Back to Journals » Journal of Inflammation Research » Volume 15
Engeletin Alleviates the Inflammation and Apoptosis in Intervertebral Disc Degeneration via Inhibiting the NF-κB and MAPK Pathways
Authors Li B, Yang X , Zhang P, Guo J, Rong K, Wang X, Cao X, Zhou T, Zhao J
Received 22 April 2022
Accepted for publication 20 September 2022
Published 10 October 2022 Volume 2022:15 Pages 5767—5783
DOI https://doi.org/10.2147/JIR.S371809
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zili You
Baixing Li,* Xiao Yang,* Pu Zhang, Jiadong Guo, Kewei Rong, Xin Wang, Xiankun Cao, Tangjun Zhou, Jie Zhao
Shanghai Key Laboratory of Orthopedic Implants, Department of Orthopedics, Ninth People’s Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, 200011, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tangjun Zhou; Jie Zhao, Shanghai Key Laboratory of Orthopedic Implants, Department of Orthopedics, Ninth People’s Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, 200011, People’s Republic of China, Tel +8621-23271159, Fax +8621-63139920, Email [email protected]; [email protected]
Purpose: Low back pain (LBP) induced by intervertebral disc degeneration (IDD) brings progressively painful status and impairs the normal daily living. Engeletin is a plant-derived medicine with anti-inflammation and antioxidant functions. Therefore, we aim to confirm its protective effects against the intervertebral disc degeneration in vivo and in vitro.
Methods: The cytotoxicity of engeletin was validated by CCK-8 tests. Using the TNF-α to simulate the inflammation status in vitro, the expression of inflammatory mediators and MMP families were determined by qPCR, Western blotting and confocal microscopy. Cell apoptosis was analyzed by flow cytometry and TUNEL assay. The expression of apoptosis-related proteins was tested by Western blotting. The activation of NF-κB and MAPK pathways was evaluated by Western blotting and confocal microscopy. In vivo, percutaneous needle puncture was used to establish the IDD model in rat, and engeletin was administrated via intradiscal injection. The therapeutic effects of engeletin were detected through imaging and histology analysis.
Results: Cell viability tests demonstrated there was little cytotoxicity of engeletin toward NP cells. Pretreatment with engeletin effectively ameliorate the TNF-α-induced up-regulation of inflammatory mediators and MMP families, promoting the anabolism of ECM meanwhile. Cell apoptosis was also attenuated with the addition of engeletin. We found that the activation of MAPK and NF-κB signaling pathways and the nuclear translocation of phosphorylated p65 and p38 were inhibited prominently with the treatment of engeletin which may be the potential molecular mechanism for its anti-inflammation effects. Finally, the IDD induced by percutaneous needle puncture was partially alleviated with the injection of engeletin in vivo.
Conclusion: As a natural compound with little cytotoxicity, engeletin possesses the outstanding anti-inflammation and anti-apoptosis effects in the process of IDD in vitro and in vivo, which may be a promising medicine for the prevention and treatment of IDD-related low back pain.
Keywards: intervertebral disc degeneration, engeletin, nucleus pulposus, inflammation, apoptosis, NF-κB and MAPK signaling pathways
Introduction
Low back pain (LBP) has become the leading cause of disability in the world, affecting an estimated 568 million people worldwide according to a recent global health report.1 Moreover, people suffering from that will continue to rise due to the population increase and aging.1,2 It is reported that about 80% of the people will experience LBP at some point in their lives.3 With the highest prevalence in the working-age population, LBP is an important reason for hospital visits and exit of jobs prematurely, which has brought a great economic burden to society.4,5 Among a variety of reasons, intervertebral disc degeneration (IDD) is a major factor contributing to LBP.6 Therefore, it is of great clinical and social significance to explore how to effectively treat IDD.
Intervertebral disc (IVD) is a flexible joint located between the adjacent vertebral bodies, composed of three integrated parts: the internal nucleus pulposus (NP), the surrounding annulus fibrosus (AF) and cartilage endplate (CEP).7 The healthy nucleus pulposus is a gelatinous tissue, consisting of abundant extracellular matrix (ECM) and scattered NP cells.8 In the majority of cases, the IDD is beginning from the nucleus pulposus, manifesting the decrease in NP cells and loss of the ECM. Subsequently, the nucleus pulposus will dehydrate and shrink, causing the height loss, impaired load-bearing capacity and various symptoms finally.9,10 The normal content of ECM is maintained relying on the homeostasis of anabolism and catabolism. But at the inflammation status, the massive inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), will disturb the metabolic homeostasis, resulting in the up-regulation of matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) family, and consequently prompting the degrading of ECM.11–13 Meanwhile, such inflammatory cytokines will also lead to the down-regulated synthesis of collagen and aggrecan, which is the principal component of ECM.8,14 The persistent inflammation will induce the NP cells apoptosis as well, further destroying the metabolic homeostasis and making the IDD irreversible.15,16 From the point of pathophysiology and molecular mechanism, inflammation is the core factor for the starting and developing of IDD; therefore, effectively controlling and relieving the inflammation may be a promising therapeutic strategy.
Engeletin is a natural compound belonging to the flavanonol glycosides, which is widely distributed in many herbs.17 As the main bioactive component of many traditional medicines, engeletin has a long history used to treat a number of inflammatory diseases.18 Modern technology has successfully purified the engeletin and confirmed its a variety of biological effects, such as anti-inflammation, antioxidant and antiulcerogenic effects.19 Recently, many studies have reported engeletin suppresses inflammatory response through blocking the MAPK and NF-κB signaling pathways in many diseases.20–22 Therefore, it has drawn our great attention to verify its therapeutic effects towards the IDD.
