Back to Journals » Journal of Inflammation Research » Volume 16
Endothelial-Related Biomarkers in Evaluation of Vascular Function During Progression of Sepsis After Severe Trauma: New Potential Diagnostic Tools in Sepsis
Authors Yang B , Wang X, Liu Z, Lu Z, Fang G, Xue X, Luo T
Received 25 April 2023
Accepted for publication 3 July 2023
Published 6 July 2023 Volume 2023:16 Pages 2773—2782
DOI https://doi.org/10.2147/JIR.S418697
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Biao Yang,1,* Xiaoyong Wang,2,* Zhaorui Liu,1,* Zhengmao Lu,1 Guoen Fang,1 Xuchao Xue,1 Tianhang Luo1
1Department of General Surgery, Changhai Hospital, The Second Military Medical University, Shanghai, 200433, People’s Republic of China; 2Department of Gastrointestinal Surgery, People’s Hospital of Haimen City, Nantong, Jiangsu Province, 226100, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tianhang Luo, Department of General Surgery, Changhai Hospital, 168 Changhai Road, Yangpu District, Shanghai, 200433, People’s Republic of China, Tel +86-21-31161589, Fax +86-21-31161593, Email [email protected]
Purpose: This study aimed to investigate the changes in endothelial-related biomarkers and their relationship with the incidence and prognosis of patients with sepsis after severe trauma.
Methods: A total of 37 severe trauma patients admitted to our hospital from Jan. to Dec. 2020 were enrolled in our research. All enrolled patients were divided into the sepsis and the non-sepsis groups. Endothelial progenitor cells (EPCs), circulating endothelial cells (CECs), and endothelial microparticles (EMPs) were detected on admission time; 24– 48 hours and 48– 72 hours after admission respectively. Demographic data, Acute Physiology, Chronic Health Evaluation (APACHE) II, and Sequential Organ Failure Assessment (SOFA) score were calculated every 24 h of admission to assess the severity of organ dysfunction. Receiver operating characteristic (ROC) curves were drawn to compare the areas under the curve (AUC) of endothelial-related biomarkers for the diagnosis of sepsis.
Results: The incidence rate of sepsis was 45.95% in all patients. The SOFA score in the sepsis group was significantly higher than that in the non-sepsis group (2 points vs 0 points, P< 0.01). The number of EPCs, CECs, and EMPs all rose quickly in the early phase after trauma. The number of EPCs was similar in both groups, but the number of CECs and EMPs in the Sepsis Group was much higher than in the non-Sepsis Group (all P< 0.01). Logistic regression analysis showed that the occurrence of sepsis was closely related to the expression of 0– 24h CECs and 0– 24h EMPs. The AUC ROC for CECs in different time periods were 0.815, 0.877, and 0.882, respectively (all P< 0.001). The AUC ROC for EMPs in 0– 24h was 0.868 (P=0.005).
Conclusion: The expression of EMPs was higher in early severe trauma, and high levels of EMPs were significantly higher in patients with early sepsis and poor prognosis.
Keywords: sepsis, severe trauma, endothelial cell, biomarkers, prognosis
Introduction
Sepsis, however, is the major cause of death in patients after severe trauma, with mortality rates of 14–60% in different reports.1,2 What’s more, sepsis remains a heterogonous syndrome with differing outcomes among patient cohorts and subtypes,3,4 especially in the cohort of patients with severe trauma.2 The incidence and outcomes from sepsis caused by severe trauma may differ from non-trauma hospitalizations.5 Plenty of studies in sepsis treatment have shown benefits in early diagnosis and treatment, which may also curtail the high mortality and cost of care. Sepsis, defined as life-threatening organ dysfunction resulting from an altered host response to infection, remains a major challenge for healthcare systems worldwide.6 Early development of effective treatment strategies through rapid and accurate diagnosis is a prerequisite for further reducing morbidity and case fatality.7
Sepsis always involves a complicated host response including the activation of several cell types and inflammatory mediators. One of the hallmarks is endothelial damage, which may be the critical event in the initiation of sepsis. But several clinical studies have shown that the assessment of inflammatory markers in peripheral circulation may not truly reflect the status of the endothelial function during the process of sepsis. The results of our former research indicate endothelial cellular surrogates such as endothelial progenitor cells (EPCs), circulating endothelial cells (CECs) and endothelial microparticles (EMPs) may reflect alterations in endothelial status which could be defined as “biomarkers” of endothelial function.8,9 The biomarkers produced by endothelial cell activation and injury can provide important information for the clinic’s diagnosis, prognosis, and treatment to improve the prognosis of sepsis.10
Therefore, we designed this research to investigate the changes in endothelial-related biomarkers after severe trauma, and whether those could act as a kind of new biomarkers to predict the incidence of sepsis and the prognosis of patients with severe trauma.
