Back to Journals » OncoTargets and Therapy » Volume 11
Endoplasmic reticulum stress-mediated autophagy contributes to 5-ethylamino-9-diethylaminobenzo[a]phenoselenazinium-mediated photodynamic therapy via the PERK–eIF2α pathway
Authors Chen J, Huang JH, Wang Z, Song X, Chen Z, Zeng Q, Zhou X, Zuo Z, Zhao S, Chen X, Kang J
Received 22 January 2018
Accepted for publication 23 May 2018
Published 24 July 2018 Volume 2018:11 Pages 4315—4325
DOI https://doi.org/10.2147/OTT.S163366
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Jing Chen,1,* Jin-Hua Huang,1,* Zhen Wang,1,* Xiangzhi Song,2,* Zeyi Chen,1,* Qinghai Zeng,1,* Xiping Zhou,1,* Zhihong Zuo,1,* Shuang Zhao,3,* Xiang Chen,3,* Jian Kang1,*
1Department of Dermatology, Third Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China; 2College of Chemistry and Chemical Engineering, Central South University, Changsha, Hunan, People’s Republic of China; 3Department of Dermatology, Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China
*All authors contributed equally to this work
Introduction: 5-ethylamino-9-diethylaminobenzo[a]phenoselenazinium (EtNBSe) is a novel synthetic bipolar photosensitizer with many promising applications. This study investigated the impact of EtNBSe-mediated photodynamic therapy (EtNBSe-PDT) on the autophagy and endoplasmic reticulum (ER) stress of squamous carcinoma cells (A-431 cells), as well as the related molecular mechanisms.
Methods: The potency of EtNBSe-PDT against squamous cell carcinoma was evaluated in BALB/c nude mice. Cell viability was evaluated using MTT. Western blotting and immunofluorescence were used to determine the expression levels of ER stress- and autophagy-related proteins.
Results: Both morphological and microscopic findings showed that the tumor on the xenograft mice exhibited an apparent reduction in volume and was replaced with fibrosis 20 days after EtNBSe-PDT. Additionally, in an in vitro study using A-431 cells, EtNBSe-PDT was found to inhibit A-431 cell survival in an EtNBSe concentration- and light dose- dependent manner, and to induce ER stress via the PERK-eIF2α signaling pathway. Additionally, EtNBSe-PDT could also induce autophagy of A-431 cells. Furthermore, the ER stress inhibitor 4-PBA and the eIF2α inhibitor salubrinal were found to inhibit the autophagy induced by EtNBSe-PDT.
Conclusion: This study demonstrated that the PERK-eIF2α signaling pathway was involved in the ER stress induced by EtNBSe-PDT. Meanwhile, the ER stress via the PERK-eIF2α pathway promoted the occurrence of autophagy in A-431 cells.
Keywords: photodynamic therapy, endoplasmic reticulum stress, squamous cell carcinoma, autophagy
Introduction
Nonmelanoma is the most prevalent skin cancer worldwide, and the incidence rate is still increasing.1 Additionally, cutaneous squamous cell carcinoma (SCC) is the most deadly of the common forms of nonmelanoma skin cancer.1 It significantly affects the quality of life, causing anxiety and isolating behaviors, particularly in patients with facial lesions. Surgery, chemotherapy, and radiotherapy have become the primary treatments for SCC.2,3 However, recurring highly invasive cancers, chemoresistance, and metastasis remain serious problems.4–6 Moreover, the marked scars resulting from extended resection significantly affect the quality of life and radio- or chemotherapy often lead to severe side effects. Therefore, there is an urgent need to develop a novel treatment with strong antitumor effects, and fewer side effects, to improve the prognosis of skin SCC patients.
