Back to Journals » Vascular Health and Risk Management » Volume 18
Enlargement of the Left Atrium Strongly Predicts Postoperative Mortality Following Heart Valve Surgery
Authors Ibrahim KS , Kheirallah KA , Megdadi MA
Received 16 July 2022
Accepted for publication 17 September 2022
Published 30 September 2022 Volume 2022:18 Pages 783—791
DOI https://doi.org/10.2147/VHRM.S380463
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Khalid S Ibrahim,1 Khalid A Kheirallah,2 Mahmoud A Megdadi2
1Division of Cardiac Surgery, Department of General Surgery and Urology, College of Medicine, Jordan University of Science and Technology, Irbid, Jordan; 2Department of Public Health and Community Health, College of Medicine, Jordan University of Science and Technology, Irbid, Jordan
Correspondence: Khalid S Ibrahim, Division of Cardiac Surgery, Department of General Surgery and Urology, College of Medicine, Jordan University of Science and Technology, Irbid, Jordan, Tel +962 79 6 42 77 00, Email [email protected]
Background: Enlargement of the left atrium has been thoroughly studied in many clinical situations, especially its association with mortality and morbidity.
Patients and Methods: The study cohort included patients with rheumatic valve pathology such as stenosis and regurgitation. All patients underwent valvular surgical procedures including mitral valve replacement (MVR), aortic valve replacement (AVR), AVR with coronary artery bypass grafting (CABG), MVR with CABG, or AVR and MVR with or without CABG. This study included patients who underwent surgery between 2002 and 2017.
Results: Three hundred and forty-six patients were included in this study. The mean patient age was 51.6± 16.1 years; 37% of the patients underwent AVR, 28% underwent MVR, and 13% underwent a combination of MVR with AVR, AVR with CABG in 6%, and MVR with CABG in 10%. The operative mortality rate was 5.8% (n=20). Univariate analysis revealed that the predictors of mortality included age (P < 0.001), body mass index (BMI) (P = 0.003), type of surgery performed (P = 0.007), hypertension (P = 0.005), emergent surgeries (P = 0.018), left atrial diameter (P = 0.003), cross-clamp time greater than 90 minutes (P = 0.007), postoperative acute kidney injury (AKI) (P = 0.044), postoperative stroke (P = 0.049), and surgical site infection (P = 0.047). Multivariate analysis revealed that predictors of mortality included age (P = 0.028, AOR=10.6), BMI (P = 0.003, AOR=3.12), re-exploration (P = 0.006, AOR=8.38), length of intensive care unit stay (P ≤ 0.002, AOR=4.55), and left atrial diameter (P = 0.003, AOR=10.64).
Conclusion: Enlargement of the left atrium has been studied extensively as a predictor of mortality and morbidity in different clinical situations, to the extent that some authors suggest adding it to risk stratification models. In this study, left atrial size > 4 cm was found to strongly predict mortality after rheumatic heart valve surgery.
Keywords: valve surgery, mortality, mitral, aortic, left atrial size
Introduction
Although its incidence is declining, rheumatic heart valve disease is still an endemic disease in developing countries and has begun to rise in developed countries due to migration of refugees. In its two pathologic presentations; stenosis and regurgitation, it causes a wide range of symptoms, like shortness of breath, fatigue, chest pain, syncope, and even sudden death. The procedure of choice is still surgical replacement, either by the open technique, trans-aortic valve implantation, or surgical valve repair. In Jordan, similar to other countries in the developing world, rheumatic valve disease is still the main pathology, rather than degenerative pathology, which is the leading cause of valve pathology in the developed world.1
Clinical factors that predict mortality after valve replacement surgery have been thoroughly investigated in several studies.2–4 Older age, coronary artery disease, high creatinine level, and congestive heart failure (HF) have been frequently found as predictors of mortality after rheumatic heart valve surgery in patients undergoing aortic or mitral valve surgery.3 In the northern part of Jordan, we previously identified clinical factors that predict mortality and morbidity following heart valve surgery, including age, use of a biological valve, emergency valve surgery, Ejection Fraction (EF) less than 35%, pump run time, cross-clamp time, use of beta-blockers for less than one month before surgery, and type of surgical procedure performed.5–7
In this study, we investigated the association between an enlarged left atrium and mortality following different types of valve replacement surgery in Jordanian patients.