Patients and Methods
Patients Enrolled
This was a nontherapeutic, observational study in patients with severe trauma (Injury Severity Score (ISS) ≥16) enrolled between Jan. 2020 and Dec. 2020 in Shanghai Changhai Hospital, China, which is a tertiary teaching hospital with more than 2500 beds serving 1,400,000 outpatients and emergencies each year. The Changhai hospital’s Institutional Review Committee on Human Research approved the study protocol (No. CHEC2019-008), and all of the patients provided written informed consent.
Eligible patients were ≥18 years and ISS ≥16 evaluated at the first visit to the emergency department of our hospital. All enrolled patients were divided into 2 groups: the sepsis group and the non-sepsis group. Sepsis was diagnosed according to the sepsis 3.0 diagnostic criteria, including infection (explicit or suspected) and the following indicators:10 (a) Whole body indicators: body temperature above 38.3 °C or below 36 °C; heart rate greater than 90 beats/min or greater than normal heart rate 2 standard deviations; tachypnea, respiratory rate greater than 20 breaths per minute; altered mental status; marked edema or positive fluid equilibrium (lasting more than 20 mL/kg for 24 hours); hyperglycaemia without diabetes (blood glucose >140 mg/dL or 7.7 mmol/L); (b) Inflammatory indicators: leukocytosis (white blood cell count >12,000/μL) or leukopenia (white blood cell count <4000/μL) or normal white blood cell count, the proportion of immature white blood cells is greater than 10%; Plasma C-reactive protein greater than normal value 2 standard deviations; plasma procalcitonin greater than normal value 2 standard deviations; (c) Hemodynamic indicators: low arterial pressure (systolic blood pressure (SBP) <90mmHg, mean artery pressure (MAP) <70mmHg, or systolic blood pressure decrease in adults > 40mmHg or 2 standard deviations below normal); (d) Organ dysfunction indicators: arterial hypoxemia (arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) <300); Acute oliguria; elevated creatinine greater than 0.5 mg/dL or 44.2 μmol/L; coagulation abnormalities (international normalized ratio (INR) >1.5 or activated partial thromboplastin time thromboplastin time (aPTT) >60s); intestinal obstruction; thrombocytopenia; hyperbilirubinemia; (e) Tissue perfusion indicators: hyperlactic acidemia (>1mmol/L); decreased capillary refill or spotted skin.
Exclusion criteria included: (1) expected survival ≤72 h; (2) recent cardiac arrest caused by severe cardiovascular diseases; (3) with a history of immunotherapy or malignant tumor; (4) conditions associated with a severe systemic inflammatory response other than sepsis. All enrolled patients were divided into 2 cohorts: sepsis (Sequential Organ Failure Assessment (SOFA) ≥2) and non-sepsis.
Clinical Treatment and Assessment
All patients with severe trauma were admitted through the emergency department, undergo blood routine examination, liver and kidney function tests, blood gas analysis, and receive symptomatic supportive care or surgical treatment according to the specific trauma causes. All patients received 24-hour electrocardiographic monitoring to dynamically monitor vital sign changes, record the amount of input and output every 24 hours, and re-examine important laboratory examinations such as blood routine, liver and kidney function, and blood gas analysis. All baseline data of patients were tested at the time of emergency admission. Medical records were prospectively recorded with standardized evaluation forms that included demographic data, Acute Physiology, Chronic Health Evaluation (APACHE) II, and Sequential Organ Failure Assessment (SOFA) score, which was calculated every 24 h of admission to assess the severity of organ dysfunction. The course of various organ dysfunctions and supportive treatments, including operation, vasoactive and ventilator therapies, and renal replacement therapies, were recorded respectively. All patients were followed until death or 28 days after discharge.