In our research, we explored the possibility of engeletin to inhibit the TNF-α-induced inflammatory cytokines synthesis and release, mitigate the disturbance of metabolic homeostasis of matrix and attenuate the apoptosis of NP cells through down-regulating the activation of MAPK and NF-κB pathways in vitro. Following, we constructed the IDD model in rat, further elucidating its protective effects for intervertebral disc in vivo.
Materials and Methods
Reagents and Antibodies
Engeletin was purchased from Selleck Chemicals (Houston, TX, United States). Dimethyl sulfoxide (DMSO), type II collagenase, ITS (Insulin Transferrin Selenium) regent and Alcian blue solution were obtained from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) culture medium, fetal bovine serum (FBS), 0.25% trypsin EDTA solution, 1% penicillin–streptomycin mixture were purchased from Gibco (Thermo Fisher Scientific, Waltham, MA, USA). Cell Counting Kit-8 (CCK-8) was obtained from Sangon Biotech Co. Ltd. (Shanghai, China). TNF-α was purchased from R&D Systems (Minneapolis, MN, USA). TRIzol reagent, BCA assay, Alexa Fluor 594 conjugate secondary antibody and Alexa Fluor 488 conjugate secondary antibody were obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). The cDNA Synthesis Kit and TB Green Premix Ex Taq Kit were obtained from Takara Bio (Otsu, Japan). The RIPA lysis buffer and phosphatase and protease inhibitors were purchased from Roche (Basel, Switzerland). The DAPI staining solution, DNase-free Proteinase K, TUNEL Apoptosis Assay Kit and Annexin V-FITC apoptosis detection kit were obtained from Beyotime Biotechnology Co. Ltd. (Shanghai, China). The primary antibodies against iNOS (AF0199), IL-1β (AF5103), COX2 (AF7003) were purchased from Affinity Biosciences (OH, United States). The primary antibodies against MMP3 (ab52915), MMP13 (ab39012), SOX9 (ab185966) were obtained from Abcam (Cambridge, MA, USA), while β-actin (66009-1-Ig), Bax (50599-2-Ig), Bcl-2 (26593-1-AP) were purchased from Proteintech (Wuhan, China). Antibodies against MMP9 (13667S), Cleaved Caspase-3 (9664S), IKKβ (8943S), phospho-IKKα/β (2694S), IκBα (4814S), phospho-IκBα (5209S), p65 (8242S), phospho-p65 (3033S), SAPK/JNK (9252S), phospho-SAPK/JNK (4668S), ERK1/2 (4695S), phospho-ERK1/2 (4370S), p38 (8690S), phospho-p38 (4511S), anti-rabbit and anti-mouse IgG (H + L; DyLight™ 800 4× PEG conjugate) secondary antibodies, HRP-linked second antibody were obtained from Cell Signaling Technology (Danvers, MA, USA).
Primary NP Cells Isolation and Culture
Six-week-old male Sprague-Dawley rats (Shanghai Lab, Animal Research Center Co. Ltd, Shanghai, China) were euthanized through an overdose of pentobarbital sodium and soaked in 75% ethanol for 15 min. After Flaying the tail skin, the intervertebral discs were exposed. Then cutting alone the cartilage endplate, the gelatinous nucleus pulposus was extracted and digested in the 1% type II Collagenase for 2 hours. Later, after the centrifuge and suspension, the primary nucleus pulposus cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 15% fetal bovine serum and 1% penicillin–streptomycin in an incubator at 37°C with 5% CO2. The NP cells used in the in vitro experiments were from the passage 2–4.
Cell Counting Kit-8 (CCK-8) Assay
The cytotoxicity and influence for proliferation of engeletin on NP cells were assessed using a CCK-8 kit. To determine the cytotoxicity, the NP cells were seeded onto 96-well plates at a density of 2×104 cells/well with engeletin at different concentrations (0, 2.5, 5, 10, 20, 40, 80, 160 µM) for 24 h. To determine the influence for proliferation, the NP cells seeded onto 96-well plates at a density of 5×103 cells/well were cultured with engeletin at different concentrations (0, 2.5, 5, 10, 20, 40, 80, 160 µM) for 24 h, 48 h and 72 h. At the end of the experimental periods, 100µl complete media containing 10 μL of CCK-8 reagent was added into each well, and mixture was also added into wells without cells as blank control. After incubating for 2h at 37°C, the absorbances (measured as optical density; OD) of each well were measured at 450nm using an Infinite M200 Pro multimode microplate reader (Tecan Life Sciences, Männedorf, Switzerland).
