Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Ensifentrine as a Novel, Inhaled Treatment for Patients with COPD
Authors Donohue JF , Rheault T, MacDonald-Berko M, Bengtsson T, Rickard K
Received 30 March 2023
Accepted for publication 3 July 2023
Published 28 July 2023 Volume 2023:18 Pages 1611—1622
DOI https://doi.org/10.2147/COPD.S413436
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
James F Donohue,1 Tara Rheault,2 Margot MacDonald-Berko,2 Thomas Bengtsson,3 Kathleen Rickard2
1Division of Pulmonary and Critical Care Medicine, University of North Carolina, School of Medicine, Chapel Hill, NC, USA; 2Verona Pharma plc, Raleigh, NC, USA; 3Stat Mind AB, Lund, Sweden
Correspondence: Tara Rheault, Verona Pharma, 8045 Arco Corporate Drive, Suite 130, Raleigh, NC, 27617, USA, Tel +1 646-530-2126, Email [email protected]
Abstract: Ensifentrine is a novel, potent, and selective dual inhibitor of phosphodiesterase (PDE)3 and PDE4 designed for delivery by inhalation that combines effects on airway inflammation, bronchodilation and ciliary function in bronchial epithelia. In Phase 2 studies in subjects with COPD, ensifentrine demonstrated clinically meaningful bronchodilation and improvements in symptoms and health-related quality of life when administered alone or in combination with current standard of care therapies. Ensifentrine is currently in late-stage clinical development for the maintenance treatment of patients with COPD. This review summarizes non-clinical data as well as Phase 1 and Phase 2 efficacy and safety results of nebulized ensifentrine relevant to the maintenance treatment of patients with COPD.
Keywords: RPL554, nebulized therapy, chronic obstructive pulmonary disease, clinical efficacy, drug development
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by progressive airflow obstruction which is largely irreversible and is associated with chronic inflammation of the respiratory tract, acute exacerbations, airway remodeling and excessive mucus production.1 Novel treatments, which can provide additional meaningful symptom relief, bronchodilation and reduction in exacerbations without side effects seen with current classes of treatments, such as corticosteroids or oral phosphodiesterase (PDE) 4 inhibitors, are needed because patients are still symptomatic despite maximal use of existing therapies.2,3
Inhibitors of PDE3 and PDE4 target a range of respiratory functions at the cellular level and tissue level: PDE3 regulates cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) concentrations in airway smooth muscle, which mediates bronchial tone.4–6 PDE4 regulates cAMP concentrations in cells associated with airway inflammation and is involved in inflammatory cell activation.7–9 Inhibitors of PDE4 have been shown to stimulate Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) and increase ciliary beat frequency in human bronchial epithelial cells.10,11 Evidence also suggests that combined inhibition of PDE3 and PDE4 may have additive or synergistic effects with respect to both anti-inflammatory and bronchodilator activity due to the expression of both PDE isoforms on inflammatory cells and airway smooth muscle.12–14
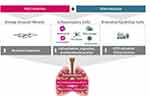
Ensifentrine (RPL554) is a novel, potent and selective, dual inhibitor of PDE3 and PDE4 designed for delivery by inhalation combining effects on airway inflammation, bronchodilation and increased ciliary beat frequency in bronchial epithelial cells (refer to Figure 1).15–20 Ensifentrine is currently in late-stage clinical development, having completed two Phase 3 trials, for the maintenance treatment of patients with COPD (NCT04535986 [with a 48-week safety subset] and NCT04542057).21
This review summarizes non-clinical ensifentrine data as well as Phase 1 and Phase 2 efficacy and safety results of nebulized ensifentrine relevant to the maintenance treatment of patients with COPD.