Blood Samples Collection
Blood samples were collected via a venous catheter with CellSave tubes (Veridex LLC, Raritan, NJ, USA) for enumeration of CECs and EMPs, EDTA tubes for culture, and enumeration of EPCs (colony forming units). Samples were drawn within 24 h of emergency department admission and 24–48 h and 48–72 h post admission. Samples collected into CellSave tubes were processed within 24 h of sample collection. The samples collected into EDTA tubes were kept at room temperature and processed within 6 h of blood collection.
Assessment of Circulating EPCs, CECs, and EMPs Levels
Enumeration of circulating EPCs was done using an in vitro culture assay for the assessment of endothelial colony-forming cells. The mononuclear cells (MNCs) were isolated by density-gradient centrifugation with Ficoll (1.077 g/mL; Sigma) from 4 mL of EDTA-anticoagulated blood. Immediately after isolation, MNCs were washed with phosphate-buffered saline (PBS) and resuspended in 4 mL of EPCs growth medium-2 (PromoCell, Heidelberg, Germany), supplemented with 10% fetal bovine serum (Hyclone, Logan, UT). Cells were plated on six-well culture dishes coated with 2% human fibronectin (Chemicon, Billerica, Massachusetts, USA) at a density of 1 × 106/cm2 and were maintained in EPCs growth medium-2 for 2 hours. After 2 hours, nonadherent cells were collected and replated. The culture medium was replaced daily and the appearance of ECFCs as well as circumscribed monolayers of cobblestone-like cells was monitored with a phase-contrast light microscope on 8th day and reported as EPCs/4 mL of blood.
Peripheral whole blood cells were prepared by a lyse/no-wash procedure and then were evaluated by three-color flow cytometric analysis, which could minimize cell loss, making it possible to perform the measurement on all of the CECs in the blood samples. We first gated CD45–cells from all cells; then looked for CD146–and CD3–cells on a 2D plot. The percentage of CECs was determined as a percentage of total events (after the exclusion of debris, Supplement Figure 1).
A total of 4 mL peripheral blood was centrifuged to obtain platelet-rich plasma (PRP). The supernatant was then centrifuged to remove suspended cells to prepare platelet-poor plasma (PPP) for three-color flow cytometric analysis.
The main indicators for detecting EMPs were CD144 (+), CD31 (+), and CD42 (-). EMPs were defined as particles ≤1.5 μm size bearing endothelial-specific antigens. Values are reported as the percentage of total events as well as absolute values per μL of original plasma.
Five samples of 50 μL PPP in a 12×75 mm polypropylene tube were incubated with either 5 μL of anti-CD51-FITC (BD Pharmingen, USA), 5 μL of anti-CD31-PE (BD Pharmingen, USA) plus 5 μL of antiCD42b-FITC (AbD Serotec, Great Britain) or 5 μL of anti-CD62E-PE (BD Pharmingen, USA) plus 1 μL of Annexin V-FITC (Miltenyi Biotec, Germany).
Statistical Analysis
R (version 4.1) was used for statistical analysis. Due to the limited sample size, measurement data (age, hospitalization days, ISS score) were presented as median [minimum, maximum]. Fisher’s precision probability test was used for all classification data. Wilcoxon rank-sum test was used for measurement data. The Chi-square test is used for the difference in expression changes of EPCs, CECs, and EMPs. Univariate and multivariate Logistic regression was used to analyze the related factors affecting the occurrence of sepsis, and the variables with clinical significance and P<0.2 in univariate regression were included in the multivariate regression analysis. Receiver operating characteristic (ROC) curve analysis was used to evaluate the predictive value. P-values <0.05 were considered statistically significant.
