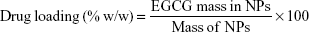Back to Journals » Drug Design, Development and Therapy » Volume 12
Enhanced oral bioavailability of EGCG using pH-sensitive polymeric nanoparticles: characterization and in vivo investigation on nephrotic syndrome rats
Received 3 May 2018
Accepted for publication 8 June 2018
Published 14 August 2018 Volume 2018:12 Pages 2509—2518
DOI https://doi.org/10.2147/DDDT.S172919
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Guojuan Zhang,1 Jianfang Zhang2
1Department of Nephrology, Beijing Tongren Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Pharmaceutics, School of Pharmacy, Fudan University, Shanghai, People’s Republic of China
Objective: Chronic kidney disease (CKD) is characterized by progressive loss of renal functions. At present, there are only limited therapeutic strategies to slow down the progress of CKD and there is an urgent need to develop new therapeutic strategies to treat CKD patients. Numerous research evidence supports the potential role of EGCG in the renal protection of CKD. However, the clinical use is still limited due to the poor oral bioavailability. The aim of this study was to develop pH-sensitive polymeric nanoparticles of EGCG to improve this deficiency.
Materials and methods: EGCG-loaded nanoparticles (EGCG NPs) were prepared by an improved emulsion evaporation method. The formulation prepared was in spherical with uniform sizes, high encapsulation efficiencies and drug loading. The therapeutic efficacy of EGCG NPs on chronic kidney disease was investigated on model of rat Nephrotic syndrome by measuring urinary protein excretion and kidney pathology score.
Results: The mean particle size was found to be 91.3±0.8 nm and the encapsulation efficiency% and drug loading% of the formulation were 80.8%±1.6% and 6.3%±1.4%, respectively. The powder X-ray diffraction and differential scanning calorimetry of EGCG NPs showed that EGCG existed in amorphous form in NPs. The release of EGCG from NPs exhibited the lower burst release at pH 1.2 (<10%) and with the increase of pH value, the release of EGCG also gradually increased. During the observation period (24 hours), the total release amount was almost 68%. EGCG NPs could significantly modify the pharmacokinetic profile and increase the bioavailability of EGCG by more than 2.4-fold in comparison with the EGCG powder group. At the end of the fourth and sixth week, proteinuria excretion of nephrotic syndrome rats treated with EGCG NPs was significantly lower than those treated with EGCG powder, and kidney pathology scores in EGCG NPs treated rats were also significantly lower than EGCG powder treated rats.
Conclusion: The results of pharmacodynamics showed that compared with EGCG powder treatment group, EGCG NPs treatment group had better efficacy and reduce kidney damage.
Keywords: oral bioavailability, EGCG, Eudragit S100, PLGA, nephrotic syndrome
Introduction
Chronic kidney disease (CKD) is characterized by progressive loss of renal functions. The most common causes of CKD are diabetes mellitus in Western countries and glomerulonephritis (GN) in China.1 Others include hypertension, obstruction, drug-induced CKD, and idiopathic nephropathy. At present, there are only limited therapeutic strategies to slow down the progress of CKD, such as controlling risk factors, angiotensin converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB) and other conservative treatments.2–4 Therefore, there is an urgent need to develop new therapeutic strategies to treat CKD patients.