Non-Clinical Data
Studies evaluating the bronchorelaxant and bronchoprotective effects of ensifentrine have been previously described.15,22–25 In vitro and in vivo anti-inflammatory experiments of ensifentrine were described by Boswell-Smith et al15 and further detailed by Rheault et al.26 In vitro human bronchial epithelial (HBE) cell experiments evaluating effects on ciliary beat function and cAMP activation have been described by Turner et al.20,27–29
In vitro Studies
In enzyme activity assays of recombinant PDE3A, PDE3B, and PDE4B2, ensifentrine inhibited PDE activity with IC50 values of 0.25, 0.29 and 290 nM, respectively.26 The inhibitory activity of ensifentrine on PDE4 was aligned with the positive control rolipram (130 nM)26 and with reported functional activity of ensifentrine on PDE4-mediated inhibition of TNFα production in lipopolysaccharide (LPS)-stimulated human monocytes (520 nM) and proliferation of human mononuclear cells stimulated with phytohemagglutinin (460 nM).15 Ensifentrine has been shown to increase levels of cAMP in human neutrophils and CFBE41o-WT cells, demonstrating the ability of ensifentrine to inhibit PDE3 and PDE4 and produce expected effects in a cellular mechanistic assay.15,28
Ensifentrine has also been shown to activate CFTR mediated chloride ion secretion in in vitro human normal and cystic fibrosis bronchial epithelial cells.20 Ensifentrine (10µM) potentiated forskolin stimulated CFTR activity approximately 10-fold. Additionally, treatment of HBE cells with ensifentrine for 30 minutes significantly increased ciliary beat frequency by 2.6 ± 0.8% (p < 0.05; n = 11) at 1 minute, 8.1 ± 1.2% (p < 0.001; n = 11) at 5 minutes and 10.4 ± 1.8% (p < 0.001; n = 11) at 30 minutes following treatment compared with time-matched controls.20
Ensifentrine has been shown to 1) produce additive effects on CFTR activation when administered in combination with ivacaftor;20 2) elevate intracellular cAMP leading to a potentiation of forskolin-stimulated current mediated by multiple class III/IV mutants of CFTR;27 and 3) stimulate a robust, CFTR-dependent secretion and a reduction in the production of pro-inflammatory cytokines in cystic fibrosis bronchial epithelial cells stimulated with Interleukin-1β.28,29 Taken together, these data suggest that ensifentrine has the potential to enhance mucociliary clearance in COPD patients where ciliary dysfunction has been described, and increased mucus production and cough are commonly reported symptoms.29
In vivo Studies
In isolated airway tissue preparations, ensifentrine produced potent, long-lasting relaxant effects on contractile responses induced by spasmogens (eg, histamine, carbachol) and electrical field stimulation (EFS).15 In guinea pig tracheal preparations, ensifentrine was shown to inhibit contractile responses to EFS by approximately 50% at a concentration of 100 nM. Ensifentrine inhibited EFS-induced contraction of isolated human bronchial tissue and isolated guinea pig trachea, with almost complete inhibition of the contractile response maintained for 5 and 6 hours, respectively, after termination of the perfusion with ensifentrine.
Ensifentrine alone induced maximal relaxation following EFS stimulation and carbachol-induced contraction, while ensifentrine administered with the muscarinic receptor antagonist, glycopyrronium, induced a concentration-dependent relaxation of the contraction induced by EFS as well as a concentration-dependent relaxation of human bronchioles.24 In addition, ensifentrine relaxed isolated human bronchial and guinea pig tracheal tissues which had been pre-contracted with spasmogens and demonstrated additional relaxation in isolated airway preparations when bronchodilator drugs such as salbutamol, ipratropium and albuterol/ipratropium combination did not achieve maximal relaxation.25 Ensifentrine alone relaxed human bronchi and produced additive or synergistic inhibition of airway smooth muscle contraction when combined with the β2-agonist, salbutamol and the muscarinic receptor antagonist, glycopyrronium.22
The bronchoprotective properties of ensifentrine demonstrated in ex vivo isolated airway tissues also translate to efficacy in vivo when administered by the inhaled route. Specifically, inhaled ensifentrine (0.01, 0.02, 0.04, 0.08 mg/kg) protected against spasmogen- and antigen-induced bronchoconstriction in guinea pigs. Ensifentrine alone caused a dose-dependent relaxation of guinea pig airways (7–65% reduction in airway obstruction) and demonstrated synergistic effects on bronchodilation when administered in combination with atropine (42–82% reduction in airway obstruction) and salbutamol (64–78% reduction in airway obstruction) in vivo.23
Ensifentrine has demonstrated anti-inflammatory activity in vivo in various allergic guinea pig models at doses that produced smooth muscle effects as described by Boswell-Smith et al.15 Ensifentrine significantly (p < 0.05) attenuated eosinophil recruitment in guinea pigs 24 hours after ovalbumin challenge following oral administration (10 mg/kg) and dry powder inhalation (25% w/w micronized lactose blend in a volumatic chamber; 1.5 hours prior to antigen challenge) compared with sham-immunized animals. A significant (p < 0.05) inhibition in eosinophil peroxidase levels in bronchoalveolar lavage (BAL) following allergen challenge was also shown.15 Treatment with aerosolized ensifentrine (0.3 mg/mL) in ovalbumin-sensitized guinea pigs resulted in a significantly reduced (p = 0.030) recruitment of total cells in BAL fluid, including reductions in neutrophils, monocytes, and eosinophil recruitment.26 Additionally, pre-treatment with inhaled ensifentrine (0.1, 0.3 and 1.0 mg/mL) resulted in a significant and dose-dependent suppression of antigen-induced eosinophil migration into nasal passages in sensitized guinea pigs, including >80% inhibition of antigen-induced eosinophil migration with inhaled ensifentrine (1.0 mg/mL).26
Clinical Efficacy Data
Primary pharmacodynamic studies demonstrate that ensifentrine is a long-lasting, reversible PDE3 and PDE4 inhibitor possessing bronchodilator, bronchoprotective, and anti-inflammatory properties at therapeutically relevant doses.