Results
Baseline Characteristics
A total of 37 cases of severe trauma were included in the study, including 28 men and 9 women. The characteristics of the patient baseline data were shown in Table 1. There were 17 cases in the sepsis group and 20 cases in the non-sepsis group. The incidence of sepsis was 45.95%. The median SOFA score for all cases was 1 point (range 0–5 points), and the SOFA score in the sepsis group was significantly higher than that in the non-sepsis group (2 points vs 0 points, P<0.01). The mean hospital stays in the sepsis group was 29 days, which was significantly higher than that of 20 days in the non-sepsis group (P=0.042). Moreover, the number of platelets was significantly lower in the sepsis group than in the non-sepsis group (P=0.004).
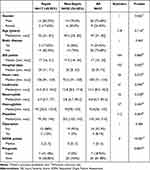 |
Table 1 The Baseline Characteristics of the Patients After Severe Trauma |
Expression Characteristics of Endothelial-Related Biomarkers
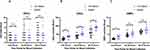
The expression of endothelial-related biomarkers were shown in Table 2. The expression of EPCs in both the sepsis and non-sepsis groups showed an upward trend in the early 72 hours after trauma. There was no statistically significant difference in expression rates between the two groups. The amount of EPCs expression varied significantly between groups of cases (Figure 1A). Similarly, the expression of CECs in the sepsis group and the non-sepsis group showed a significant upward trend in the early traumatic period. Moreover, the number of CECs expressed in the sepsis group was significantly higher than that in the non-sepsis group (all P<0.01, Figure 1B). The expression of EMPs increased within 72 hours in both groups. The expression of EMPs in 0–24 h in the sepsis group was significantly higher than that in the non-sepsis group (P<0.001, Figure 1C).
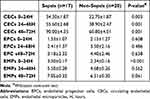 |
Table 2 The Expression of Endothelial-Related Biomarkers After Severe Trauma |
The Logistic Regression Analyses of Endothelial-Related Biomarkers Expression
The univariate logistic regression analysis showed that the occurrence of sepsis was closely related to the expression of 0–24h CEC (Odds Ratio (OR): 1.17, 95% Confidence Interval (CI): 1.06–1.29; P=0.002), and the expression of 0–24h EMPs (OR: 12.83, 95% CI: 2.16–76.0; P=0.005) (Table 3). The multivariable logistic regression analysis showed that the occurrence of sepsis was closely related to the expression of 0–24h EMPs (OR: 9.49, 95% CI: 1.23, 73.10; P=0.031).
 |
Table 3 Regression Analysis of Multiple Factors Affecting the Occurrence of Sepsis After Severe Trauma |
The Value of Endothelial-Related Biomarkers Expression Predicting the Occurrence of Sepsis
The AUC ROC for CECs in different time periods were 0.815 (95% CI: 0.659–0.971), 0.877 (95% CI: 0.758–0.995), and 0.882 (95% CI: 0.772–0.993), respectively (all P<0.001 Table 4). The AUC ROC for EMPs in 0–24h was 0.868 (95% CI: 0.744–0.991, P=0.005, Figure 2).
 |
Table 4 ROC Curve of Predicting the Occurrence of Sepsis After Severe Trauma |
Discussion
Patients with severe trauma have a high probability of developing sepsis. Once sepsis occurs, it is easy to induce multiple organ dysfunction syndromes (MODS), which seriously threatens the prognosis of patients. In recent years, it has been found that endothelial cell damage and activation may be the core link in the development of sepsis, and play an important role in the pathophysiology of sepsis.10 Severe and persistent endothelial changes impair microcirculatory blood flow and cause tissue hypoperfusion, leading to life-threatening organ failure.11 In sepsis, vascular endothelial injury may be the initial step of the incidence of multi-organ dysfunction caused by sepsis. Therefore, focusing on the damage and activation pathways of endothelial cells in sepsis and exploring more intuitive and reliable biomarkers have become new targets for the diagnosis and treatment of sepsis.12,13 This study detected biomarkers that reflect endothelial function, including EPCs, CECs, and EMPs. We found that the expression of EMPs was significantly elevated in the early stages of severe trauma.