Patients and Methods
Patients
The cohort of patients studied included 346 patients who were admitted to our institute for valve surgery between July 2002 and December 2017. Among these, 29% underwent isolated mitral valve replacement surgery (MVR), 37% underwent replacement of the aortic valve (AVR), 6% underwent AVR combined with coronary artery bypass grafting surgery (CABG), 10% underwent MVR combined with CABG, and 14% underwent combined surgery of AVR and MVR with or without CABG. Rheumatic heart disease was diagnosed by a supervising consultant cardiologist, based on the patient’s history, clinical findings, echocardiographic examination, and postoperative histopathological examination. Exclusion criteria were repeat cardiac surgery and aortic dissection requiring aortic root replacement.
The electronic medical records of eligible patients were used to retrieve clinical, echocardiographic, and surgical data, which were reviewed and analyzed later. Patients’ previous medication use was documented, and the duration of use was adopted to stratify them (one month or less or more than a month before surgery). An ALT (HD1) 6000 ht (2–4 MHz probe, Philips Medical Systems Inc., Bothell, WA, USA) was used to perform preoperative two-dimensional transthoracic echocardiography imaging studies. These studies were used to evaluate the left ventricular ejection fraction, degree of regurgitation in the mitral/aortic/tricuspid valves, and dimensions of the left atrium (diameters) as part of the preoperative evaluation of the patients. To measure the left atrial diameter, the long-axis view was calculated by measuring the antero-posterior dimension in M-mode. The normal diameters range from 2.5 to 4 cm. Coronary catheterization was performed for all patients aged 35 years, while in patients aged less than 35 years, it was performed only if there was an indication. The primary endpoint was mortality within 30 days of surgery. The Institutional Review Board (IRB) at KAUH and Jordan University of Science and Technology (JUST) approved the study. The study was performed in accordance with the principles of the Declaration of Helsinki, 1975. Informed consent was waived by the IRB committee due to the retrospective nature of the study, as the research could not be carried out practically without the waiver. In addition, the research involves no more than minimal risk to the patients, and it will not adversely affect the rights or privacy of the participants given the importance of the knowledge to be gained. Patient data were anonymized and confidentiality was maintained.
Operative Procedure
Median sternotomy was the standard approach for all the patients. All the patients underwent surgery using a cardiopulmonary bypass machine. Arterial cannulation was performed centrally on the distal ascending aorta, while venous cannulation was performed through two-stage cannulation in AVR patients, and bicaval cannulation was used in patients undergoing MVR. In patients who underwent combined valve replacement and CABG, all distal anastomoses using vein grafts were performed first, starting with the right coronary artery, followed by the circumflex artery, and then the diagonals were performed. Continuous anastomoses were performed using 7/0 polypropylene sutures. Valve replacement was performed next, and left internal thoracic artery-to-left anterior descending anastomoses (LIMA-LAD) were performed subsequently. A transverse incision in the proximal part of the ascending aorta, approximately 1.5 cm from the origin of the right coronary artery, was made to approach the aortic valve, while MVR was performed through an incision in the interatrial groove. In cases of valve replacement, complete excision of the valve leaflets, and proper decalcification of the annulus, interrupted pledged polyester [2/0] sutures were used in both the aortic and mitral positions. Proper sizing was performed and the valve was downloaded using the parachuting technique. Mechanical valves were offered for all patients younger than 65 years, except if patients chose the biologic valve, whereas biological valves were offered for those 65 years or older.
Statistical Analysis
SPSS version 22 was used for data analysis. Frequencies and percentages were used to summarize categorical variables, while mean ± standard deviation was used for continuous variables. Left atrial diameter was dichotomized into >4 cm or ≤4 cm.
Independent sample t-tests or X2 tests were used to analyze independent variables for 30-day mortality, as appropriate. P-values are reported for bivariate analyses. All independent variables that were significantly associated with mortality (P < 0.05) were included in a backward conditional logistic regression model (entry at P = 0.05, removal at P = 0.2). Adjusted odds ratios (AOR) and P-values are reported. The alpha level for all analyses was set at 0.05.
A logistic regression model was used to include variables that were significantly associated with 30-day mortality at the bivariate level. These included age, body mass index (BMI), surgery performed on emergency bases, type of surgery performed, hypertension, aortic cross-clamp time >90 minutes, use of B-blockers for > a month before surgery, use of biological valves, re-exploration for bleeding, postoperative acute kidney injury (AKI), intensive care unit (ICU) stay of >3 nights, postoperative stroke, sternal wound infection, and left atrial diameter >4 cm.