Healthy Volunteers
A randomized, double-blind, placebo-controlled, cross-over study in healthy volunteers was conducted to evaluate the anti-inflammatory effects of nebulized ensifentrine solution or placebo on bacterial LPS-induced inflammatory cell recruitment to the airways (LPS-challenge), a model of COPD-like inflammation (Table 1).16 Inhaled ensifentrine solution (approximately 1.5 mg dosed once-daily for 6 days) resulted in statistically significant reductions in inflammatory cells of sputum samples 6 hours after LPS-challenge. Absolute numbers of neutrophils were reduced by 40% (6.81 × 106 cells vs placebo 11.38 × 106 cells/g sputum; p = 0.002), macrophages by 27% (1.16 × 106 cells vs 1.58 × 106 cells/g sputum; p = 0.044), eosinophils by 44% (0.014 × 106 cells vs 0.025 × 106 cells/g sputum; p = 0.001), lymphocytes by 67% (0.014 × 106 cells vs 0.042 × 106 cells/g sputum; p = 0.001), and total cells by 36% (8.66 × 106 cells vs placebo 13.57 × 106 cells/g sputum; p = 0.002).
 |
Table 1 Summary of Clinical Study Designs of Nebulized Ensifentrine in Healthy Volunteers and in Subjects with COPD |
Subjects with COPD
Nebulized inhaled ensifentrine was evaluated in a series of Phase 2a and Phase 2b clinical trials in subjects with moderate-to-severe COPD. The design features of these completed Phase 2 are described in Table 1. The clinical profile of nebulized ensifentrine suspension included statistically significant and clinically relevant bronchodilation over 12 hours following administration as a single dose, twice daily over 3-days, and twice daily over 4 weeks either alone or in combination with a short-acting β2-agonist, short-acting muscarinic antagonist or long-acting muscarinic antagonist. Significant reductions in lung volumes (residual volume, RV) were also demonstrated after single doses with ensifentrine alone or added on to short-acting bronchodilators and over 3-days of twice daily treatment with ensifentrine added on to a long-acting muscarinic antagonist compared with placebo. Ensifentrine has been well tolerated up to 6 mg twice daily for 4 weeks. A summary of the primary endpoint results of the Phase 2 studies is included in Table 2.
 |
Table 2 Summary of Phase 2 Clinical Study Efficacy Data of Nebulized Ensifentrine by Dose in Subjects with COPD |
Ensifentrine Monotherapy
RPL554-CO-203 was a 4-week Phase 2b, randomized, double-blind, placebo-controlled, parallel-group study in 403 subjects with moderate-to-severe COPD who were not taking concomitant maintenance (long-acting) bronchodilator therapies. Subjects were randomized to receive nebulized ensifentrine suspension (0.75, 1.5, 3 or 6 mg) or placebo twice daily for 4 weeks.18
All doses of ensifentrine met the primary endpoint, showing a statistically significant increase in peak FEV1 versus placebo (p < 0.001) with placebo-corrected changes from baseline >200 mL in peak FEV1 after Day 1 and after 4 weeks of dosing. A dose-response was evident up to the 3-mg dose, with the 6-mg mg dose appearing to not provide additional effects beyond the 3-mg dose. In addition, statistically significant improvements in average FEV1 over 12 hours were observed at all doses on Day 1 and Week 4 that were also dose-ordered up to 3 mg. The ensifentrine 3-mg dose also demonstrated a consistent improvement in morning trough FEV1 (68 to 89 mL, p < 0.05 for weeks 1 through 4) compared to placebo. Notably, statistically significant and clinically meaningful improvements in COPD symptoms were shown using the Evaluating Respiratory Symptoms in COPD (E-RS™: COPD)33 measure, a validated daily assessment of stable COPD symptoms.18,33 Ensifentrine showed a meaningful reduction in symptoms on the Total Score and all subscales of the E-RS (breathlessness, chest symptoms, cough/sputum) at or near the minimal clinically important difference (MCID) of −2 units at Week 4. A statistically significant improvement in dyspnea as measured via the Transition Dyspnea Index (TDI)34 compared with placebo at Week 4 was observed for all ensifentrine doses (p < 0.05) with improvements that exceeded the MCID of 1.0 unit. Health-related quality of life (QoL) was assessed using the St. George’s Respiratory Questionnaire-COPD (SGRQ-C),35 and nominal improvements from baseline to Week 4 were also observed compared to placebo, although statistical significance was not demonstrated.