RNA Extraction and Real-Time Quantitative PCR (qPCR) Analyses
NP cells were seeded in 6-well plates and incubated with different treatments for 24h at 37°C. Then, total RNA was extracted from cells using TRIzol reagent according to the manufacturer’s protocol. The complementary DNA (cDNA) was reversed transcribed from the obtained total RNA using the cDNA Synthesis Kit. Real-time qPCR was performed using the TB Green Premix Ex Taq Kit on an Applied Biosystems QuantStudio 6 Flex Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). The β-actin was used as the internal reference gene and the expression of other genes were normalized using the 2−ΔΔCT method. The primer sequences were designed using NCBI BLAST and listed in Table 1.
 |
Table 1 Primer Sequences |
Protein Extraction and Western Blotting
Total cellular proteins were extracted from the pretreatment NP cells using RIPA lysis buffer containing phosphatase and protease inhibitors, and the content of proteins was quantified with BCA assay. Then, the equal amounts of proteins were resolved on 4–20% gels and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electroblotted onto 0.22 μm PVDF membrane (Merck-Millipore). After blocking with 5% BSA-TBST at room temperature for 1h, the membranes were washed for 30 min with TBST buffer and subsequently incubated with targeted primary antibodies (diluted 1:1000) overnight at 4°C. Membranes were washed for another 30 min with TBST and then incubated with anti-rabbit or anti-mouse secondary antibodies (diluted 1:5000) at RT for 1 hour in the dark. Later, the membranes were washed again and protein immunoreactivity was detected on a LI-COR Odyssey fluorescence imaging system (LI-COR Biosciences, Lincoln, NE, United States). The β-actin was used as internal reference and semi-quantitative analysis was achieved using ImageJ V1.8.0 software (National Institutes of Health).
Immunofluorescence
NP cells were seeded on the 35-mm Nunc™ glass-based dish (Thermo Fisher Scientific) and stimulated using TNF-α with or without engeletin addition for 24h. As for the assessment of p-p65 and p-p38 nuclear translocation, the cells were pretreated with or without engeletin for 3h and then stimulated with TNF-α for 15min. The normal NP cells without treatment were served as the control group. At the end of the experimental periods, NP cells were washed three times with PBS buffer and then fixed for 30min with 4% paraformaldehyde. After another three times wash, NP cells were permeabilized with 0.5% Triton X-100 at room temperature for 10min and blocked with 5% BSA for another 1h. Subsequently, NP cells were incubated with primary antibodies (diluted 1:200) at 4°C overnight. The next day, after washing with PBS three times, NP cells were incubated with Alexa Fluor 594 conjugate secondary antibody or Alexa Fluor 488 conjugate secondary antibody (diluted 1:1000) for 1h in the dark, and then stained with DAPI solution for 5min in the dark. Finally, the digital fluorescence images were obtained using a laser scanning microscope (Leica SP8 confocal microscope, Germany). The integrated optical density (IOD) of targeted genes and DAPI were measured for semi-quantitative analysis.
High-Density Culture
NP cells were harvested and suspended at a density of 1.5×107 cells/mL. Then, a 10µl solution containing about 1.5×105 NP cells was seeded at the bottom of the 24-well plate. After 2 hours at 37°C, the DMEM supplemented with 2.5% FBS, 10ng/mL ITS (Insulin Transferrin Selenium) with or without TNF-α and engeletin was added into each well. The medium was refreshed every 2 days. After a week, the NP cells clusters were stained with Alcian blue solution overnight at room temperature to evaluate the ECM synthesis capacity, and the digital images were captured using a Leica DM4000B epifluorescence microscope (Leica Microsystems).
Flow Cytometry
The Annexin V-FITC apoptosis detection kit was used to evaluate the rate of apoptosis. The NP cells with different treatments were collected and washed with cold PBS two times, then 1.0×105 cells in each group were harvested and suspended with 195µl Annexin V-FITC binding buffer. Subsequently, 5µl Annexin V-FITC and 10µl propidium iodide (PI) were added and incubated for 20min at RT in the dark. Finally, the rate of apoptosis was detected using a flow cytometer (BD Co., USA).
TdT-Mediated dUTP Nick End Labeling (TUNEL) Assay
The TUNEL apoptosis assay kit was also used to assess the anti-apoptotic ability of engeletin. Briefly, the NP cells at different groups were fixed with 4% paraformaldehyde for 30min at room temperature and treated with 0.5% Triton X-100 for another 10min. As for the TUNEL staining for tissues, the sections were deparaffinized and rehydrated, followed by incubated with DNase-free Proteinase K for 30min at room temperature. Then, after washing three times, NP cells or sections were incubated with TUNEL detection solution at RT for 1h in the dark. The nucleus was stained with DAPI staining solution for another 5min. Finally, the digital fluorescence images were captured using a confocal microscope or epifluorescence microscope. The semi-quantitative analysis was performed through counting the TUNEL-positive cells.
Animals and Surgical Procedures
Ten 12-week-old male Sprague-Dawley rats were housed in pathogen-free conditions with appropriate temperature, humidity and nutrition before and after the surgery. The percutaneous needle puncture was demonstrated as a credible method to construct IDD model on rat previously.23 Briefly, before the surgical procedures, rats were anesthetized through intraperitoneal injections of pentobarbital sodium (40 mg/kg of body weight) and their tails were disinfected with 75% ethanol. Then, the Co7/8 and Co8/9 discs were punctured using a 20-gauge sterile needle from the dorsal skin into the central of nucleus pulposus and rotated 360° with the following 30s kept in position before extraction. Finally, the tail skin was resterilized after extracting the needle to avoid infection. The Co8/9 discs were given 50µl engeletin with a concentration of 80µM via intradiscal injection every week using a microsyringe. Meanwhile, the Co7/8 discs were given the same volume of saline every week as the treatment group. The Co6/7 discs were chosen as the control group without any treatment.