Results
Three hundred and forty-six patients met the inclusion criteria were included in this study. The mean patient age was 51.6±16.1 years. Male sex constituted 51% of patients (n=178); 21% of the patients were diabetic, while 44.6% had hypertension (Tables 1 and 2). Approximately 32% of patients had CAD and 23% had HF.
 |
Table 1 Distribution of Participants by Pre-Operative Mortality and Background Characteristics |
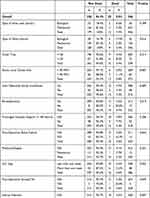 |
Table 2 Distribution of Participants by Intra and Post-Operative Mortality Characteristics |
The thirty-day mortality rate was 5.8% (n=20); 6 patients died as a result of sepsis, 8 as a result of multi-organ failure, and 3 due to stroke. In 3 patients, the cause of death was not documented in their files. The medications used were as follows: aspirin (33%), clopidogrel (16%), statins (25%), ACEi (28%), β-blockers (35%), and loop diuretics (49%). Clopidogrel was the only medication that was stopped 5–7 days before surgery.
Predictors of Mortality
The univariate predictors of mortality after surgery are presented in (Tables 1 and 2). These variables included age (P < 0.001), BMI (P = 0.003), operation type (P = 0.007), hypertension (P = 0.005), emergency surgery (P = 0.018), left atrial diameter (P = 0.003), use of B-blockers (none or less than a month) before surgery (P = 0.01), use of biological valve in the mitral position (P = 0.016), length of stay in the ICU after surgery (P = 0.022), re-exploration for bleeding (P = 0.019), cross-clamp time greater than 90 minutes (P = 0.007), postoperative AKI (P = 0.044), postoperative stroke (P = 0.049), and surgical site infection (P = 0.047).
Table 3 shows the logistic regression analysis performed to identify the independent predictors of mortality after adjusting for other variables and potential confounders. Age (P = 0.028, AOR=10.6), BMI (P = 0.003, AOR=3.12), re-exploration (P = 0.006, AOR=8.38), length of ICU stay (P ≤ 0.002, AOR=4.55), and LA diameter >4 cm (P = 0.003, AOR=10.64) were independent predictors of postoperative mortality.
 |
Table 3 Adjusted Effect of Selected Variables on Perioperative Mortality |
Discussion
Following CABG, valve surgery is second in terms of frequency of performance as open-heart surgery in Jordan. Our studies results regarding mortality and morbidity after valve operations have been published previously (5–7). According to these studies, age, emergency valve surgery, EF<35%, use of beta-blockers for more than a month before surgery, use of a tissue valve, type of surgery performed, pump run time, and cross-clamp time were all found to be independent predictors of mortality after valve heart surgery.5–7
In practice, one can be considered a preoperative predictor of mortality. Left atrium enlargement has been shown in many clinical situations to be a predictor of morbidity and mortality8,9 Ferreira et al8 showed that, in terms of echocardiographic criteria, the increase in left atrium volume was the only determinant associated with a need for mechanical ventricular assist device, heart transplantation and death in patients with dilated cardiomyopathy. Incident HF,10 atrial fibrillation,11 stroke,12 and even death12,13 have been reported to be associated with left atrial enlargement. In patients with type II diabetes mellitus, enlargement of the left atrium has also been reported as an independent predictor of cardiovascular morbidity and mortality.9
In patients with aortic valve stenosis (AS), regardless of the mode of treatment, whether medical or surgical, left atrial enlargement was found to be a strong predictor of mortality, in addition to known predictors of outcome,14 and the authors suggested that it can be assessed in clinical practice for preoperative risk stratification. This conclusion was replicated in this study. Even in patients with asymptomatic aortic valve stenosis, left atrial enlargement as a measure of left ventricular diastolic dysfunction has been shown to be a predictor of mortality.15