Ensifentrine in Combination with Standard of Care β2-Agonist or Muscarinic Antagonist Bronchodilators
RPL554-009-2015 was a single-dose Phase 2, randomized, double-blind, placebo-controlled, double-dummy, complete-block, 6-way cross-over study in 33 subjects with moderate-to-severe COPD randomized to receive single doses of nebulized ensifentrine suspension (6 mg) or placebo, inhaled salbutamol (200 μg) or placebo, and inhaled ipratropium (40 μg) or placebo.17
Ensifentrine alone (6 mg) produced a statistically significant improvement in peak and average FEV1 over 8 hours compared to placebo (p < 0.001, Table 2). Furthermore, ensifentrine alone was as effective as salbutamol and ipratropium as a bronchodilator as measured by change from baseline in peak FEV1 (p < 0.001). Ensifentrine (6 mg) produced significant additive bronchodilation when dosed with salbutamol or ipratropium compared to placebo dosed with salbutamol or ipratropium. Improvement in absolute peak FEV1 over 8 hours was 302 mL for ensifentrine alone (6 mg), 384 mL when added to salbutamol (geometric least-squares mean ratio %: 104.53; 95% CI: 101.41–107.75; p = 0.004 vs ensifentrine alone) and 385 mL when added to ipratropium (geometric least-squares mean ratio %: 104.53; 95% CI: 101.41–107.76; p = 0.005 vs ensifentrine alone).
Additionally, ensifentrine produced a statistically significant reduction in residual lung volumes and airway resistance when dosed as a monotherapy compared to placebo and also when added on to salbutamol or ipratropium compared to either bronchodilator. Finally, the onset of action (defined as the median timepoint when 50% of subjects reached a 10% improvement in FEV1) was significantly faster for the ensifentrine added on to salbutamol or ipratropium compared to the individual component (3.6 minutes with ensifentrine + salbutamol, 6 minutes with salbutamol, 4.8 minutes with ensifentrine + ipratropium, 18.4 minutes with ipratropium and 14.6 minutes with ensifentrine).
RPL554-CO-202 was a 3-day Phase 2, randomized, double-blind, placebo-controlled, complete-block, 3-way cross-over study in 29 subjects with moderate-to-severe COPD randomized to receive nebulized ensifentrine suspension (1.5 and 6 mg) or placebo BID in addition to open-label tiotropium (Spiriva® Handihaler®) once daily (QD) for 3-days.17
Ensifentrine (1.5 and 6 mg) BID added on to tiotropium QD for 3 days demonstrated statistically significant and clinically meaningful improvements in bronchodilation in subjects with ensifentrine (1.5 and 6 mg) compared to placebo. Ensifentrine (1.5 and 6 mg) + tiotropium increased peak FEV1 measured over 4 hours by 104 mL and 127 mL on Day 3, respectively, compared to placebo + tiotropium. Morning trough FEV1 on Day 3 was increased from baseline by 116 mL (p < 0.001) and 54 mL (p = 0.347) with ensifentrine 6 mg + tiotropium and 1.5 mg + tiotropium compared to placebo + tiotropium.
Both doses of ensifentrine added on to tiotropium significantly reduced residual lung volumes and airway resistance compared to placebo + tiotropium. Ensifentrine 6 mg + tiotropium significantly reduced residual volume at 1.25h post-dose on Day 2 (198 mL, p = 0.005) and specific airway conductance (sGaw, 0.252/(kPa*sec), p < 0.001) compared with placebo + tiotropium. Ensifentrine (1.5 and 6 mg) + tiotropium was also shown to significantly increase the speed of onset (time to 10% improvement in FEV1) of the bronchodilator effect to under 5 minutes compared to 37.6 minutes with placebo + tiotropium (p < 0.001).
RPL554-CO-205 was a 4-week Phase 2b, randomized, double-blind, placebo-controlled, parallel-group study in 413 subjects with moderate-to-severe COPD added on to steady state tiotropium. Subjects were enrolled who remained symptomatic and with impaired lung function following a 2-week tiotropium run-in, and once-daily tiotropium was taken throughout the 4-week study. Subjects were randomized to receive nebulized ensifentrine suspension (0.375, 0.75, 1.5 and 3 mg) or placebo BID in addition to tiotropium (Spiriva® Respimat®) QD.19
All doses of ensifentrine met the primary endpoint, showing a statistically significant increase in peak FEV1 (all p < 0.05) which was sustained over 4 weeks. A dose-ordered improvement in FEV1 was observed with maximal effects achieved with ensifentrine 3 mg (124 mL, p < 0.001) compared to placebo + tiotropium. In addition, a statistically significant and meaningful improvement in average FEV1 AUC0-12h was shown with ensifentrine 3 mg at Week 4 (87 mL, p = 0.011). Ensifentrine 3 mg + tiotropium showed a nominal improvement in morning trough FEV1 at Week 4 that was not significant compared to placebo + tiotropium. Notably, statistically significant and clinically meaningful improvements in health-related QoL as measured by SGRQ-C were observed at Week 4 with ensifentrine (1.5 and 3 mg) + tiotropium (placebo-corrected −4.8 [p = 0.012] and −4.0 [p = 0.033 respectively]), exceeding the MCID of −4 units for both doses. Improvements in COPD symptoms (measured via E-RS™:COPD) from baseline to Week 4 were observed that were nominally greater than placebo + tiotropium, although the improvements did not achieve statistical significance. Similarly, numerical improvements in dyspnea were observed with ensifentrine (0.375, 1.5 and 3 mg) as measured by TDI compared with placebo + tiotropium at Week 4. While both 4-week Phase 2b studies, RPL554-CO-20318,31 and RPL554-CO-20519 were not powered to show a statistical benefit with ensifentrine on symptom or QoL measures, consistent improvements compared to placebo were observed in both studies on these important aspects of COPD treatment.