(−)-Epigallocatechin-3-gallate (EGCG), the major catechin of green tea extract, is believed to be one of the physiologically active agents in tea due to their abundance in brewed tea and reported biological activities in animals and humans including antioxidant activities and the modulation of plasma lipid profiles.5 It helps enhance vasodilatation6 and increase fatty acid oxidation and insulin sensitivity.7 In the past 10 years, the health promoting role of EGCG has been extensively studied. It can inhibit leukocyte chemotaxis, quench free radicals, chelate transition metals, and interrupt lipid peroxidation chain reaction.8,9 Kidney, exposed to harmful agents, is at a high risk of oxidative stress such as reactive oxygen species.10 Therefore, oxidative stress and inflammation play a key role in the progress of CKD.11 Numerous research evidence supports the potential role of EGCG in the renal protection of CKD. The results showed that EGCG decreased serum creatinine12 in rats with adenine-induced renal failure and improved glucose toxicity and renal injury in diabetic nephropathy animal models.13
It is noteworthy that the oral bioavailability of EGCG is believed to be relatively poor. In humans, the maximum plasma EGCG concentrations of 0.15 μM are achieved after consumption of two cups of green tea.14 Several factors are considered to limit the bioavailability of a complete EGCG: 1) the potential sensitivity of EGCG to digestive conditions;15 2) low intestinal absorbance;16 and 3) a high degradation rate in the gastrointestinal environment.17 For the above reasons, the use of natural sources of EGCG in traditional preparations does not seem to reach the therapeutic concentration of EGCG. The application of nanotechnology in medicine, more specifically the use of nanocarrier systems to incorporate EGCG, is well known, and some nanocomposites are being developed at the moment.18
A polymeric nanoparticle (NP) is a circular structure of nanometer size, in which the drug is distributed evenly in the matrix.19 With regard to intestinal absorbance, pH-sensitive systems represent a leading approach because the pH value differs along the gastrointestinal tract.20 Eudragit S100 (ES100), the most commonly investigated biocompatible polymer for colon-release drug delivery, has been accepted for oral administration by the regulatory agencies.21 It selectively dissolves in aqueous media of pH 6–7, releasing any loaded drug to the colon. The formulation of EGCG in nanostructured lipid carriers has already been performed in other studies.22 However, those studies did not verify the effectiveness of the preparations in animal models. The aim of this study was to develop pH-sensitive polymeric NPs of EGCG in order to improve oral bioavailability. Physicochemical physiognomies of the NPs were perused using powder X-ray diffraction (XRD), differential scanning calorimetry (DSC) and transmission electron microscopy (TEM). Moreover, their dissolution rates, pharmacokinetics and pharmacodynamics in rats were studied in comparison with the drug powder.
Materials and methods
Materials
Poly(lactic-co-glycolic acid) (PLGA) (Mw=12,000 Da, lactic acid:glycolic acid=50:50) and poly(vinyl alcohol) (PVA, 90% hydrolyzed, low molecular weight) were purchased from Sigma-Aldrich (Shanghai, China). The EGCG was received as a gift from Rongkai Herb Ltd, Co (Huzhou, China). ES100 was purchased from Sigma-Aldrich (Shanghai, China). All other solvents and chemical substances were of reagent grade. Deionized water (Thermo Fisher Scientific, Waltham, MA, USA) was used throughout the study to prepare the solution and mobile phase.
Preparation
EGCG-loaded nanoparticles (EGCG NPs) were prepared by an improved emulsion evaporation method.23 Some 80 mg of ES100 and PLGA with a weight ratio of (1–2) were dissolved in a solvent system composed of dichloromethane/methanol. The obtained polymeric solution was added to 5 mL of 2.5% (w/v) acidic PVA solution (pH 2.0, containing 10 mg EGCG) under vortex. The emulsion was immediately injected into 50 mL of 2.5% (w/v) acidic PVA solution (pH 2.0). After that, the organic solvent was evaporated under low vacuum conditions (Rotary evaporator, R-1001-VN, Zhengzhou, China). The EGCG NPs prepared by this method were collected by centrifugation at 3,000g for 10 minutes, washed twice with acidic solution (pH 2.0), and dried in a freeze-drying machine. The obtained NPs were stored in an airtight container at −20°C. Blank NPs with ES100/PLGA at weight ratio of 1:2 were prepared as control.
Characterization of EGCG-loaded NPs
The particle size of the freshly prepared NPs was determined by the laser diffraction method. The particle size and zeta potential were measured using the zeta analyzer (Microtrac Inc, Montgomeryville, PA, USA). A specific amount of EGCG NPs-lyophilized powder was dissolved in 100 mL of methanol using a probe sonicator and subjected to high performance liquid chromatography (HPLC) analysis. Drug loading and encapsulation efficiency were determined by equations 1 and 2, respectively.24
|
|
|
|
The surface morphology of the prepared EGCG NPs was observed by TEM. Morphological examination was performed using TEM (Philips CM120, the Netherlands). In practice, NPs solution containing 0.1% (w/v) phosphotungstic acid was placed on the carbon film and observed by electron microscope at 80 kV.
DSC and XRD were used to determine the crystalline form of NPs and EGCG dispersed in the polymer materials. DSC analysis was carried out using a DSC8000 differential scanning calorimeter. Accurate weighing samples were placed in aluminum pots and sealed with a lid. Al2O3 was used as a control. During the scanning process, a heating rate of 5°C/min was applied with a temperature range from 20°C to 150°C. XRD studies were performed using the Phillips X-ray diffractometer and Cu-Kα radiation. The samples were scanned over a 2θ range of 0°–90° at a scan rate of 0.05°/s.