From a pathophysiologic point of view, as the left atrial contraction at the end-diastole “left atrial kick” is considered to contribute significantly to cardiac output and as this “kick” is reduced when the left atrium is enlarged, left atrial enlargement consequently has adverse hemodynamic consequences due to impairment of left ventricular filling during the diastolic phase in these patients. Conversely, increased left ventricular stiffness as a consequence of progressive left ventricular dysfunction can lead to progressive mitral regurgitation, and thus, increased LA size.16 In a vicious cycle, enlargement of the left atrium can further impair left ventricular filling due to increased filling pressure or atrial fibrillation. Systolic dysfunction and left atrial enlargement can also be a consequence of mitral regurgitation,17 so the “history” of mitral regurgitation in terms of severity and duration can be reflected at the size of the left atrium. As left ventricular myocardial ischemic remodeling can result in mitral regurgitation, which in turn can lead to left atrial enlargement, mitral regurgitation can be considered a strong indicator of mortality. Moreover, ischemia and dilatation both enhance endothelin-1 (ET-1) synthesis. We have previously demonstrated that atrial ET-1 levels are strongly and independently correlated with LA enlargement of the left atrium as well as the severity of mitral regurgitation in atrial fibrillation patients.16
Postoperative atrial fibrillation is a predictor of mortality and morbidity,13 as left atrial enlargement is associated with postoperative atrial fibrillation,18 suggesting a common mechanism by which left atrial enlargement can contribute to postoperative morbidity and mortality. In a previous study on the same cohort, we showed that LA enlargement was associated with postoperative atrial fibrillation.7
It has been suggested that rheumatic heart disease has been eradicated in developed countries due to improved lifestyles and use of antibiotics.19 However, migration in many developed countries and refugee crises have led to an increase in rheumatic heart disease, a pathology that has not been treated in these areas for a long time.20 Our findings emphasize that rheumatic valve surgery should be performed earlier to achieve better clinical outcomes.
Limitations of the Study
This study was limited by its retrospective nature and missing data. Elevated red cell distribution width, which reflects variability in the size of red blood cells (anisocytosis) due to erythrocyte dysfunction or inflammation, has been found to be a predictor of mortality after valvular surgery.21,22 Future prospective studies are recommended to include complete data on red blood cell count and anisocytosis. The European System for Cardiac Operative Risk Evaluation (EUROScore II) is a helpful risk classification model that has been widely used to predict in-hospital mortality in patients undergoing heart surgery. Future studies that consider EuroScore are recommended.
Conclusion
Enlargement of the left atrium has been studied extensively as a predictor of mortality and morbidity in different clinical situations, to the extent that some authors suggest adding it to risk stratification models. In this study, left atrial size >4 cm was considered a strong predictor of mortality after rheumatic heart valve surgery.
Abbreviations
AVR, aortic valve replacement; MVR, mitral valve replacement; CABG, coronary artery bypass grafting; AKI, acute kidney injury; BMI, body mass index; LA, left atrium; AOR, adjusted odds ratio; TAVI, trans-aortic valve implantation; HF, heart failure; EF, ejection fraction; LVEF, left ventricular ejection fraction; LIMA, left internal mammary artery; LAD, left anterior descending; ICU, intensive care unit; CAD, coronary artery disease; ACE-i, angiotensin converting enzyme-inhibitor; ET-1, endothelin 1; IRB, Institutional Research Board.
Ethics Approval
Approved by the IRB.
Informed Consent/Consent to Publish
Waived by IRB.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
1. Chen LW, Chen J, Zheng JN, et al. Prediction of short-term mortality after valve surgery: a single center’s perspective. Chin Med J. 2018;131(20):2499–2502. doi:10.4103/0366-6999.243553
2. Enriquez-Sarano M, Schaff HV, Orszulak TA, Tajik AJ, Bailey KR, Frye RL. Valve repair improves the outcome of surgery for mitral regurgitation. J Multivar Anal Circulation. 1995;91(4):1022–1028.
3. Nowicki ER, Birkmeyer NJ, Weintraub RW, et al. Multivariable prediction of in-hospital mortality associated with aortic and mitral valve surgery in Northern New England. Ann Thorac Surg. 2004;77(6):1966–1977. doi:10.1016/j.athoracsur.2003.12.035
4. Taylor NE, O’Brien S, Edwards FH, Peterson ED, Bridges CR. Relationship between race and mortality and morbidity after valve replacement surgery. Circulation. 2005;111(10):1305–1312. doi:10.1161/01.CIR.0000157737.92938.D8
5. Ibrahim KS, Kheirallah KA, Mayyas FA, Alwaqfi NR, Alawami MH, Aljarrah QM. Predictors of short-term mortality after rheumatic heart valve surgery: a single-center retrospective study. Ann Med Surg. 2021;26(62):395–401. doi:10.1016/j.amsu.2021.01.077
6. Ibrahim KS, Kheirallah KA, Mayyas FA, Nizar A. Predictors of acute kidney injury following surgical valve replacement. Thorac Cardiovasc Surg. 2020;69(5):396–404.