Clinical Safety Data
Healthy Volunteers
Nebulized ensifentrine solution (approximately 1.5 mg), when dosed once daily over six days in healthy volunteers, was shown to be well tolerated with an adverse event profile similar to placebo.16
Single doses of nebulized ensifentrine suspension (3 and 9 mg) in healthy volunteers in a Phase 1, randomized, double-blind, placebo- and positive-controlled, 4-way crossover thorough QT (TQT) study did not prolong the QT interval or otherwise have a clinically significant effect on cardiac conduction parameters including the PR and QRS intervals at the therapeutic dose of 3 mg or the supratherapeutic dose of 9 mg.30
Subjects with COPD
Clinical safety of nebulized ensifentrine in subjects with COPD has been assessed at doses ranging from 0.375 mg to 6 mg in single dose studies, BID over 3-days, and BID over 28-days, including the incidence of adverse events, pre- and post-dose vital signs, pre- and post-dose ECGs, 24-hour Holter monitoring and laboratory safety measures. To date, no significant medical risks or areas of particular medical safety concern to patients with COPD have been identified.
Overall, adverse events (AEs) reported in clinical studies were generally comparable in frequency and severity to placebo, with no trends noted in AE terms or system organ class. There has been no evidence of significant AEs related to ensifentrine, including those involving the cardiovascular or gastrointestinal systems up to and including doses of 6 mg BID for 4 weeks.18,19 Additionally, adverse effects reported with other marketed PDE4 inhibitors, including nausea, vomiting and diarrhea, have been rarely observed in studies of nebulized ensifentrine (incidence similar to placebo).
In both 4-week studies of ensifentrine (RPL554-CO-203, RPL554-CO-205), a total of 165 subjects received nebulized ensifentrine suspension (3 mg) BID and 163 received nebulized placebo BID. Table 3 lists the most-commonly reported pooled AEs in subjects that received either ensifentrine 3 mg or placebo over 4 weeks. The most-commonly reported AEs (by more than one subject) were headache 5% (compared to 2.5% for placebo), worsening COPD 3.6% (compared to 3.7% for placebo), cough 4.2% (compared to 1.2% for placebo) and dyspnea 1.8% (compared to 3.1% for placebo).
 |
Table 3 The Most-Commonly Reported AEs (by More Than One Subject) with Nebulized Ensifentrine (3 mg) Twice Daily in Subjects with COPD from Two 4-Week Phase 2 Dose-Ranging Studies RPL554-CO-203 and RPL554-CO-205a,32 |
Overall, serious adverse events (SAEs) have been uncommon with no apparent pattern in the SAEs reported to date and no significant safety concerns identified with ensifentrine. No significant medical risks to subjects have been identified thus far with ensifentrine in healthy volunteers, or in subjects with COPD.
Consistent changes in vital signs (blood pressure and pulse) and ECG parameters including QTcF have not been observed with ensifentrine at any dose, except for a small and transient increase in peak heart rate with the 6-mg dose of approximately 3 beats per minute in subjects with COPD dosed twice daily for 4 weeks.32 Twenty-four-hour Holter monitoring conducted pre-dose before and after -weeks of treatment in 324 subjects (RPL554-CO-203) showed no arrhythmogenic effects in subjects dosed with ensifentrine from 0.75 mg to 6 mg twice daily. Effects on laboratory safety parameters associated with ensifentrine have not been observed.