X-Ray Analysis
After 4 weeks of surgery, these rats received X-ray to evaluate the changes of intervertebral space. The digital imaging was captured in the anteroposterior axis with a 21 lp/mm detector that provides up to 5× geometric magnification (Faxitron VersaVision; Faxitron Bioptics LLC, Tucson, AZ, USA). The Disc height indexes (DHIs) were calculated according to the formula provided previously.23
Magnetic Resonance Imaging (MRI) Scan
MRI was commonly used to assess the degree of IDD according to the signal and structure changes of discs. At 4 weeks post-surgery, these anesthetized rats were fixed and the coccygeal IVD signal was obtained using a 1.5 T magnetic resonance scanner (Philips Eclipse, Cleveland, OH, USA) in sagittal T2-weighted images (T2WI). Later, the Pfirrmann grades were measured to evaluate the degree of IDD.
Histology Analysis
The coccygeal IVD tissues were collected and fixed in 4% paraformaldehyde at least 48h. Then, the samples were embedded into paraffin and 5µm serial sections were obtained in the midsagittal region. Subsequently, the sections were stained with hematoxylin and eosin (HE), safranin O-fast green (SO-FG) and alcian blue (AB) to evaluate the structural changes of the IVD. Histological score was calculated according to a modified histologic grading system.24
Immunohistochemistry
The sections were deparaffinized and rehydrated first, and then dealt with 3% hydrogen peroxide (H2O2) 10min at room temperature to block the endogenous peroxidase. After blocking with the 10% goat serum for 1h at RT, the primary antibodies were incubated overnight at 4°C. Finally, the sections were washed with PBS twice and incubated with HRP conjugated secondary antibody for 1h at RT the next day. Digital images were obtained under a Leica DM4000 B microscope (Leica Microsystems). The integrated optical density (IOD) was measured for semi-quantitative analysis using Image Pro Plus 6.0 software.
Statistical Analysis
All experiments presented in this study were conducted at least three times to obtain the data. The measurement data were shown as the mean ± standard deviation. t-test was used to analyze the difference between the two groups, while multiple groups were analyzed using one-way analysis of variance (ANOVA). p values <0.05 was considered statistical significance. GraphPad Prism 8.3 (GraphPad Software, San Diego, CA, United States) was used to analyze the data and draw relevant figures.
Results
Effects of Engeletin on NP Cells Viability and Proliferation
Engeletin is a natural compound with a chemical structure shown in Figure 1A. The CCK-8 tests were performed in order to determine the effects of engeletin on NP cells viability and proliferation. For cytotoxicity analysis, NP cells were treated with different concentrations (0, 2.5, 5, 10, 20, 40, 80, 160uM) of engeletin for 24h. As shown in Figure 1B, the cell viability manifested no significant difference between different groups. For proliferation analysis, NP cells with a lower density were treated with engeletin for 24, 48 and 72h. Engeletin showed no influence on NP cells proliferation for 24h (Figure 1C), but a little proliferation inhibition was shown at a concentration of 160µM for 48 and 72h (Figure 1D and E). Therefore, 80µM of engeletin was used in the following experiments.
Engeletin Ameliorated TNF-α-Induced Inflammation on NP Cells
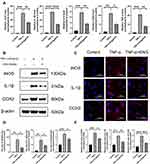
To detect the anti-inflammation effects of engeletin, TNF-α was used to simulate the inflammation status in vitro and the expression of several inflammatory mediators were determined by Western blotting, RT-qPCR and Immunofluorescence. NP cells were seeded in 6-well plates at a density of 2×105 cells/well, followed by incubated with engeletin (80uM) and TNF-α (20ng/mL) for 24h. As shown in Figure 2A, the TNF-α stimulation prominently improved the expression of iNOS, IL-1β, IL-6, COX2 and TNF-α on mRNA level, but engeletin could block the increment effectively. Meanwhile, the expression of iNOS, IL-1β and COX2 was also up-regulated on protein levels after stimulating by TNF-α (Figure 2B and D), while the addition of engeletin reversed these changes. The results of immunofluorescence further demonstrated the effects of engeletin on inhibiting the expression of inflammatory mediators (Figure 2C and E).
Engeletin Protected NP Cells from the Disturbance of Metabolic Homeostasis Induced by TNF-α
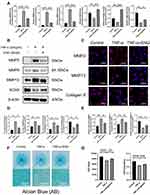
The disturbance of metabolic homeostasis is the critical factor of IDD, manifested as the enhancement of catabolism and destroying of normal anabolism, leading to the reduction of ECM finally. As shown in Figure 3A, B and D, the expression of catabolic mediators of ECM, such as MMP3, MMP9 and MMP13, was elevated on mRNA and protein levels after the stimulation of TNF-α, while the anabolic mediators (Collagen II, Aggrecan and SOX9) presented an opposite trend. However, in the treatment group, engeletin could attenuate the degradation and promote the synthesis of ECM. The results of immunofluorescence on MMP3, MMP13 and Collagen II were consistent with Western blotting and RT-qPCR, which also elucidated the protective effects of engeletin on ECM (Figure 3C and E). In order to further validate the promoting effects on the synthesis of ECM, high-density culture was performed to simulate the functions of engeletin on nucleus pulposus in vivo. As shown in Figure 3F and G, engeletin could notably block the TNF-α-induced loss of ECM.
Engeletin Alleviated TNF-α-Induced Apoptosis of NP Cells
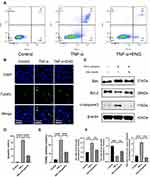
After treating with engeletin and TNF-α for 48h, flow cytometry was used to evaluate the rate of NP cells apoptosis. As shown in Figure 4A and D, TNF-α stimulation promoted NP cells apoptosis distinctly, while engeletin could reverse such effects. The anti-apoptotic function of engeletin was further determined using TUNEL assay. Similar results were also observed in the treatment group (Figure 4B and E). In order to explore the anti-apoptotic molecular mechanism of engeletin, the expression of apoptosis-related proteins were detected by Western blotting (Figure 4C and F). Engeletin treatment could inhibit the TNF-α-induced up-regulation of Bax and cleaved-caspase 3, which were pro-apoptotic protein and apoptosis mediators. Meanwhile, the expression of Bcl-2, an anti-apoptotic protein, was elevated notably.