7. brahim KS, Kheirallah K, Mayyas F, Waqfi N, Al-Zoubi N, Van WD. Atrial fibrillation after rheumatic heart valve surgery: incidence, predictors and outcomes. Thorac Cardiovasc Surg. 2022. doi:10.1055/s-0041-1740985
8. Ferreira F, Galrinho A, Soares R, et al. Prognostic value of left atrial volume in patients with dilated cardiomyopathy. Rev Port Cardiol. 2013;32:865–872. doi:10.1016/j.repc.2012.12.017
9. Paoletti E, Zoccali C. A look at the upper heart chamber: the left atrium in chronic kidney disease. Nephrol Dial Transplant. 2014;29:1847–1853. doi:10.1093/ndt/gft482
10. Takemoto Y, Barnes ME, Seward JB, et al. Usefulness of left atrial volume in predicting first congestive heart failure in patients > or = 65 years of age with well-preserved left ventricular systolic function. Am J Cardiol. 2005;96:832–836. doi:10.1016/j.amjcard.2005.05.031
11. Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994;89:724–730. doi:10.1161/01.CIR.89.2.724
12. Laukkanen JA, Kurl S, Eranen J, et al. Left atrium size and the risk of cardiovascular death in middle-aged men. Arch Intern Med. 2005;165:1788–1793. doi:10.1001/archinte.165.15.1788
13. Benjamin EJ, D’Agostino RB, Belanger AJ, et al. Left atrial size and the risk of stroke and death. The Framingham heart study. Circulation. 1995;92:835–841. doi:10.1161/01.CIR.92.4.835
14. Rusinaru D, Bohbot Y, Kowalski C, Ringle A, Maréchaux S, Tribouilloy C. Left atrial volume and mortality in patients with aortic stenosis. J Am Heart Assoc. 2017;6:11. doi:10.1161/JAHA.117.006615
15. Casaclang‐Verzosa G, Malouf JF, Scott CG, Juracan EM, Nishimura RA, Pellikka PA. Does left atrial size predict mortality in asymptomatic patients with severe aortic stenosis? Echocardiography. 2010;27(2):105–109. doi:10.1111/j.1540-8175.2009.01002.x
16. Mayyas F, Niebauer M, Zurick A, et al. Association of left atrial endothelin-1 with atrial rhythm, size, and fibrosis in patients with structural heart disease. Circ Arrhythm Electrophysiol. 2010;3:369–379. doi:10.1161/CIRCEP.109.924985
17. Rusinaru D, Tribouilloy C, Grigioni F, et al. Left atrial size is a potent predictor of mortality in mitral regurgitation due to flail leaflets: results from a large international multicenter study. Circ Cardiovasc Imaging. 2011;4:473–481. doi:10.1161/CIRCIMAGING.110.961011
18. Tsai YT, Lai CH, Loh SH, et al. Assessment of the risk factors and outcomes for postoperative atrial fibrillation patients undergoing isolated coronary artery bypass grafting. Acta Cardiol Sin. 2015;31:436–443. doi:10.6515/acs20150609a
19. Iung B, Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol. 2014;30(9):962–970. doi:10.1016/j.cjca.2014.03.022
20. Mutagaywa RK, Wind A-M, Kamuhabwa A, Cramer MJ, Chillo P, Chamuleau S. Rheumatic heart disease anno 2020: impacts of gender and migration on epidemiology and management. Eur J Clin Invest. 2020;50(12). doi:10.1111/eci.13374
21. Duchnowski P, Hryniewiecki T, Stokłosa P, Kuśmierczyk M, Piotr S. Number of erythrocytes as a prognostic marker in patients undergoing heart valve surgery. Kardiol Pol. 2018;76(4):791–793. doi:10.5603/KP.2018.0076
22. Duchnowski P, Hryniewiecki T, Kuśmierczyk M, Piotr S. Anisocytosis predicts postoperative renal replacement therapy in patients undergoing heart valve surgery. Cardiol J. 2020;27(4):362–367. doi:10.5603/CJ.a2019.0020
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
