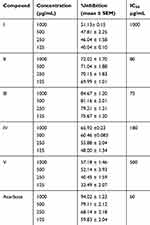 |
Table 4 Effect Of The Compounds On Activity Of Enzyme Alpha Glucosidase |
 |
Table 5 Effect Of The Compounds On Activity Of Enzyme Glucose-6-Phosphatase |
 |
Table 6 Estimation Of Free Radical Scavenging Potential Through DPPH And ABTS Assays |
A totally different inhibitory pattern was observed in case of ABTS free radical. Compound I was more potent with IC50 value of 66 µg/mL, followed by compound III and V (IC50 = 80 and 85 µg/mL respectively). Compound IV was moderately effective while compound II was the least effective one.
Anti-Bacterial Activities Of The Compounds
The antibacterial spectrum of the synthesized compounds was determined against A. tumefaction, P. vulgaris, and S. aureus. The results have been presented in Table 7. Among the tested compounds, compound I was highly active against A. tumefaction with MIC value of 4.02 µg/mL followed by compound II (MIC = 4.02 µg/mL). Against the same bacteria, compound III, IV and V were also quite effective with MIC values of 11.62, 13.49, and 11.59 µg/mL respectively.
 |
Table 7 Evaluation Of Antibacterial Potential Of The Synthesized Compounds |
Against P. vulgaris, compound V was more effective followed by compound I (MIC = 8.94 and 9.76 µg/mL respectively). The rest of the compounds were also quite effective against this bacterial strain.
The growth of S. aureus was more effectively inhibited by compound IV (MIC= 4.03 µg/mL). The rest of the compounds were moderately active. Comparatively, all these compounds exhibited good antibacterial actions against all the selected bacterial strains.
Conclusion
The wide applicability of thioureas in various fields, especially in the field of medicines, makes it quite practical to study biological activities of an already synthesized compound or to synthesize some more and determine their therapeutic significance, if any. In this study, five new thioureas were synthesized from chiral amines in the presence of sunlight (hence eco-friendly and economic procedure) which were then used as starting material in the synthesis of N-heterocyclic thiourea compounds. The compounds were tested for their antibacterial, antioxidant, anticholinesterase, and antidiabetic potentials. Glucose-6-phosphatase was potently inhibited by compound V and II, α-amylase by compound IV and I, α-glucosidase by compound III and II while acetylcholinesterase and butyrylcholinesterase were inhibited by compound III and II. As free radical scavengers, compound IV was a more potent inhibitor of DPPH radical while compound II was the most effective against ABTS. Their antibacterial evaluation revealed that all these compounds were effective against A. tumefaction. Even though the in vitro experiments pointed out the good biological activities of these compounds, it is still not safe to assume that they are good therapeutic agents and further testing and in vivo experiments are needed to fully assess its pharmacological applicabilities.
Availability Of Data And Material
The data in the form of the thesis will be provided on demand.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no RG-1440-100.
Author Contributions
S.N. and M.Z. conceived and designed the experiments; M.N.U., B.A., R.U. and M.U.K.S. performed the experiments; M.Z., A.A.S and H.M.M. analyzed the data; M.Z., and M.U.K.S contributed reagents and materials. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bhanja C, Jena S. Rational synthesis design of a potent anti-diabetic therapeutic’pioglitazone’using retrosynthetic analysis. J Chem Pharm Res. 2012;4(9):4323–4333.
2. Kulshrestha AA. Physicochemical Studies of Some Compounds. Saurashtra University; 2009.
3. Didwagh SS, Piste PB, Burungale AS, Nalawade AM. Synthesis and antimicrobial evaluation of novel 3-(4, 6-diphenyl-6H-1, 3-thiazin-2-yl)-2-(4-methoxyphenyl) thiazolidin-4-one derivatives. J Appl Pharm Sci. 2013;3(11):122–127.
4. Alkherraz AM, Lusta ZI, Zubi AE. Synthesis and use of thiourea derivative (1-phenyl-3-benzoyl-2-thiourea) for extraction of cadmium ion. Int J Chem Nucl Metall Mater Eng. 2014;8:118–120.
5. Shaik T, Chennamsetty S, Devineni S, et al. Catalyst-free green synthesis of urea and thiourea derivatives of tetramethylguanidine (TMG) and evaluation of biological activity. Published Bulgarian Chem Comm. 2014;46(4):724–730.
6. Wermuth C, Aldous D, Raboisson P, Rognan D. Medicinal chemistry: definitions and objectives, drug activity phases, drug classification systems. In: The Practice of Medicinal Chemistry. Academic Press; 2015.
7. Nagalakshmi G, Maity T, Maiti B. Synthesis, characterization and anti-HIV evaluation of some novel 2-[(substitutedphenyl/heteroaryl) imino]-3-phenyl-1, 3-thiazolidin-4-ones. Synthesis. 2013;5(5):2079–2089.
8. Pattan S, Kedar M, Pattan J, et al. Synthesis and evaluation of some novel 2, 4-thiazolidinedione derivatives for antibacterial, antitubercular and antidiabetic activities. Indian J Chem. 2012;51B:1421–1425. doi:10.1094/PDIS-11-11-0999-PDN.