Discussion
Patients with COPD rely on inhaled maintenance therapies (eg, bronchodilators and ICS) for long-term symptom control.36,37 The dual inhibition of PDE3/4 following inhaled ensifentrine administration directly to the lungs maximizes local effects in the lung (bronchodilation and anti-inflammatory effects) and minimizes the possibility of systemic effects observed with orally- delivered PDE3 and PDE4 inhibitors.13,38–40
Ensifentrine has been shown to inhibit PDE3 and PDE4 in vitro, showing expected mechanistic effects in enzyme and cellular assays on cAMP and downstream functional effects of PDE4 inhibition on inflammatory cells. In vivo, ensifentrine has demonstrated relaxation of isolated human bronchial and guinea pig tracheal tissues pre-contracted with spasmogens demonstrating functional effects of PDE3 inhibition.22–25 The bronchodilator and bronchoprotective properties of ensifentrine in ex vivo isolated airway tissues also translate to efficacy in vivo when administered by the inhaled route. Ensifentrine has shown dose-dependent effects in animal models of lung inflammation at the same doses that produced bronchoprotective/smooth muscle effects in vivo; thus, ensifentrine has demonstrated bronchodilator, bronchoprotective, and anti-inflammatory effects in the airways consistent with a mechanism of action of dual inhibition of PDE3 and PDE4.
In clinical studies to date up to 4 weeks, twice daily nebulized ensifentrine suspension has demonstrated consistent and sustained bronchodilation that is clinically meaningful, reduction in residual volumes and improvements in COPD symptoms and quality of life either alone or in combination with other classes of bronchodilators.17–19,41 Reduction in residual volume via effects on small airways is associated with an improvement of shortness of breath, a major symptom present in patients with COPD. The anti-inflammatory effects of ensifentrine demonstrated clinically with reduction in inflammatory cells in the sputum in healthy volunteers challenged with LPS are likely also contributing to this clinical profile.16
Furthermore, clinical studies in subjects with COPD over a short duration up to 4 weeks have shown a favorable safety profile of ensifentrine.17–19,41 Ensifentrine nebulizer suspension has been well tolerated, with an adverse event incidence and severity generally similar to those treated with placebo. Doses up to and including ensifentrine 6 mg BID are well tolerated, with no consistent dose-related adverse events. To date, use of nebulized ensifentrine has resulted in limited reports of SAEs with no apparent pattern or dose-relationship. Gastrointestinal and cardiovascular adverse events have not been associated with nebulized ensifentrine to date. A TQT study demonstrated that single doses of nebulized ensifentrine (3 mg or 9 mg) did not result in a clinically relevant increase in QT interval or have an effect on cardiac conduction parameters. Clinically relevant effects associated with ensifentrine have not been observed on blood pressure, vital signs, ECGs including Holter monitoring or laboratory safety parameters.
Conclusion
Ensifentrine is currently in Phase 3 development as a novel maintenance therapy in individuals with COPD to be administered twice daily via standard jet nebulizer. No dual inhibitors of PDE3 and PDE4 are currently-approved for the treatment of COPD, thus ensifentrine is a novel therapeutic class with the ability to provide significant bronchodilation when dosed as monotherapy or in addition to other currently approved classes of bronchodilators, as well as provide non-steroidal broad anti-inflammatory effects and promote ciliary function, potentially improving symptoms associated with sputum. This clinical profile of inhaled ensifentrine, combined with the safety profile to date, makes ensifentrine a promising addition to the limited treatment class options available for patients with COPD.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
All clinical studies described in this review were funded by Verona Pharma plc.
Disclosure
Margot MacDonald-Berko, Tara Rheault, PhD, and Kathleen Rickard, MD are employees and shareholders of Verona Pharma. Thomas Bengtsson is a paid statistical consultant for Verona Pharma. James F Donohue, MD has consultant and advisory board relationships with Verona Pharma. Dr Kathleen Rickard has a patent Various pending to unknown. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2023 report; 2023. Available from: https://goldcopd.org/2023-gold-report-2/.
2. Cazzola M, Rogliani P, Matera MG. The future of bronchodilation: looking for new classes of bronchodilators. Eur Respir Rev. 2019;28:190095. doi:10.1183/16000617.0095-2019
3. Matera MG, Cazzola M, Page C. Prospects for COPD treatment. Curr Opin Pharmacol. 2021;56:74–84. doi:10.1016/j.coph.2020.11.003
4. Banner KH, Press NJ. Dual PDE3/4 inhibitors as therapeutic agents for chronic obstructive pulmonary disease. Br J Pharmacol. 2009;157:892–906. doi:10.1111/j.1476-5381.2009.00170.x
5. de Boer J, Philpott AJ, Van amsterdam RG, Shahid M, Zaagsma J, Nicholson CD. Human bronchial cyclic nucleotide phosphodiesterase isoenzymes: biochemical and pharmacological analysis using selective inhibitors. Br J Pharmacol. 1992;106:1028–1034. doi:10.1111/j.1476-5381.1992.tb14451.x
6. Page CP, Spina D. Phosphodiesterase inhibitors in the treatment of inflammatory diseases. In: Francis S, Conti M, Houslay M, editors. Phosphodiesterases as Drug Targets. Handbook of Experimental Pharmacology. Berlin: Springer; 2011:391–414.