Photodynamic therapy (PDT) has become an established efficacious, safe, and affordable method for the treatment of tumors.7 It is based on a photosensitizer being located in the target tissue, along with appropriated light, resulting in the oxidation of various biological components and induction of several biological processes.8 Previous studies have revealed that the following mechanisms contribute to PDT by promoting the destruction of tumor cells: direct cytotoxicity to the tumor cells, induction of cell stress responses, and damage to tumor vessels.9,10 Furthermore, it has been reported that endoplasmic reticulum (ER) stress is regarded as one of the main mechanisms mediating PDT-induced tumor cell death.11 The intracellular stimulations induced by PDT could lead to the accumulation of unfolded or misfolded proteins in the ER lumen and lead to ER stress. This adaptation response relieves the burden on the ER and directly regulates the survival of the stressed cells. In addition, PDT could induce autophagy in cells.12 In autophagy, cells break down and recycle their cytoplasmic contents. Thus, the severely damaged cells are relieved or eliminated. Although ER stress and autophagy could function independently, they share a number of common features, including protecting cells and inducing cell death under extreme conditions. However, the relationships between these two complicated systems are controversial and ER stress has been found to have the ability to induce, inhibit, or select autophagy.13
The photosensitizer is regarded as the key factor in PDT. The effectiveness and safety of this promising treatment relies on the availability of photosensitizers that have a high degree of specificity, phototoxicity, and low dark toxicity. 5-Ethylamino-9-diethylaminobenzo[a]phenoselenazinium (EtNBSe) is a novel synthetic bipolar photosensitizer and its chemical structure is shown in Figure 1. It has been proven to exceed >10,000 times the phototoxicity of photofrin, and other photosensitizers in the chalcogen series, in microorganisms and parasites.14,15 However, the antitumor effects of EtNBSe have not been testified and the detailed mechanism of the cell destruction induced by this promising photosensitizer remains to be demonstrated. In this study, we explored the effect of this novel photosensitizer, EtNBSe, on ER stress and autophagy of SCC cells and explored the related molecular mechanisms and relationships between autophagy and ER stress induced by EtNBSe-mediated PDT.
  | Figure 1 The chemical structure of EtNBSe. |
Materials and methods
Reagents
EtNBSe was synthesized by the Department of Chemistry of Central South University (Changsha, People’s Republic of China) using the synthetic method reported in our previous study.14 Fetal bovine serum (FBS) and Dulbecco’s Modified Eagle’s Medium (DMEM) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). The anti-GRP78 (sc-13968), anti-GADD153 (sc-7351), anti-ATF4 (sc-22800), anti-Beclin-1 (sc-48341), anti-LC3-I (sc-134226), anti-LC3-II (sc-28266), anti-Lamp (sc-5570), anti-PERK (sc-13073), anti-p-PERK (sc-32577), anti-eIF2α (sc-11386), anti-p-eIF2α (sc-12412), and anti-GAPDH (sc-293335) antibodies were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). The anti-IRE1 (ab-37037) and anti-p-IRE1 (ab-124945) antibodies were purchased from Abcam (Cambridge, MA, USA). 3-(4,5-Dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT; M2128) was purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Detailed information about the antibodies is listed and characterized in Table 1. Salubrinal (sc-3506) and 4-phenylbutyrate (4-PBA; sc-200652) were purchased from Santa Cruz Biotechnology Inc. The autophagy activator rapamycin (#9004) was purchased from Cell Signaling Technology (Shanghai, People’s Republic of China).
  | Table 1 Antibodies used in the present study |
Cell culture
A-431 cells were purchased from Shanghai Cell Bank of the Chinese Academy of Science (Shanghai, People’s Republic of China). The tumor cells were cultured in 25 cm2 culture dishes supplemented with 10% FBS, 100 U/mL penicillin, and 0.1 mg/mL streptomycin and maintained at 37°C under a 5% CO2 atmosphere.
PDT protocol in vitro
A-431 cells were transplanted to six-well culture plates at 5×105 cells/well and were incubated for 24 h. For the cell-viability assay and some protein expression levels, A-431 cells were randomly divided into the following seven groups: the blank control group, the photosensitizer alone group, the LED alone group, and four EtNBSe-PDT groups. The cells, in the photosensitizer alone group and the LED alone group, were exposed to only EtNBSe or LED light. The cells in the EtNBSe-PDT groups were treated with various concentrations of EtNBSe (100, 200, 400, and 600 nmol/L). After washing three times with PBS and replacing the culture medium, the cells in the LED alone group and EtNBSe-PDT groups were irradiated with LED light (635 nm, 2.8 J/cm2).