A related circulating cell population is EPCs, which originates from the bone marrow, rather than from vessel walls. When the body is damaged or stimulated, it can be mobilized to there through blood circulation and promotes the repair of vascular endothelial damage through directional differentiation and secretion of cytokines. Guldner et al found that expanded endothelial progenitor cells can reduce the release of inflammatory factors such as IL-1β, IL-6, and TNF-α in vivo and promote the increase of serum IL-10 levels, thereby reducing vascular damage and lung tissue damage caused by inflammation.14 Zahran et al found in clinical trials that the number of endothelial progenitor cells in children with sepsis was negatively correlated with white blood cell count, SOFA score, and CRP level, and positively correlated with serum albumin level. At the same time, it was believed that the combination of the number of endothelial progenitor cells and the above indicators can indicate the prognosis of children with sepsis.15 Our former research also showed that autologous transplantation of EPCs can prevent MODS in vivo.8 The results of this study showed that EPCs were raised early in severe trauma, regardless of the development of sepsis. There were differences between the sepsis and non-sepsis groups, but the differences were not statistically significant. Moreover, the difference between EPCs expression between the two groups of cases was large. We analyzed that this may be related to the low number of EPCs expressions and the decline in the number of EPCs expressions in older patients. However, EPCs cannot be identified morphologically alone, and their recognition mainly relies on special markers on the cell surface. So far, there is no unified identification standard for EPCs. In the physiological state, the content of EPCs in peripheral blood is also very small. At the same time, a large number of EPCs have the risk of promoting tumor vascularization and leading to vascular fibrosis, so the application of EPCs in the diagnosis and treatment of sepsis needs further research.
The presence of CECs has recently been recognized as a useful marker of vascular damage. Usually absent in the blood of healthy individuals, CECs counts are elevated in diseases hallmarked by the presence of a vascular insult, such as sickle cell anemia, acute myocardial infarction, Cytomegalovirus (CMV) infection, endotoxemia, and neoplastic processes. CECs can be used as an important indicator of sensitive and specific vascular endothelial damage in the human body.16 Li et al studied the relationship between CECs and acute myocardial infarction (AMI) and measured the plasma levels of CECs in 61 AMI patients, 45 healthy volunteers, and 19 AMI patients who received 1 month of treatment.17 The results showed that the CECs level of AMI patients was significantly higher than that of healthy volunteers, and the CECs level in the blood plasma of AMI patients treated for 1 month was significantly lower than that of untreated. Martínez Sales et al studied the relationship between CECs and heart failure, and the results showed that the CECs level of heart failure patients was significantly higher than that of healthy people, and the CECs count was increased in the acute stage, suggesting that CECs can be used as an indicator to evaluate the progression of heart failure.18 The results of this study showed that early CECs were significantly higher in the sepsis group than in the non-sepsis group. Moreover, the number of early CECs expressions was closely related to the incidence of sepsis, the score of SOFA, and the prognosis of patients. There is also a lack of uniform standards for the phenotypic identification of CECs. Due to the separation method and individual differences, there is no gold standard for the number of CECs in healthy people. The detection and separation of CECs are expensive and difficult to operate, and it is difficult to apply them to clinical practice at present.
Recently, a kind of new endothelial marker associated with vascular dysfunction, EMPs, has been identified.19 EMPs are not only a biomarker of endothelial function, elevated EMPs can in turn promote endothelial dysfunction, creating a vicious circle. In animal experiments, aortic endothelial diastolic function was significantly reduced in mice receiving intravenous EMPs.20 Ci et al also found that EMPs can cause decreased NO production by inhibiting the AKT/eNOS-Hsp90 signaling pathway, resulting in endothelial cell dysfunction.21 EMPs can also be used as a biomarker of inflammatory response and has a large impact on the pathogenesis of inflammatory diseases. Elevated circulating Elevated EMPs were first reported in patients with sepsis due to meningococcal infection.22 Mostefai et al conducted a trial on 36 patients with sepsis and 18 healthy controls and found that the level of EMPs in patients with sepsis was significantly higher than that in healthy people.23 Studies had confirmed that the EMPs produced by TNF-α stimulation can be used as a paracrine mediator to induce an inflammatory response in endothelial cells by upregulating the expression of intercellular adhesion molecule-1 and microRNA, while EMPs produced under non-stimulated conditions had no effect.24,25 Thus, this suggested that EMPs were both a cause and a consequence of the inflammatory response. In addition, EMPs act as a signal for angiogenesis and play a key role in tissue remodeling and angiogenesis by activating matrix metalloproteinases involved in extracellular matrix degradation and growth factor release.26 In the study, we found that EMPs expression was higher relative to EPCs and CECs, and high levels of EMPs were significantly higher in patients with early disease and poor prognosis. A large number of studies also show that EMPs are a new biological information transmitter and are closely related to a variety of disease pathological processes.27–29 Therefore, EMPs are expected to become an important biomarker of endothelial functional status and disease prognosis.