9. Bielenica A, Stefańska J, Stępień K, et al. Synthesis, cytotoxicity and antimicrobial activity of thiourea derivatives incorporating 3-(trifluoromethyl) phenyl moiety. Eur J Med Chem. 2015;101:111–125. doi:10.1016/j.ejmech.2015.06.027
10. Kulakov I, Nurkenov O, Akhmetova S, Seidakhmetova R, Zhambekov Z. Synthesis and antibacterial and antifungal activities of thiourea derivatives of the alkaloid anabasine. Pharm Chem J. 2011;45(1):15–18.
11. Hosseinzadeh H, Nassiri‐Asl M. Avicenna’s (Ibn Sina) the canon of medicine and saffron (Crocus sativus): a review. Phytother Res. 2013;27(4):475–483. doi:10.1002/ptr.4784
12. Kumar V, Chimni SS. Recent developments on thiourea based anticancer chemotherapeutics. Anticancer Agents Med Chem. 2015;15(2):163–175.
13. Bharate SB, Thompson CM. Antimicrobial, antimalarial, and antileishmanial activities of mono‐and bis‐quaternary pyridinium compounds. Chem Biol Drug Des. 2010;76(6):546–551.
14. Choi J, Jee J-G. Repositioning of thiourea-containing drugs as tyrosinase inhibitors. Int J Mol Sci. 2015;16(12):28534–28548. doi:10.3390/ijms161226114
15. Suyoga Vardhan D, Shantharam C, Suhas R, Sridhara M, Channe Gowda D. Synthesis and urease inhibition studies of ureas and thioureas derived from amino acids conjugated heterocycle. International Journal of Chemical and Pharmaceutical Sciences. 2013;4(3):54–58.
16. Imran S, Taha M, Ismail NH, Fayyaz S, Khan KM, Choudhary MI. Synthesis, biological evaluation, and docking studies of novel thiourea derivatives of bisindolylmethane as carbonic anhydrase II inhibitor. Bioorg Chem. 2015;62:83–93. doi:10.1016/j.bioorg.2015.08.001
17. Antunes S, Corre J-P, Mikaty G, Douat C, Goossens PL, Guichard G. Effect of replacing main-chain ureas with thiourea and guanidinium surrogates on the bactericidal activity of membrane active oligourea foldamers. Bioorg Med Chem. 2017;25(16):4245–4252.
18. Bari WU, Zahoor M, Zeb A, et al. Anticholinesterase, antioxidant potentials, and molecular docking studies of isolated bioactive compounds from Grewia optiva. Int J Food Prop. 2019;22(1):1386–1396.
19. Zahoor M, Zafar R, Rahman NU. Isolation and identification of phenolic antioxidants from Pistacia integerrima gall and their anticholine esterase activities. Heliyon. 2018;4(12):e01007. doi:10.1016/j.heliyon.2018.e01007
20. Nazir N, Zahoor M, Nisar M, et al. Phytochemical analysis and antidiabetic potential of Elaeagnus umbellata (Thunb.) in streptozotocin-induced diabetic rats: pharmacological and computational approach. BMC Complement Altern Med. 2018;18(1):332. doi:10.1186/s12906-018-2317-3
21. Zahoor M, Shafiq S, Ullah H, Sadiq A, Ullah F. Isolation of quercetin and mandelic acid from Aesculus indica fruit and their biological activities. BMC Biochem. 2018;19(1):5.
22. Ovais M, Ayaz M, Khalil AT, et al. HPLC-DAD finger printing, antioxidant, cholinesterase, and α-glucosidase inhibitory potentials of a novel plant Olax nana. BMC Complement Altern Med. 2018;18(1):1. doi:10.1186/s12906-018-2317-3
23. Saeed A, Zaib S, Ashraf S, et al. Synthesis, cholinesterase inhibition and molecular modelling studies of coumarin linked thiourea derivatives. Bioorg Chem. 2015;63:58–63. doi:10.1016/j.bioorg.2015.09.009
24. Zahoor M,Jan R, Naz S. Investigation of repressive and enhancive effects of fruit extracts on the activity of glucose-6-phophatase. Pak J Phar Sci. 2016;29(6):1985–1991.
25. Gleason CA, Juul SE. Inborn Errors of Carbohydrate, Ammonia, Amino Acid, and Organic Acid Metabolism. In: J Lawrence Merritt II, Renata C Gallagher, editors. Avery's Diseases of the Newborn (Tenth Edition). Elsevier; 2018:230–252.
26. Lee M-W, Chanda D, Yang J, et al. Regulation of hepatic gluconeogenesis by an ER-bound transcription factor, CREBH. Cell Metab. 2010;11(4):331–339. doi:10.1016/j.cmet.2010.02.016
27. Nugent TC, Umar MN, Bibi A. Picolylamine as an organocatalyst template for highly diastereo-and enantioselective aqueous aldol reactions. Org Biomol Chem. 2010;8(18):4085–4089.
28. Peng L, Rong Z, Wang H, et al. A novel assay to determine acetylcholinesterase activity: the application potential for screening of drugs against Alzheimer’s disease. Biomed Chromatogr. 2017;31(10):e3971. doi:10.1002/bmc.3882
29. Fiske CH, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66(2):375–400.
30. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9–10):1231–1237. doi:10.1016/s0891-5849(98)00315-3
31. Cheruiyot K, Olila D, Kateregga J. In-vitro antibacterial activity of selected medicinal plants from Longisa region of Bomet district, Kenya. Afr Health Sci. 2009;9(2):S42–S46.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