Stability study
Recommendations for stability studies are mainly revised according to the guidelines of the Chinese Pharmacopeia. The NPs was placed in a stable box at the room temperature and saturated with sodium chloride solution (relative humidity=75%±5%). At the end of 0, 1, 2, 3 and 6 months of testing, the samples were evaluated to determine whether the particle size, zeta potential, encapsulation efficiency% and drug loading% changed or not.
In vitro drug release study
The dialysis bag method was used to study the in vitro release of EGCG NPs. In brief, EGCG NPs (10 mg) were placed in the dialysis bags (molecular weight=8,000–10,000) and immersed in a 27 mL release medium and in a dissolution apparatus with a paddle (75 rpm, 37°C). One milliliter aliquots were withdrawn at different time intervals and centrifuged at 4,000g for 10 minutes. At the same time, the same amount of fresh medium was replenished to maintain the sink condition. In vitro drug release was initiated in a buffer system at pH 1.2. After 2 and 4 hours, the pH was changed to 6.8 and 7.2, respectively, corresponding to the pH in the stomach, upper small intestine, and both ileum and colon, respectively.25 The ingredients of the release medium included hydrochloric acid/potassium chloride (pH=1.2), acetic acid/sodium acetate (pH=6.8) and PBS (pH=7.2). The samples from in vitro release were analyzed using HPLC as described. All experiments were performed in triplicate.
Animal illustration
All in vivo experimental protocols were approved by the animal care committee of the Faculty of Medicine, Fudan University Animal Center and all experiments were conducted in strict accordance with the laboratory animal care and use guidelines adopted by the National Institutes of Health (Shanghai, People’s Republic of China).
Pharmacokinetic study
Forty Sprague Dawley rats were divided into four groups and orally administered a single dose of EGCG powder (20 mg/kg) and EGCG NPs (10, 20 and 50 mg/kg). Blood samples (1 mL) were collected at 0.5, 1, 2, 3, 4, 6, 8, 10, 12 and 24 hours after the administration and centrifuged at 12,000 rpm for 5 minutes. Supernatants (plasma) were obtained and immediately stored at −80°C until liquid chromatography-mass spectrometry (LC-MS/MS) analysis.
The LC-MS/MS analysis of the concentration of EGCG in blood samples and the pharmacokinetic data analysis were performed using the same method used in our previous study. The plasma samples (100 μL) were mixed with 20 μL methanol, 20 μL (Vit C [20%, w/v])+EDTA-Na2 (0.05%, w/v) mixed solution, 20 μL of an internal standard solution (5 μg/mL vanillin in methanol) and 1,000 μL ethyl acetate. After centrifugation at 12,000 rpm for 10 minutes at 4°C, the supernatants were evaporated to dryness under nitrogen. The residual was dissolved in 100 μL mobile phase and 10 μL of the dissolved sample was analyzed. Detected transition ions from a precursor ion to a specific product ion for EGCG was at a mass to charge ratio (m/z) of 457.0–m/z 168.8.3, and vanillin (IS) was m/z 150.9–m/z 135.8. Separation was carried out at 30°C using a reverse-phase C18 column (5 μm, 2.1×150 mm). The mobile phase consisted of methanol and water (30:70). A flow rate of 0.5 mL/minute was employed.
Pharmacodynamic studies
Two hundred Sprague Dawley rats were randomly divided into five groups including a control group (Group 1), a nephropathy group (Group 2), a steroid therapy (Group 3), an EGCG powder treatment group (Group 4) and an EGCG NPs treatment group (Group 5). On the first day of experiment, in the control group, 0.1 mL physiological saline was injected into the tail vein; in other groups, doxorubicin solution (5 mg/kg) was injected into the tail vein to establish a model of rat nephrotic syndrome. Starting from the second week of the experiment, Group 3 started the subcutaneous injection of dexamethasone (2 mg/kg·d) until the end of experiment in the sixth week. Groups 4 and 5 started EGCG lavage (50 mg/kg·d) from week 2 to the end of week 6 when the experiment ended. Groups 1 and 2 started lavage of the same dose of physiological saline from week 2 to the end of the experiment at week 6.