There were some limitations in our study. First, the data from our study was limited to a single center with a relatively small amount of patients. Multi-center studies were also needed to increase the number of study cases in the future. Second, our study was conducted only on Asian patients. And the cellular and molecular mechanisms of endothelial-related biomarkers in the inflammatory process need to be further studied.
Conclusion
In summary, the expression of EMPs was higher in early severe trauma, and high levels of EMPs were significantly higher in patients with early sepsis and poor prognosis. EMPs can be used to evaluate the degree of organ damage, predict the prognosis, and provide a new detection method for the clinical assessment of sepsis, and the relevant mechanisms that need to be further explored.
Ethical Approval and Consent to Participate
This study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by the ethics committee of the Changhai Hospital (No. CHEC2019-008). Written informed consent was obtained from all subjects and/or their legal guardian(s).
Acknowledgments
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81671886).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi:10.1056/NEJMoa022139
2. Osborn TM, Tracy JK, Dunne JR, et al. Epidemiology of sepsis in patients with traumatic injury. Crit Care Med. 2004;32(11):2234–2240. doi:10.1097/01.CCM.0000145586.23276.0F
3. Bhavani SV, Carey KA, Gilbert ER, et al. Identifying novel sepsis subphenotypes using temperature trajectories. Am J Respir Crit Care Med. 2019;200(3):327–335. doi:10.1164/rccm.201806-1197OC
4. Seymour CW, Kennedy JN, Wang S, et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019;321(20):2003–2017. doi:10.1001/jama.2019.5791
5. Dimaggio C, Ayoung-Chee P, Shinseki M, et al. Traumatic injury in the United States: in-patient epidemiology 2000–2011. Injury. 2016;47(7):1393–1403. doi:10.1016/j.injury.2016.04.002
6. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
7. Schenz J, Weigand MA, Uhle F. Molecular and biomarker-based diagnostics in early sepsis: current challenges and future perspectives. Expert Rev Mol Diagn. 2019;19(12):1069–1078. doi:10.1080/14737159.2020.1680285
8. Tianhang L, Bo W, Zhengmao L, et al. Autologous transplantation of endothelial progenitor cells to prevent multiple organ dysfunction syndromes in pig. J Trauma Acute Care Surg. 2013;74(2):508–515. doi:10.1097/TA.0b013e3182703420
9. T L, J S, Lu Z, et al. Potential role of caveolin-1 in regulating the function of endothelial progenitor cells from experimental MODS model. Mediators Inflamm. 2019;2019(8297391). doi:10.1155/2019/8297391
10. Joffre J, Hellman J, Ince C, et al. Endothelial responses in sepsis. Am J Respir Crit Care Med. 2020;202(3):361–370. doi:10.1164/rccm.201910-1911TR
11. Pons S, Arnaud M, Loiselle M, et al. Immune consequences of endothelial cells’ activation and dysfunction during sepsis. Crit Care Clin. 2020;36(2):401–413. doi:10.1016/j.ccc.2019.12.001
12. H F, Goodwin AJ, Chang E, et al. Endothelial progenitor cells and a stromal cell-derived factor-1alpha analogue synergistically improve survival in sepsis. Am J Respir Crit Care Med. 2014;189(12):1509–1519. doi:10.1164/rccm.201312-2163OC
13. X S, Seidle KA, Simms KJ, et al. Endothelial progenitor cells in the host defense response. Pharmacol Ther. 2022;108315. doi:10.1016/j.pharmthera.2022.108315