Engeletin Down-Regulated the TNF-α-Induced NF-κB and MAPK Signaling Pathways Activation in NP Cells
The NF-κB and MAPK signaling pathways were closely related to inflammation and apoptosis, and many studies have confirmed the abnormal activation of NF-κB and MAPK pathways are important mechanisms for the beginning and development of various inflammatory diseases.25–27 Therefore, we also tested if engeletin mitigated inflammation and apoptosis through inhibiting NF-κB and MAPK pathways.
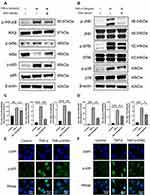
After pretreating with engeletin for 2h, TNF-α was added for another 15min. As shown in Figure 5A and C, NF-κB pathways were activated after stimulating by TNF-α, increasing the phosphorylation of IKKα/β, IκBα and p65, while pretreating with engeletin obviously inhibited their phosphorylation. As for the MAPK pathways, similar results were obtained (Figure 5B and D). Engeletin could down-regulated the TNF-α-induced phosphorylation of JNK, ERK and p38, showing the inhibitory function on the activation of MAPK pathways. The results of immunofluorescence manifested that engeletin suppressed the phosphorylation and nuclear translocation of p65 and p38 (Figure 5E and F). In general, engeletin exerted its anti-inflammation and anti-apoptotic effects via inhibiting NF-κB and MAPK pathways.
Engeletin Ameliorated IDD in a Rat Model in vivo
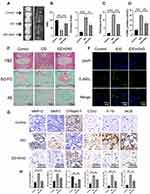
In order to evaluate the therapeutic effects of engeletin in vivo, we constructed a percutaneous needle puncture induced-IDD model in rat. The results of X-ray showed that the IVD degenerated much severely in the simple surgery group compared with the control group, manifested by decreased DHI and formation of osteophyte. Meanwhile, the MRI results also revealed that the signal intensity on T2WI and Pfirrmann grade was much lower in the IDD group than in the control group (Figure 6A and B). However, the height of IVD and signal intensity on T2WI were partially maintained in the treatment group, demonstrating engeletin could inhibit the development of IDD to some extent in vivo (Figure 6A and B). The results of H&E, SO-FG and AB staining showed that the structure of IVD were destroyed seriously in the IDD group, but intradiscal injection of engeletin could reverse such destruction notably, which further elucidated the therapeutic functions of engeletin in vivo (Figure 6C and E). Besides, the results of Immunohistochemistry staining for COX2, IL-1β, iNOS, MMP3, MMP13 and Collagen II also illustrated that engeletin could inhibit the expression of inflammatory mediators and catabolic mediators, protecting the ECM from degrading and promoting the synthesis of ECM (Figure 6G and H). Finally, the TUNEL staining for IVD tissues was conducted to further confirm the anti-apoptotic effects of engeletin in vivo. The results were consistent with the results in vitro, implying engeletin could also alleviate NP cells apoptosis in vivo (Figure 6D and F).
Discussion
Low back pain induced by IDD influences increasing people worldwide, leading to limited activities of daily living and poor life quality. It is reported that IVD undergoes the degenerative process very early after skeletal maturity, manifested as loss of ECM and the increasing cell death.8,28 Attributing to its specific structure and microenvironment, the IVD is prone to degenerating. As the largest avascular tissue, the microenvironment of IVD is hostile, implying low nutrition, hypoxia, acidic environment, high compression loading and so on.3,29,30 Under such condition, some NP cells will undergo phenotypic changes, manifested as abnormal production and accumulation of pro-inflammatory factors, such as TNF-α and IL-1β.31–33 It is believed that inflammation is the initial step for IDD. These inflammatory cytokines will lead to the dysfunction of normal NP cells characteristic by up-regulated expression of inflammatory mediators and pro-catabolic factors, such as MMPs and ADAMTS.32 Subsequently, the inflammatory cytokines will act on other normal NP cells, and pro-catabolic factors will promote the degradation of ECM, leading to the loss of ECM. More seriously, the matrix degradation production will further induce the production of inflammatory mediators and resulting in a vicious cycle.30,34 Therefore, the disturbance of metabolic homeostasis for ECM is the critical step for the development of IDD. Afterwards, the prominent and consistent inflammation will stimulate the apoptosis and senescence of NP cells, leading to the functioned NP cells further decreasing.35 Finally, the anabolism of ECM, mainly composed by type II collagen and aggrecan, is destructed due to the reduced number and dysfunction of NP cells, which is the symbol of irreversible IDD through the conventional therapy.36 In general, from the perspective of the pathophysiology, it may be a promising therapeutic strategy for IDD to inhibit the inflammation and catabolism of NP in the early stage.