A 24-hour urine specimen of each group was collected at the end of weeks 2, 4 and 6 for use before the experiment, After the experiment ended, kidney specimens of each group were collected, and put under detection by light microscopy after fixed by formalin. Coomassie brilliant blue was used to measure 24 hours urine protein excretion during different times. The semi-quantitative scoring method was used to score and evaluate pathological changes of glomerular and tubulointerstitial.26
The evaluation standards of glomerular pathological damage:
- Mesangial cell proliferation was scored 0 point, 1 point, 2 points, 3 points if the mesangial cell number in each mesangial area was smaller than 3, equal to 3, equal to four and larger than 5.
- Mesangial matrix widening compared with capillary lumen diameter and openness, 0 point, 1 point, 2 points, or 3 points were given if: capillary lumen opened well, no obvious hyperplasia was observed on matrix; matrix hyperplasia was slightly smaller than capillary lumen diameter with segmental distribution; matrix hyperplasia was slightly larger than capillary lumen diameter with diffuse distribution, capillary lumen with poor segmental openness; matrix hyperplasia with diffuse distribution, most capillary lumen were poorly opened.
- Cirrhosis.
- Basement membrane changes; e cyst wall adhesion; 0 point, 1 point, 2 points, or 3 points were given if: no obvious changes on c, d, and e regarding disease area; disease area was <30%; disease area was between 30% and 60% or disease area was larger than 60%.
- Tubular interstitium pathological damage evaluation standard: 0 point, 1 point, 2 points, or 3 points were given if: non, light, medium or severe tubular dilatation, tubular atrophy, vascular degeneration of tubulointerstitial cells, tubular stromal cell necrosis, degree of interstitial fibrosis and renal interstitial inflammatory cells degree of infiltration.
Statistical analysis
Statistical analysis was performed using analysis of variance test followed by Student’s t-test. Data were expressed as mean±standard error. Statistical significance was represented by P<0.05.
Result and discussion
Characterizations
In this study, EGCG NPs were prepared using an improved emulsion evaporation method. The aim of adding an emulsifier in this NP system was to separate the oil and water phases and was necessary to prevent aggregation of the NPs. PVA, as an emulsifier for the preparation of NPs, was widely used. During the formation of NPs, the hydrophobic fragments of PVA penetrated into the organic phase and remained entrapped in the NPs polymeric matrix (ES100 and PLGA). The hydrophilic fragments of PVA then surrounded the NPs, stabilizing them through steric hindrance. The formulation prepared was in spherical NPs with uniform sizes, high encapsulation efficiencies and drug loading, as shown in Table 1 and Figure 1A and C. The mean particle size was found to be 91.3±0.8 nm. The encapsulation efficiency% and drug loading% of the formulation were 80.8%±1.6% and 6.3%±1.4%, respectively. Normally, NPs with high molecular polymers have negative-charge properties. The higher the absolute value of zeta potential, the more stable the system.27,28 In this study, the zeta potential of EGCG NPs was −21.3 mV, which might be related to the formation of molecular polarization and charge adsorption in water. Similar to the structure of the ionic double layer, it produced a higher zeta potential value.
  | Table 1 Physical and chemical properties and stability data of EGCG NPs |
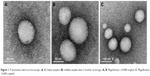  | Figure 1 Transmission electron microscopy. (A, C) Initial samples; (B) stability samples after 6 months of storage. (A, B: Magnification ×10,000 original, C: Magnification ×5,000 original). |
Figure 2 shows the XRD profiles of drugs with EGCG powder and NP systems. EGCG provided sharp characteristic peaks at diffraction angles, suggesting typical crystallization properties of the drug (Figure 2A). In blank ES100/PLGA NPs, the intensity was diminished (Figure 2C). The physical mixture in Figure 2B showed distinct and intense peaks at 2θ scale indicating crystallization property of the drug. However, after nanoencapsulation with ES100/PLGA, the intensity of drug crystallinity was diminished significantly (Figure 2D). This indicated that the drug was transformed into the amorphous state in ES100/PLGA NPs.