14. Guldner A, Maron-Gutierrez T, Abreu SC, et al. Expanded endothelial progenitor cells mitigate lung injury in septic mice. Stem Cell Res Ther. 2015;6(230). doi:10.1186/s13287-015-0226-7
15. Zahran AM, Elsayh KI, Mohamad IL, et al. Circulating endothelial cells and endothelial progenitor cells in pediatric sepsis. Pediatr Emerg Care. 2016;32(3):163–167. doi:10.1097/PEC.0000000000000727
16. Moussa MD, Santonocito C, Fagnoul D, et al. Evaluation of endothelial damage in sepsis-related ARDS using circulating endothelial cells. Intensive Care Med. 2015;41(2):231–238. doi:10.1007/s00134-014-3589-9
17. Li C, Wu Q, B L, et al. Detection and validation of circulating endothelial cells, a blood-based diagnostic marker of acute myocardial infarction. PLoS One. 2013;8(3):e58478. doi:10.1371/journal.pone.0058478
18. Martinez-Sales V, Sanchez-Lazaro I, Vila V, et al. Circulating endothelial cells in patients with heart failure and left ventricular dysfunction. Dis Markers. 2011;31(2):75–82. doi:10.1155/2011/757840
19. Takei Y, Yamada M, Saito K, et al. Increase in circulating ACE-positive endothelial microparticles during acute lung injury. Eur Respir J. 2019;54(4):1801188. doi:10.1183/13993003.01188-2018
20. Paudel KR, Panth N, Kim DW. Circulating endothelial microparticles: a key hallmark of atherosclerosis progression. Scientifica. 2016;2016(8514056):1–9. doi:10.1155/2016/8514056
21. Han WQ, Chang FJ, Wang QR, et al. Microparticles from patients with the acute coronary syndrome impair vasodilatation by inhibiting the Akt/eNOS-Hsp90 signaling pathway. Cardiology. 2015;132(4):252–260. doi:10.1159/000438782
22. Nieuwland R, Berckmans RJ, Mcgregor S, et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95(3):930–935. doi:10.1182/blood.V95.3.930.003k46_930_935
23. Mostefai HA, Meziani F, Mastronardi ML, et al. Circulating microparticles from patients with septic shock exert protective role in vascular function. Am J Respir Crit Care Med. 2008;178(11):1148–1155. doi:10.1164/rccm.200712-1835OC
24. Jansen F, Yang X, Baumann K, et al. Endothelial microparticles reduce ICAM-1 expression in a microRNA-222-dependent mechanism. J Cell Mol Med. 2015;19(9):2202–2214. doi:10.1111/jcmm.12607
25. Curtis AM, Wilkinson PF, Gui M, et al. p38 mitogen-activated protein kinase targets the production of proinflammatory endothelial microparticles. J Thromb Haemost. 2009;7(4):701–709. doi:10.1111/j.1538-7836.2009.03304.x
26. Lacroix R, Sabatier F, Mialhe A, et al. Activation of plasminogen into plasmin at the surface of endothelial microparticles: a mechanism that modulates angiogenic properties of endothelial progenitor cells in vitro. Blood. 2007;110(7):2432–2439. doi:10.1182/blood-2007-02-069997
27. Vitkova V, Zivny J, Janota J. Endothelial cell-derived microvesicles: potential mediators and biomarkers of pathologic processes. Biomark Med. 2018;12(2):161–175. doi:10.2217/bmm-2017-0182
28. Mahmoud AM, Wilkinson FL, Mccarthy EM, et al. Endothelial microparticles prevent lipid-induced endothelial damage via Akt/eNOS signaling and reduced oxidative stress. FASEB J. 2017;31(10):4636–4648. doi:10.1096/fj.201601244RR
29. Pernomian L, Moreira JD, Gomes MS. In the view of endothelial microparticles: novel perspectives for diagnostic and pharmacological management of cardiovascular risk during diabetes distress. J Diabetes Res. 2018;2018(9685205):1–7. doi:10.1155/2018/9685205
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.