Tumor xenograft mouse model
All animal experiments were approved by the Institutional Animal Care and Use Committee of Third Xiangya Hospital, Central South University, and all procedures were performed according to the guidelines approved by the regulation of the Institutional Animal Care and Use Committee of Third Xiangya Hospital, Central South University. Six-week-old female mice (BALB/c nude mice) were obtained from the Laboratory Animal Center of Xiangya Medical School, Central South University (Changsha, People’s Republic of China). Animals were maintained at 22°C under specific pathogen-free conditions and fed with standard food and water ad libitum. A-431 cells (8.0×106) re-suspended in 200 μL of PBS were injected into the hypodermis of both axillary fossae of the mice. The tumors were allowed to grow for 2 weeks, and then, the left underarm site received EtNBSe-PDT. In the EtNBSe-PDT groups, 100 μL of EtNBSe (500 μmol/L) was injected into the tumor tissue and the treated site was irradiated with LED light (635 nm, 4.8 J/cm2) 1 h later. The initial administration was set as day 1. The size of the tumor was measured and photographed every day, and the mean volume was calculated according to the formulation: volume = width × high × length ×0.5328.
Histological assay
For the histological assay, tumors in the xenograft mice models were removed and subjected to pathological examinations. The tumors were transected as serial sections and embedded in paraffin. After routine dewaxing, hematoxylin and eosin (H&E) staining was carried out and the microscopic findings were captured with appropriate objective lenses (20× or 40×).
Immunofluorescent staining
The cells were grown on coverslips, and at indicated time points after PDT, the cells were washed with PBS for 2 min, fixed with 4% PFA for 20 min, and gently rinsed in 0.3% Triton X-100 for 15 min at room temperature. After washing with PBS for 3 min, the cells were blocked with 5% BSA for 1 h. The cells were then incubated with anti-Lamp2 and anti-LC3-II antibodies (dilution 1:500) overnight at 4°C and washed with PBS for 5 min. Afterward, Alexa Fluor 488-labeled (AB150073; Abcam) and Alexa Fluor 647-labeled (AB150131; Abcam) secondary antibodies (dilution 1:200) were added to develop the fluorescence staining at room temperature. The fluorescence intensity and the co-localization between Lamp2 and LC3-II were observed under a fluorescence microscope (BX41; Olympus Corporation, Tokyo, Japan) and quantitatively analyzed by Image-Pro Plus 6.0.
MTT
A-431 cells were plated in 96-well plates at 5×103 cells/well, followed by incubation for 24 h. After the attachment, the cells were treated according to the corresponding requirements. The cells were then incubated for another 24 h at 37°C under a 5% CO2 atmosphere, and 10 μL of MTT solution (5 mg/mL) was added to each well. The crystals of formazan precipitate were dissolved using 150 μL of DMSO, and the absorbance was then detected using a Microplate Reader (Bio-Rad Laboratories Inc., Hercules, CA, USA). Cell viability compared with the DMSO control was calculated.
Western blotting analysis
For the Western blotting assay, the cells were washed three times with precooled PBS and then lysed in buffer containing 20 mM Tris-HCl (pH 8.8), 2.5 mM EDTA, 150 mM NaCl, 10% glycerine, 10% SDS, 1% Triton X-100, 10 mM sodium pyrophosphate, and 1 mM PMSF for 30 min. The samples were separated by 10% SDS-PAGE and transferred onto a polyvinylidene difluoride (PVDF) membrane. The resulting membrane was blocked with 5% BSA for 1 h and then incubated with the designated primary antibody at 4°C overnight, followed by HRP-conjugated secondary antibodies. The signals were detected using the Quantity One 1-D Analysis Software (Bio-Rad Laboratories Inc.).
Statistical analyses
All quantitative data were presented as the mean ± SD from at least three independent experiments. The SPSS 19.0 software package was used to perform all statistical analyses. Comparisons between two groups were performed using the Student’s t-test, and comparisons between multiple groups were performed using ANOVA. A value of P<0.05 was considered statistically significant.