The thermal properties of EGCG powders and NPs are displayed in Figure 3. EGCG powder generated a deep endotherm, corresponding to its melting point at about 209°C, which confirmed its characteristic crystalline properties (Figure 3A). Similarly, in the physical mixture (Figure 3B), a sharp endotherm was also produced at the melting point of EGCG, proving that the drug existed in the crystalline state in the physical mixture of EGCG and blank ES100/PLGA NPs. In contrast, the blank ES100/PLGA NPs (Figure 3C) did not show any endotherm. Thermogram of EGCG NPs showed endothermic peak at 207°C suggesting that EGCG existed in amorphous form in NPs (Figure 3D).
Stability study
The physical stability of the EGCG NPs was also periodically verified by analyzing the changes in particle size, zeta potential, encapsulation efficiency% and drug loading% during storage conditions at room temperature for 6 months (Table 1 and Figure 1B). It was observed that there was an increase in the particle size of EGCG NPs in storage. On the other hand, a slight decrease was observed in the zeta potential of the preparation in storage. In addition, there was no significant difference in the entrapment efficiency% and drug loading% (P<0.05), indicating that the form of the ES100/PLGA NPs system was suitable for EGCG storage at room temperature.
In vitro drug release study
The in vitro release profiles of EGCG from NPs were studied in a buffer that underwent gradual changes in pH, as shown in Figure 4. Free EGCG exhibited a quicker drug release in pH 1.2 or 6.8 PBS and 96% of EGCG was released in the first 4 hours. In contrast, the EGCG ES100/PLGA NPs exhibited a lower burst release at pH 1.2 (<10%). With the increase of the pH value, the release of EGCG also gradually increased. During the observation period (24 hours), the total release amount was almost 68%. These increased release profiles and stability in acidic conditions could affect the bioavailability. In addition, there was no significant difference in the release curves between the samples obtained from the stability test and the initial samples in the in vitro release test.
LC-MS/MS analysis
EGCG and IS peaks were clearly separated in both chromatograms (Figure 5). The retention times of the EGCG and IS in chromatograms were 1.82 and 2.95 minutes, respectively. These results suggested that both assays were simple, fast, and specific. The calibration curve of the HPLC assay for EGCG in neat solution has good linearity (r2=0.996) over the range of 10–10,000 ng/mL. The lower limit of the quantitation of EGCG in the LC-MS/MS assay was found to be 10 ng/mL. These results indicated that the assays provided good linearity and sensitivity for their specific applications.
Pharmacokinetic study
Pharmacokinetic studies were carried out in rats using different EGCG preparations. Mean plasma concentration time profiles of EGCG after p.o. administration of EGCG powder and EGCG NPs to rats are summarized in Figure 6. Table 2 lists the pharmacokinetic parameters calculated from the plasma drug concentration vs time profiles. After the first hour, the concentration of all EGCG NPs group was lower than that of the EGCG powder group. In the group of EGCG powder, there was a high burst release at the initial stage. This action continued until reaching the Cmax in <0.25 hours, followed by a sharp decrease in plasma levels. This is mainly due to the shorter half-life and higher clearance rate of EGCG. However, EGCG NPs could significantly modify the pharmacokinetic profile and increase the bioavailability of EGCG by more than 2.4-fold in comparison with the EGCG powder group in the same dosage. Meanwhile, the area under the curve (AUC) presented a dose-dependent characteristic.
These results indicate that the formulation of EGCG as NPs enhanced its absorption across the wall of the gastrointestinal tract. This could be attributed to the preparation of EGCG in the form of ES100/PLGA, which could protect NPs from the influence of gastric acid environment and increase the absorption of intestinal tract. In addition, the NPs introduced the formulated drug as a fine dispersion rather than coarse particles of oral powder suspension, thus resulting in increased surface area and a reduced diffusion path length. These results confirmed the promising properties of ES100/PLGA NPs and enhanced the oral bioavailability of EGCG in vivo.
Pharmacodynamic studies
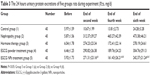
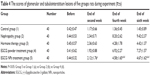
At the end of the experiment, the excretion of 24 hours urine protein was recorded as follows: Group 2> Group 4> Group 3> Group 5> Group 1. The EGCG NPs treatment group compared with the steroid therapy group had no statistical significance (P>0.05). But compared with the nephropathy group and EGCG powder treatment group, there was a significant statistical difference (P<0.05) (Table 3). Kidney damage in five groups of rats was recorded as follows: Group 2> Group 4> Group 5> Group 3> Group 1. No statistical significance was shown when comparing the steroid therapy group and the EGCG NPs treatment group. However, if compared with the EGCG powder treatment group, there was a very significant statistical significance (P<0.05) (Table 4). After analysis it was found that 24 hours urine protein excretion had highly positive correlation with the kidney pathology score (r=0.856, P<0.01). It proved that EGCG NPs could reduce kidney damage and delay the chronic progression of kidney pathology by reducing the excretion of urine protein.