Flavonoids are a big family of natural polyphenolic compounds, which are widely distributed in the fruits, vegetables or herbs and mostly possess anti-inflammation, antioxidant or anticancer activities, used to treat many kinds of diseases for a long history.37–39 As a kind of flavanonol glycosides, engeletin has superior anti-inflammation and antioxidant functions with little toxicity, but its therapeutic effect for IDD is still unknown up to now. In our studies, NP cells were stimulated with TNF-α to mimic the inflammatory environment in vitro, followed by incubated with engeletin in the treatment group. We found that engeletin could ameliorate inflammation, ECM degradation and NP cells apoptosis via inhibiting NF-κB and MAPK pathways. In the following rat models in vivo, engeletin also exerted its protective effects for IVD and partially reversed the structural destruction compared with the IDD group.
The degenerated IVD is featured by a higher expression of inflammatory mediators, especially TNF-α and IL-1β, compared to the normal IVD.33 Meanwhile, there is a positive correlation between the degree of IDD and the expression of such mediators.40 Except for the TNF-α, IL-1β and IL-6, iNOS and COX2 also play an important role in the development of IDD, as they can mediate the expression of downstream nitric oxide (NO) and prostaglandin E2 (PGE2), which can promote the degradation of ECM.41 In our studies, we confirmed that engeletin could inhibit the expression of such inflammatory mediators on mRNA and protein levels. Our further studies will validate if engeletin could also inhibit the secretion of such mediators in vitro and in vivo.
The functioned NP cells decrease was commonly found in the progressive stage of IDD. Our studies confirmed that engeletin maintained the functioned NP cells via alleviating the TNF-α-induced apoptosis. In order to explore the anti-apoptotic mechanism of engeletin, we also detected the expression of apoptosis-related proteins. The results indicated engeletin effectively elevated the expression of Bcl-2, a classical anti-apoptotic protein, and down-regulated the expression of Bax, which is a critical executioner in apoptosis.42 Besides, other researchers have reported engeletin could inhibit the production of ROS and maintain the mitochondrial membrane potential in chondrocytes, which may be also an important mechanism for its anti-apoptotic effects, requiring our further confirming in NP cells in the future.22 In addition, our following studies will also focus on if engeletin possesses the effects on mitigating the senescence of NP cells.
NF-κB and MAPK pathways are closely related to the regulation of inflammation and apoptosis. After stimulating by inflammatory factors, the p65 and p38 will be phosphorylated and transfer into the nucleus, followed by regulating the expression of many genes involved in the inflammation and apoptosis. Other studies also confirmed the activation of NF-κB and MAPK pathways could lead to the production of inflammatory mediators and matrix degrading enzymes in IVD.43 In our study, engeletin successfully blocked the phosphorylation of IKKα/β, IκBα, p65, JNK, ERK and p38, then down-regulated the nucleus translocation of p65 and p38 to exert its protective effects. However, the limitation is that we did not find the precise targets of engeletin for the inhibition of NF-κB and MAPK pathways. Based on our available results, the targets may be in the upstream of IKKα/β and JNK/ERK/p38, which should be further explored.
With the in-depth understanding of the mechanism in IDD, many novel therapeutic strategies for disc regeneration come out, including cell-based therapy, growth factors treatment, gene therapy, bioactive compound therapy and so on, which are aimed to increase the cell number and balance the metabolism of ECM.44–46 In our studies, we validated the therapeutic effects in vivo through intradiscal injection of engeletin in rat models. However, in clinical practice, the intradiscal injection is not a conventional route of administration. Therefore, we will test the protective functions of engeletin in vivo via other route of administration in the following studies, but the effect is not distinct due to the avascular characteristic of IVD.
This study has three limitations. First, the underlying anti-apoptotic mechanism of engeletin is not well explored. In our studies, although we discovered the expression of apoptosis-related proteins was influenced by engeletin, the specific target and regulating pathways are still indistinct and further investigation should focus on this point. Second, the rats used to construct IDD models were pretty young. Generally, the IDD and related low back pain are more prevalent in elderly persons. Thus, the older rats are more appropriate to explore the therapeutic effects of engeletin towards aging-related IDD. Third, the in vivo toxicity of engeletin was not definite. The local toxicity towards IVD and systemic toxicity should be further researched in the following studies.
Conclusion
This article elucidated that engeletin could inhibit the TNF-α-induced up-regulation of inflammatory mediators and NP cells apoptosis, maintaining the metabolic homeostasis of ECM through blocking the NF-κB and MAPK pathways in vitro (Figure 7). In addition, we further confirmed that engeletin exerted protective effects in percutaneous needle puncture induced-IDD models in vivo. In general, our studies highlight engeletin is a promising drug for the prevention and treatment of IDD.
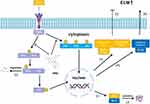 |
Figure 7 Potential molecular mechanism involved in the therapeutic effects of engeletin for IDD. |
Abbreviations
LBP, low back pain; IDD, intervertebral disc degeneration; IVD, intervertebral disc; NP, nucleus pulposus; AF, annulus fibrosus; CEP, cartilage endplate; ECM, extracellular matrix; MMP, matrix metalloproteinase; ADAMTS, a disintegrin and metalloproteinase with thrombospondin motifs; NF-κB, nuclear factor kappa-B; MAPK, mitogen-activated protein kinase; DMSO, Dimethyl Sulfoxide; ITS, Insulin Transferrin Selenium; DMEM, Dulbecco’s modified Eagle’s medium; FBS, fetal bovine serum; CCK-8, Cell Counting Kit-8; TUNEL, TdT-mediated dUTP nick end labeling; OD, optical density; WB, Western blotting; RT-qPCR, real-time quantitative PCR; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; RT, room temperature; DHI, Disc height indexes; MRI, Magnetic Resonance Imaging; T2WI, T2-weighted images; HE, hematoxylin and eosin; SO-FG, safranin O-fast green; AB, alcian blue; ENG, engeletin; TNF-α, tumor necrosis factor alpha; IL-1β, interleukin-1β; ROS, reactive oxygen species.