Results
EtNBSe-PDT inhibited A-431 cell survival
The influence of the EtNBSe-PDT irradiated with 635 nm light on the survival of A-431 cells is shown in Figure 2, from which the EtNBSe concentration– and light dose–response curve can be observed. Compared with the control group (0 nmol/L EtNBSe, 0 J/cm2), the EtNBSe alone group and the LED alone group did not significantly inhibit A-431 cell survival (P>0.05). In the EtNBSe-PDT group, different concentrations (100, 200, 400, 600, and 800 nmol/L) of EtNBSe combined with LED light exposure at different light energy densities (1.4, 2.8, and 5.6 J/cm2) were used to treat the A-431 cells. The viability of the A-431 cell was significantly inhibited in all EtNBSe-PDT groups, except for those treated with 100 nmol/L of EtNBSe and the group treated with 200 nmol/L of EtNBSe combined with the 1.4 J/cm2 light dose. Additionally, the inhibition rate in the group that was exposed to 400 nmol/L EtNBSe combined with a light dose of 2.8 J/cm2 was 51.24%±2.33%. Therefore, an EtNBSe concentration of 400 nmol/L and a light dose of 2.8 J/cm2 were chosen for the subsequent experiments.
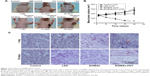
EtNBSe-PDT inhibited tumor growth in vivo
The antitumor effects of EtNBSe-PDT in vivo were investigated using a xenograft mice model. The apparent necrosis and fibrosis of the tumor treated with EtNBSe-PDT are shown in Figure 3A. The volume of tumor in the blank control group gradually increased from 347.21±38.17 to 407.94±62.67 mm3. However, the volume of tumor treated with EtNBSe-PDT decreased by 77.26%±9.72% (from 332.75±46.58 to 75.66±18.91 mm3) 20 days after receiving PDT (Figure 3B and Table 2). In addition, a significant tumor-suppressing effect was observed 5 days after the initial administration versus the blank control group (Figure 3B).
  | Table 2 The effects of EtNBSe-PDT on tumor growth |
All tumors in the mice models were removed and subjected to pathological examination. At 1 day post-treatment, several blue granules were observed throughout the cytoplasm of the A-431 cells in the EtNBSe alone and EtNBSe-PDT groups. The tumor tissue exhibited a significant reduction in volume and was replaced with fibrosis 20 days after PDT (Figure 3C). Comparatively, the tumor cells were tightly packed in the control group and showed no apparent changes. These results showed that EtNBSe-PDT had antitumor effects in vivo.
EtNBSe-PDT induced autophagy in A-431 cells
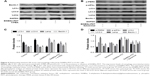
To detect whether EtNBSe-PDT induced autophagy in A-431 cells, the expression levels of autophagy-related proteins were measured. LC3 is a reliable autophagy marker and has two subtypes, such as LC3-I and LC3-II. Upon the induction of autophagy, LC3-I was transformed into LC3-II, which was involved in the formation of autophagosome. Thus, the expression of LC3-I and LC3-II indicated the level of autophagy. Lamp and Beclin-1 played an important role in inducing autophagy, and the increasing expression levels of these proteins also revealed the initiation of autophagy.16 The autophagy activator rapamycin was used as the positive control. In this study, the Western blotting showed that the protein levels of Lamp, LC3-II/LC3-I, and Beclin-1 were notably increased in the groups treated with 200, 400, and 600 nmol/L EtNBSe combined with a 2.8 J/cm2 light dose (Figure 4A and B). Additionally, the relative fluorescence level of LC3-II was evaluated to monitor autophagosome formation. As shown in Figure 4C and D, the staining of LC3-II was distributed evenly throughout the cell in the control group, whereas EtNBSe-PDT and rapamycin resulted in more distinctive LC3-II spots and a significant increase in the relative fluorescence intensity of LC3-II in the A-431 cells. Moreover, Lamp2 staining was significantly colocalized with LC3-II-positive staining 2 h after EtNBSe-PDT, indicating the occurrence of autophagic flux (Figure 4C). These findings suggested that EtNBSe-PDT could induce autophagy in A-431 cells.