A large amount of proteinuria was the main clinical manifestation of the nephrotic syndrome. Continuous massive proteinuria could lead to persistent kidney damage and thus chronic progressive kidney damage. Therefore, effective control of proteinuria excretion is essential to the treatment of kidney disease. In addition, excessive accumulation of reactive oxygen radicals was the main cause of the chronic progression of kidney disease. Currently the preferred drug for the clinical treatment of nephrotic syndrome is glucocorticoid, but a large number of research found that long-term use of glucocorticoid could cause the decrease in glomerular entry arteriolar resistance, increase pressure in the glomerulus, change glomerular permeability to macromolecules, increase urinary protein excretion and accelerate kidney damage.
In recent years, domestic scholars also discovered that glucocorticoid could not only increase the patient’s urine protein, but also lead to increase in glomerular filtration rate and effective renal plasma flow. Thus, it has become one of the most important topics for the current clinical professionals in kidney disease treatment to find an alternative or adjuvant steroid therapy, shorten the treatment time and reduce side effects caused by glucocorticoids.
The study of EGCG mechanism of prevention and treatment of rat nephrotic syndrome in this research has led to the findings that EGCG has a good effect on the prevention of chronic progression of glomerular diseases. Further system research and observation of the EGCG NPs’ effect of nephrotic syndrome rat proteinuria and kidney pathological score have found that the EGCG powder treatment group, EGCG NPs treatment group and steroid treatment group all could reduce proteinuria excretion and kidney pathology scores to certain degrees but also with differences. Compared with EGCG powder treatment group, the EGCG NPs’ treatment group had better efficacy. This may be related to the fact that EGCG NPs have better stability and bioavailability in rats than free EGCG.29 These findings have provided theoretical and experimental evidence for the use of EGCG NPs to prevent and treat clinical nephrotic syndrome, and also provided a new method for the development of nephropathy therapeutic drugs.
Disclosure
The authors report no conflicts of interest in this work.
References
Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41(1):1–12. | ||
Sharma P, Blackburn RC, Parke CL, McCullough K, Marks A, Black C. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for adults with early (stage 1 to 3) non-diabetic chronic kidney disease. Cochrane Database Syst Rev. 2011;5(10):CD007751. | ||
Fraser SD, Taal MW. Multimorbidity in people with chronic kidney disease: implications for outcomes and treatment. Curr Opin Nephrol Hypertens. 2016;25(6):465–472. | ||
Meguid El Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005;365(9456):331–340. | ||
Erba D, Riso P, Bordoni A, Foti P, Biagi PL, Testolin G. Effectiveness of moderate green tea consumption on antioxidative status and plasma lipid profile in humans. J Nutr Biochem. 2005;16(3):144–149. | ||
Kim JA, Formoso G, Li Y, et al. Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J Biol Chem. 2007;282(18):13736–13745. | ||
Venables MC, Hulston CJ, Cox HR, Jeukendrup AE. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am J Clin Nutr. 2008;87(3):778–784. | ||
Takano K, Nakaima K, Nitta M, Shibata F, Nakagawa H. Inhibitory effect of (−)-Epigallocatechin 3-gallate, a polyphenol of green tea, on neutrophil chemotaxis in vitro and in vivo. J Agric Food Chem. 2004;52(14):4571–4576. | ||
Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010;501(1):65–72. | ||
Pedraza-Chaverri J, Sánchez-Lozada LG, Osorio-Alonso H, Tapia E, Scholze A. New pathogenic concepts and therapeutic approaches to oxidative stress in chronic kidney disease. Oxid Med Cell Longev. 2016;2016:6043601–6043621. | ||