Ethics Approval
All animal experimentations were approved by the Institutional Animal Care and Ethics Committee of Ninth People’s Hospital, Shanghai Jiaotong University School of Medicine (Shanghai, China) and performed in accordance with the principles and procedures of the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and the Guidelines for Animal Treatment of Shanghai Jiaotong University.
Acknowledgments
We sincerely appreciate the support we received from the Institute of Precision Medicine of Shanghai Ninth People’s Hospital.
Funding
The present study was supported by grants from The National Natural Science Foundation of China (grant nos. 82130073, 81871790, 81572768 and 81972136), and Shanghai Frontiers Science Center of Degeneration and Regeneration in Skeletal System.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cieza A, Causey K, Kamenov K, Hanson SW, Chatterji S, Vos T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2021;396(10267):2006–2017. doi:10.1016/S0140-6736(20)32340-0
2. Jin Z, Wang D, Zhang H, et al. Incidence trend of five common musculoskeletal disorders from 1990 to 2017 at the global, regional and national level: results from the global burden of disease study 2017. Ann Rheum Dis. 2020;79(8):1014–1022. doi:10.1136/annrheumdis-2020-217050
3. Liu Y, Li Y, Nan LP, et al. Insights of stem cell-based endogenous repair of intervertebral disc degeneration. World J Stem Cells. 2020;12(4):266–276. doi:10.4252/wjsc.v12.i4.266
4. Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi:10.1016/S0140-6736(20)30925-9
5. Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356–2367. doi:10.1016/S0140-6736(18)30480-X
6. Vo NV, Hartman RA, Patil PR, et al. Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res. 2016;34(8):1289–1306. doi:10.1002/jor.23195
7. Binch ALA, Fitzgerald JC, Growney EA, Barry F. Cell-based strategies for IVD repair: clinical progress and translational obstacles. Nat Rev Rheumatol. 2021;17(3):158–175. doi:10.1038/s41584-020-00568-w
8. Frapin L, Clouet J, Delplace V, Fusellier M, Guicheux J, Le Visage C. Lessons learned from intervertebral disc pathophysiology to guide rational design of sequential delivery systems for therapeutic biological factors. Adv Drug Deliv Rev. 2019;149–150:49–71. doi:10.1016/j.addr.2019.08.007
9. Gruber HE, Hanley EN. Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine. 1998;23(7):751–757. doi:10.1097/00007632-199804010-00001
10. Urban JPG, Fairbank JCT. Current perspectives on the role of biomechanical loading and genetics in development of disc degeneration and low back pain; a narrative review. J Biomech. 2020;102:109573. doi:10.1016/j.jbiomech.2019.109573
11. Wang Y, Che M, Xin J, Zheng Z, Li J, Zhang S. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed Pharmacother. 2020;131:110660. doi:10.1016/j.biopha.2020.110660
12. Johnson ZI, Schoepflin ZR, Choi H, Shapiro IM, Risbud MV. Disc in flames: roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur Cell Mater. 2015;30:104–117. doi:10.22203/eCM.v030a08
13. Bachmeier BE, Nerlich A, Mittermaier N, et al. Matrix metalloproteinase expression levels suggest distinct enzyme roles during lumbar disc herniation and degeneration. Eur Spine J. 2009;18(11):1573–1586. doi:10.1007/s00586-009-1031-8
14. Lang G, Liu Y, Geries J, et al. An intervertebral disc whole organ culture system to investigate proinflammatory and degenerative disc disease condition. J Tissue Eng Regen Med. 2018;12(4):e2051–e2061. doi:10.1002/term.2636
15. Zhang X-B, Hu Y-C, Cheng P, et al. Targeted therapy for intervertebral disc degeneration: inhibiting apoptosis is a promising treatment strategy. Int J Med Sci. 2021;18(13):2799–2813. doi:10.7150/ijms.59171
16. Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):10–14. doi:10.2106/JBJS.F.00019
17. Huang Z, Ji H, Shi J, Zhu X, Zhi Z. Engeletin attenuates Aβ1-42-induced oxidative stress and neuroinflammation by Keap1/Nrf2 pathway. Inflammation. 2020;43(5):1759–1771. doi:10.1007/s10753-020-01250-9
18. Zhao X, Chen R, Shi Y, Zhang X, Tian C, Xia D. Antioxidant and anti-inflammatory activities of six flavonoids from roxb. Molecules. 2020;25(22):5295. doi:10.3390/molecules25225295
19. Alam F, Mohammadin K, Shafique Z, Amjad ST, Asad MHHB. Citrus flavonoids as potential therapeutic agents: a review. Phytother Res. 2022;36(4):1417–1441. doi:10.1002/ptr.7261
20. Wang C, La L, Feng H, et al. Aldose reductase inhibitor engeletin suppresses pelvic inflammatory disease by blocking the phospholipase C/Protein kinase C-Dependent/NF-κB and MAPK cascades. J Agric Food Chem. 2020;68(42):11747–11757. doi:10.1021/acs.jafc.0c05102
21. Wu H, Zhao G, Jiang K, Li C, Qiu C, Deng G. Engeletin alleviates lipopolysaccharide-induced endometritis in mice by inhibiting TLR4-mediated NF-κB activation. J Agric Food Chem. 2016;64(31):6171–6178. doi:10.1021/acs.jafc.6b02304