7. Calverley PM, Rabe KF, Goehring UM, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685–694. doi:10.1016/S0140-6736(09)61255-1
8. Singh D, Nandeuil MA, Pigeon-Francisco C, et al. Efficacy and safety of CHF6001, a novel inhaled PDE4 inhibitor in COPD: the pioneer dose finding study. Am J Respir Crit Care Med. 2019;199:A4529.
9. DALIRESP® (roflumilast) [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2020. Accessed from: https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/704932ce-e104-4c6f-840a-575170971344/704932ce-e104-4c6f-840a-575170971344_viewable_rendition__v.pdf.
10. Thomas B, Koh MS, O’Callaghan C, et al. Dysfunctional bronchial cilia are a feature of Chronic Obstructive Pulmonary Disease (COPD). COPD. 2021;18(6):657–663. doi:10.1080/15412555.2021.1963695
11. Dransfield M, Rowe S, Vogelmeier CF, et al. Cystic fibrosis transmembrane conductance regulator: roles in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2022;205(6):631–640. doi:10.1164/rccm.202109-2064TR
12. Abbott-Banner KH, Page CP. Dual PDE3/4 and PDE4 inhibitors: novel treatments for COPD and other inflammatory airway diseases. Basic Clin Pharmacol Toxicol. 2014;114(5):365–376. doi:10.1111/bcpt.12209
13. Zuo H, Cattani-Cavalieri I, Musheshe N, Nikolaev V, Schmidt M. Phosphodiesterases as therapeutic targets for respiratory diseases. Pharmacol Ther. 2019;197:225–242. doi:10.1016/j.pharmthera.2019.02.002
14. Matera MG, Page CP, Calzetta L, Rogliani P, Cazzola M. Pharmacology and therapeutics of bronchodilators revisited. Pharmacol Rev. 2020;72(1):218–252. doi:10.1124/pr.119.018150
15. Boswell-Smith V, Spina D, Oxford AW, Comer MB, Seeds EA, Page CP. The pharmacology of two novel long-acting phosphodiesterase ¾ inhibitors, RPL554 [9,10-dimethoxy-2(2,4,6-trimethylphenylimino)-3-(n-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-A]isoquinolin-4-one] and RPL565 [6,7-dihydro-2-(2,6-diisopropylphenoxy)-9,10-dimethoxy-4H-pyrimido[6,1-A]isoquinolin-4-one]. J Pharmacol Exp Ther. 2006;318(2):840–848. doi:10.1124/jpet.105.099192
16. Franciosi LG, Diamant Z, Banner KH, et al. Efficacy and safety of RPL554, a dual PDE3 and PDE4 inhibitor, in healthy volunteers and in patients with asthma or chronic obstructive pulmonary disease: findings from four clinical trials. Lancet Respir Med. 2013;1(9):714–727. doi:10.1016/S2213-2600(13)70187-5
17. Singh D, Abbott-Banner K, Bengtsson T, Newman K. The short-term bronchodilator effects of the dual phosphodiesterase 3 and 4 inhibitor RPL554 in COPD. Eur Respir J. 2018;52(5):1801074. doi:10.1183/13993003.01074-2018
18. Singh D, Martinez FJ, Watz H, Bengtsson T, Maurer BT. A dose-ranging study of the inhaled dual phosphodiesterase 3 and 4 inhibitor ensifentrine in COPD. Respir Res. 2020;21(1):47. doi:10.1186/s12931-020-1307-4
19. Ferguson GT, Kerwin EM, Rheault T, Bengtsson T, Rickard K. A dose-ranging study of the novel inhaled dual PDE 3 and 4 inhibitor ensifentrine in patients with COPD receiving maintenance tiotropium therapy. Int J Chron Obstruct Pulmon Dis. 2021;16:1137–1148. doi:10.2147/COPD.S307160
20. Turner MJ, Matthes E, Billet A, et al. The dual phosphodiesterase 3 and 4 inhibitor RPL554 stimulates CFTR and ciliary beating in primary cultures of bronchial epithelia. Am J Physiol Lung Cell Mol Physiol. 2016;310(1):L59–70. doi:10.1152/ajplung.00324.2015
21. Anzueto A, Barjaktarevic IZ, Siler TM, et al.; for the ENHANCE Investigators. Ensifentrine, a novel PDE3 and PDE4 inhibitor for the treatment of COPD: randomized, double-blind, placebo-controlled, multicenter, phase III trials (The ENHANCE Trials). Am J Respir Crit Care Med. 2023. doi:10.1164/rccm.202306-0944OC
22. Calzetta L, Page CP, Spina D, et al. Effect of the mixed phosphodiesterase ¾ inhibitor RPL554 on human isolated bronchial smooth muscle tone. J Pharmacol Exp Ther. 2013;346(3):414–423. doi:10.1124/jpet.113.204644
23. Keir S, Page C. RPL554, a dual phosphodiesterase (PDE) ¾ inhibitor acts synergistically with muscarinic receptor antagonists and beta-adrenoceptor agonists to produce bronchodilation in vivo. Am J Respir Crit Care Med. 2014;189:A4218.