Kim HJ, Vaziri ND. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol. 2010;298(3):F662–F671. | ||
Nakagawa T, Yokozawa T, Sano M, Takeuchi S, Kim M, Minamoto S. Activity of (−)-Epigallocatechin 3-O-gallate against oxidative stress in rats with adenine-induced renal failure. J Agric Food Chem. 2004;52(7):2103–2107. | ||
Yoon SP, Maeng YH, Hong R, et al. Protective effects of Epigallocatechin gallate (EGCG) on streptozotocin-induced diabetic nephropathy in mice. Acta Histochem. 2014;116(8):1210–1215. | ||
Zhang J, Nie S, Wang S. Nanoencapsulation enhances Epigallocatechin-3-gallate stability and its antiatherogenic bioactivities in macrophages. J Agric Food Chem. 2013;61(38):9200–9209. | ||
Green RJ, Murphy AS, Schulz B, Watkins BA, Ferruzzi MG. Common tea formulations modulate in vitro digestive recovery of green tea catechins. Mol Nutr Food Res. 2007;51(9):1152–1162. | ||
Smith A, Giunta B, Bickford PC, Fountain M, Tan J, Shytle RD. Nanolipidic particles improve the bioavailability and alpha-secretase inducing ability of Epigallocatechin-3-gallate (EGCG) for the treatment of Alzheimer’s disease. Int J Pharm. 2010;389(1–2):207–212. | ||
Wang D, Taylor EW, Wang Y, Wan X, Zhang J. Encapsulated nano-Epigallocatechin-3-gallate and elemental selenium nanoparticles as paradigms for nanochemoprevention. Int J Nanomedicine. 2012;7:1711–1721. | ||
Fangueiro JF, Andreani T, Fernandes L, et al. Physicochemical characterization of Epigallocatechin gallate lipid nanoparticles (EGCG-LNs) for ocular instillation. Colloids Surf B Biointerfaces. 2014;123:452–460. | ||
Mora-Huertas CE, Fessi H, Elaissari A. Polymer-based nanocapsules for drug delivery. Int J Pharm. 2010;385(1–2):113–142. | ||
Mura C, Nácher A, Merino V, et al. Design, characterization and in vitro evaluation of 5-aminosalicylic acid loaded N-succinyl-chitosan microparticles for colon specific delivery. Colloids Surf B Biointerfaces. 2012;94:199–205. | ||
Seremeta KP, Chiappetta DA, Sosnik A. Poly(ε-caprolactone), Eudragit® RS 100 and poly(ε-caprolactone)/Eudragit® RS 100 blend submicron particles for the sustained release of the antiretroviral efavirenz. Colloids Surf B Biointerfaces. 2013;102:441–449. | ||
Frias I, Neves AR, Pinheiro M, Reis S. Design, development, and characterization of lipid nanocarriers-based Epigallocatechin gallate delivery system for preventive and therapeutic supplementation. Drug Des Devel Ther. 2016;10:3519–3528. | ||
Xiao B, Zhang M, Viennois E, et al. Inhibition of MDR1 gene expression and enhancing cellular uptake for effective colon cancer treatment using dual-surface-functionalized nanoparticles. Biomaterials. 2015;48:147–160. | ||
Zhang HX, Wang JX, Zhang ZB, Le Y, Shen ZG, Chen JF. Micronization of atorvastatin calcium by antisolvent precipitation process. Int J Pharm. 2009;374(1–2):106–113. | ||
Vandamme T, Lenourry A, Charrueau C, Chaumeil J. The use of polysaccharides to target drugs to the colon. Carbohydr Polym. 2002;48(3):219–231. | ||
Zeng L, Yan J, Luo L, Ma M, Zhu H. Preparation and characterization of (−)-Epigallocatechin-3-gallate (EGCG)-loaded nanoparticles and their inhibitory effects on human breast cancer MCF-7 cells. Sci Rep. 2017;7:45521. | ||
Juère E, Florek J, Bouchoucha M, et al. In vitro dissolution, cellular membrane permeability, and anti-inflammatory response of resveratrol-encapsulated mesoporous silica nanoparticles. Mol Pharm. 2017;14(12):4431–4441. | ||
Tyagi N, de R, Begun J, Popat A. Cancer therapeutics with Epigallocatechin-3-gallate encapsulated in biopolymeric nanoparticles. Int J Pharm. 2017;518(1–2):220–227. | ||
Pujara N, Jambhrunkar S, Wong KY, Mcguckin M, Popat A. Enhanced colloidal stability, solubility and rapid dissolution of resveratrol by nanocomplexation with soy protein isolate. J Colloid Interface Sci. 2017;488:303–308. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.