22. Wang H, Jiang Z, Pang Z, et al. Engeletin protects against TNF-α-induced apoptosis and reactive oxygen species generation in chondrocytes and alleviates osteoarthritis in vivo. J Inflamm Res. 2021;14:745–760. doi:10.2147/JIR.S297166
23. Han B, Zhu K, Li F-C, et al. A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine. 2008;33(18):1925–1934. doi:10.1097/BRS.0b013e31817c64a9
24. Ji M-L, Jiang H, Zhang X-J, et al. Preclinical development of a microRNA-based therapy for intervertebral disc degeneration. Nat Commun. 2018;9(1):5051. doi:10.1038/s41467-018-07360-1
25. Chen Z, Yang X, Zhou Y, et al. Dehydrocostus lactone attenuates the senescence of nucleus pulposus cells and ameliorates intervertebral disc degeneration via inhibition of STING-TBK1/NF-κB and MAPK signaling. Front Pharmacol. 2021;12:641098. doi:10.3389/fphar.2021.641098
26. Hossain MA, Alam MJ, Kim B, Kang C-W, Kim J-H. Ginsenoside-Rb1 prevents bone cartilage destruction through down-regulation of p-Akt, p-P38, and p-P65 signaling in rabbit. Phytomedicine. 2022;100:154039. doi:10.1016/j.phymed.2022.154039
27. Zhao H, Kong L, Shao M, et al. Protective effect of flavonoids extract of Hippophae rhamnoides L. on alcoholic fatty liver disease through regulating intestinal flora and inhibiting TAK1/p38MAPK/p65NF-κB pathway. J Ethnopharmacol. 2022;292:115225. doi:10.1016/j.jep.2022.115225
28. Urban JPG, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5(3):120–130. doi:10.1186/ar629
29. Grunhagen T, Shirazi-Adl A, Fairbank JCT, Urban JPG. Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthop Clin North Am. 2011;42(4):465–477. doi:10.1016/j.ocl.2011.07.010
30. Sakai D, Grad S. Advancing the cellular and molecular therapy for intervertebral disc disease. Adv Drug Deliv Rev. 2015;84:159–171. doi:10.1016/j.addr.2014.06.009
31. Gruber HE, Hoelscher GL, Ingram JA, Bethea S, Hanley EN. Growth and differentiation factor-5 (GDF-5) in the human intervertebral annulus cells and its modulation by IL-1ß and TNF-α in vitro. Exp Mol Pathol. 2014;96(2):225–229. doi:10.1016/j.yexmp.2014.02.005
32. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. doi:10.1038/nrrheum.2013.160
33. Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9(4):R77. doi:10.1186/ar2275
34. Vergroesen PPA, Kingma I, Emanuel KS, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthr Cartil. 2015;23(7):1057–1070. doi:10.1016/j.joca.2015.03.028
35. Kepler CK, Anderson DG, Tannoury C, Ponnappan RK. Intervertebral disk degeneration and emerging biologic treatments. J Am Acad Orthop Surg. 2011;19(9):543–553. doi:10.5435/00124635-201109000-00005
36. Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20(11):1307–1314. doi:10.1097/00007632-199506000-00022
37. Khan H, Belwal T, Efferth T, et al. Targeting epigenetics in cancer: therapeutic potential of flavonoids. Crit Rev Food Sci Nutr. 2021;61(10):1616–1639. doi:10.1080/10408398.2020.1763910
38. Mozaffarian D, Wu JHY. Flavonoids, dairy foods, and cardiovascular and metabolic health: a review of emerging biologic pathways. Circ Res. 2018;122(2):369–384. doi:10.1161/CIRCRESAHA.117.309008
39. Rengasamy KRR, Khan H, Gowrishankar S, et al. The role of flavonoids in autoimmune diseases: therapeutic updates. Pharmacol Ther. 2019;194:107–131. doi:10.1016/j.pharmthera.2018.09.009
40. Weiler C, Nerlich AG, Bachmeier BE, Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine. 2005;30(1):44–53. doi:10.1097/01.brs.0000149186.63457.20
41. Lin Z, Fu C, Yan Z, et al. The protective effect of hesperetin in osteoarthritis: an in vitro and in vivo study. Food Funct. 2020;11(3):2654–2666. doi:10.1039/C9FO02552A
42. Spitz AZ, Gavathiotis E. Physiological and pharmacological modulation of BAX. Trends Pharmacol Sci. 2022;43(3):206–220. doi:10.1016/j.tips.2021.11.001
43. Hiyama A, Sakai D, Mochida J. Cell signaling pathways related to pain receptors in the degenerated disk. Global Spine j. 2013;3(3):165–174. doi:10.1055/s-0033-1345036
44. Clouet J, Fusellier M, Camus A, Le Visage C, Guicheux J. Intervertebral disc regeneration: from cell therapy to the development of novel bioinspired endogenous repair strategies. Adv Drug Deliv Rev. 2019;146:306–324. doi:10.1016/j.addr.2018.04.017
45. Sampara P, Banala RR, Vemuri SK, Av GR, Gpv S. Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: a review. Gene Ther. 2018;25(2):67–82. doi:10.1038/s41434-018-0004-0
46. Hu ZL, Li HY, Chang X, et al. Exosomes derived from stem cells as an emerging therapeutic strategy for intervertebral disc degeneration. World J Stem Cells. 2020;12(8):803–813. doi:10.4252/wjsc.v12.i8.803
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.