24. Calzetta L, Cazzola M, Page CP, Rogliani P, Facciolo F, Matera MG. Pharmacological characterization of the interaction between the dual phosphodiesterase (PDE) ¾ inhibitor RPL554 and glycopyrronium on human isolated bronchi and small airways. Pulm Pharmacol Ther. 2015;32:15–23. doi:10.1016/j.pupt.2015.03.007
25. Venkatasamy R, Spina D. Novel relaxant effects of RPL554 on Guinea pig tracheal smooth muscle contractility. Br J Pharmacol. 2016;173(15):2335–2351. doi:10.1111/bph.13512
26. Rheault T, MacDonald-Berko M. Anti-inflammatory pharmacology of ensifentrine. CHEST J. 2020;158(4):A2284. doi:10.1016/j.chest.2020.08.1936
27. Turner MJ, Luo Y, Thomas DY, Hanrahan JW. The dual phosphodiesterase 3/4 inhibitor RPL554 stimulates rare class III and IV CFTR mutants. Am J Physiol Lung Cell Mol Physiol. 2020;318(5):L908–L920. doi:10.1152/ajplung.00285.2019
28. Turner MJ, Dauletbaev N, Lands LC, Hanrahan JW. The phosphodiesterase inhibitor ensifentrine reduces production of proinflammatory mediators in well differentiated bronchial epithelial cells by inhibiting PDE4. J Pharmacol Exp Ther. 2020;375(3):414–429. doi:10.1124/jpet.120.000080
29. Turner MJ, Abbott-Banner K, Thomas DY, Hanrahan JW. Cyclic nucleotide phosphodiesterase inhibitors as therapeutic interventions for cystic fibrosis. Pharmacol Ther. 2021;224:107826. doi:10.1016/j.pharmthera.2021.107826
30. Rickard K, Darpo B, Xue H, Bengtsson T, Rheault T. The dual phosphodiesterase (PDE) 3 and 4 inhibitor ensifentrine does not prolong QT interval in healthy volunteers. Am J Respir Crit Care Med. 2022;205:A5605.
31. Watz H, Rickard K, Rheault T, Bengtsson T, Singh D. Symptom improvement following treatment with the inhaled dual phosphodiesterase 3 and 4 inhibitor ensifentrine in patients with moderate to severe COPD – a detailed analysis. Int J Chron Obstruct Pulmon Dis. 2020;15:2199–2206. doi:10.2147/COPD.S263025
32. Rickard K, Bengtsson T, Rheault T. Cardiovascular safety profile of ensifentrine in patients with COPD: results from two four-week, dose-ranging, randomized, placebo-controlled trials. Am J Respir Crit Care Med. 2021;2021:A2256.
33. Leidy NK, Murray LT, Monz BU, et al. Measuring respiratory symptoms of COPD: performance of the EXACT- Respiratory Symptoms Tool (E-RS) in three clinical trials. Respir Res. 2014;15:124. doi:10.1186/s12931-014-0124-z
34. Mahler DA, Witek TJ. The MCID of the transition dyspnea index is a total score of one unit. COPD. 2005;2(1):99–103. doi:10.1081/COPD-200050666
35. Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved, COPD-specific version of the St. George respiratory questionnaire. CHEST J. 2007;132(2):456–463. doi:10.1378/chest.06-0702
36. Terry PD, Dhand R. Inhalation therapy for stable COPD: 20 years of GOLD reports. Adv Ther. 2020;37(5):1812–1828. doi:10.1007/s12325-020-01289-y
37. Barjaktarevic IZ, Milstone AP. Nebulized therapies in COPD: past, present, and the future. Int J Chron Obstruct Pulmon Dis. 2020;15:1665. doi:10.2147/COPD.S252435
38. Terry PD, Dhand R. Maintenance therapy with nebulizers in patients with stable COPD: need for reevaluation. Pulm Ther. 2020;6(2):177–192. doi:10.1007/s41030-020-00120-x
39. Packer M, Carver JR, Rodeheffer RJ, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med. 1991;325(21):1468–1475. doi:10.1056/NEJM199111213252103
40. Lipworth BJ. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet. 2005;365(9454):167–175. doi:10.1016/S0140-6736(05)17708-3
41. Martin C, Burgel PR, Roche N. Inhaled dual phosphodiesterase 3/4 inhibitors for the treatment of patients with COPD: a short review. Int J Chron Obstruct Pulmon Dis. 2021;16:2363–2373. doi:10.2147/COPD.S226688
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.