EtNBSe-PDT induced ER stress via the PERK–eIF2α pathway
Previous studies have reported that ER stress serves as an important mechanism for mediating PDT-induced tumor cell death. It is well documented that the PERK–eIF2α signaling pathway is an important mechanism in the process of ER stress induction.17 To determine whether the PERK–eIF2α signaling pathway was involved in the ER stress in A-431 cells induced by EtNBSe-PDT, the expression levels of several ER stress-related proteins and PERK–eIF2α signaling pathway activation were evaluated. As shown in Figure 5A and B, the expression levels of GRP78, GADD153, p-eIF2α, and p-PERK were significantly increased in the 200, 400, and 600 nmol/L EtNBSe-PDT-treated groups, compared with the controls. Furthermore, the expression of upstream and downstream signals of the PERK–eIF2α pathway was assessed. In 100, 200, 400, and 600 nmol/L EtNBSe-PDT-treated groups, the protein expression of ATF4 and p-IRE1 was significantly increased (Figure 5A and B) revealing an EtNBSe-PDT-induced activation of the IRE1–PERK–eIF2α signaling pathway. Meanwhile, we examined the expression levels of total IRE1, PERK, and eIF2α. As shown in Figure 5C and D, there was no significant change in the expression of these proteins after EtNBSe-PDT treatment. Taken together, these results demonstrated that EtNBSe-PDT could induce ER stress via the PERK–eIF2α pathway.
Relationship between ER stress and autophagy induced by EtNBSe-PDT in A-431 cells
The relationship between ER stress and autophagy is complicated and differs with cellular genotype, photosensitizer, and stimulus types. To determine whether the EtNBSe-PDT-induced ER stress promoted or inhibited autophagy, we pretreated some A-431 cells with 20 μmol/L of 4-PBA, which inhibited ER stress and then performed EtNBSe-PDT. Western blotting was used to detect the influence of 4-PBA pretreatment on the expression levels of autophagy-related proteins induced by EtNBSe-PDT. The results showed that the autophagy level was significantly inhibited, in contrast to the EtNBSe-PDT group after pretreatment with 4-PBA (Figure 6A and C). Based on the above results, we decided to further explore the relationship between the PERK–eIF2α signaling pathway and autophagy in A-431 cells. We adapted the same method as earlier while salubrinal was used to inhibit eIF2α. It was demonstrated that salubrinal had an apparent inhibitory effect on the phosphorylation of eIF2α and PERK. Additionally, LC3-II, Beclin1, and Lamp were significantly suppressed by salubrinal, in contrast to the EtNBSe-PDT group (Figure 6B and D). Taken together, these results demonstrated that the inhibition of ER stress in A-431 cells had the potential to reduce EtNBSe-PDT-induced autophagy.
Discussion
EtNBSe is a novel synthetic water soluble, lipophilic photosensitizer. Recently, this selenium dye has been proven to be highly effective as a broad-spectrum antimicrobial photosensitizer.14,18 However, it remains to be elucidated as to whether EtNBSe-PDT is also efficacious and safe for human malignancies and if so, the precise molecular mechanisms that occur. In this study, the antitumor effects of EtNBSe were observed for the first time in vivo. Significantly, on the first day after treatment, several blue granules in the cytoplasm of A-431 cells showed the ability of EtNBSe to accumulate in cancer cells. This result was consistent with previous findings that the benzophenoxazinium family, to which EtNBSe belongs, can strongly and specifically stain solid murine tumors.19,20 Moreover, in the in vitro study, the results showed that EtNBSe-PDT significantly decreased A-431 cells’ viability in an EtNBSe concentration- and light dose-dependent manner. Additionally, it was proven that this leads to much more oxidative stress than 5-ALA, which is a frequently used clinical photosensitizer in tumor cells.21 In contrast, the treatment with EtNBSe or light alone did not reduce A-431 cell viability. All of these results indicated that EtNBSe possessed the ability for specific tissue accumulation, high phototoxicity, and low dark toxicity, which makes it a promising photosensitizer.
Next, we studied the effects of the combination of 400 nmol/L EtNBSe and a light dose of 2.8 J/cm2 on A-431 cells and found that EtNBSe-PDT induced ER stress and autophagy in A-431 cells. Both ER stress and autophagy are important biological cell processes. They can be induced by the same stimulus, including PDT. However, the relationship between ER stress and autophagy, as well as the related molecular mechanisms, was complicated and remains to be understood. The clarification of how autophagy could be manipulated via ER stress to favor prosurvival or prodeath signaling is necessary to improve the antitumor effects of PDT.
Previous studies by us and others have shown that the ER is an important target for PDT. During the course of PDT, a large amount of proteins are synthesized and accumulate in the ER, increasing the demand for proteins supporting the folding machinery.22,23 PERK is one of the ER-localized transmembrane proteins with luminal stress-sensing domains. In the unstressed state, it combines with the abundant ER chaperone immunoglobulin-binding protein (GRP78/Bip), which locks PERK into the inactive state and is regarded as an important ER stress marker. ER stress dissociates the inhibited complex and incorporates PERK into active complexes, which can phosphorylate the α-subunit of eukaryotic translation initiation factor-2 (eIF2α). This phosphorylation inactivates the eIF2 required for protein synthesis, thereby resulting in lower levels of translation initiation and reducing the ER stress.24 Furthermore, recent studies have identified that the phosphorylation of eIF2α induces the activation of ATF4 that directly regulates the survival of the stressed cells. In addition, this phosphorylation provokes a paradoxical increase in the translation of the selected mRNAs and the CCAAT/enhancer-binding protein homology protein (CHOP/GADD153) is one of the most induced.17 In this study, the ER stress-related proteins, such as GRP78 and GADD153, were found to increase in an EtNBSe dose-dependent manner. Furthermore, the levels of ATF4, p-IRE1, p-PERK, and p-eIF2α significantly increased after EtNBSe-PDT. All these results suggested that EtNBSe-PDT could induce ER stress in A-431 cells via the PERK–eIF2α pathway, which was consistent with previous studies.32,33 However, in the present study, there was no significant increase in the expression levels of total IRE1, PERK, and eIF2α after EtNBSe-PDT. This demonstrated that EtNBSe-PDT activated the PERK–eIF2α signaling pathway through upregulating the phosphorylation of signaling proteins and had little impact on total expression levels, which was different from the ER stress induced by some antitumor drugs and heavy metal ion.25,26
Autophagy-dependent cell death is one of the regulated cell deaths when cells are subjected to environmental stress. It is reported that proficient autophagic responses could mediate cytoprotective effects. However, in numerous pathological conditions, the molecular machinery for autophagy contributes to cellular demise responding to stress. And it is largely dependent on the complicated autophagic machinery and related components.27 In autophagy, LC3-I is transformed into LC3-II. Together with the lysosome membrane protein Lamp2, LC3-II is involved in the formation of autophagosomes. Additionally, the stress results in the upregulation of free Beclin-1 levels by releasing it from the Beclin-1/Bcl-2 complex. In our study, obvious Lamp2–LC3-II merged staining was observed 2 h after PDT and Western blotting showed that EtNBSe-PDT could significantly increase the levels of LC3-II, Lamp and Beclin-1. These results suggested that obvious autophagy was induced by EtNBSe-PDT in A-431 cells.
Thus, we proved that EtNBSe-PDT could induce ER stress and autophagy in A-431 cells. However, both of the two complicated processes with opposing theories of their involvements in carcinogenesis and tumor progression are controversial areas of investigation in cancer and the relationship between them is still unclear.28 Lin et al29 found that severe ER stress induced by hypericin-PDT could lead to the induction of CHOP and triggered autophagic flux and cell death. However, a growing body of evidence has begun to shed light on the mechanisms of ER stress-mediated inhibition of autophagy. Lee et al30 reported that the pharmacological induction of ER stress via thapsigargin or tunicamycin inhibited ERN1-mediated autophagy, suggesting that the ER stress had an antiautophagy effect. In our study, the pretreatment of salubrinal, which inhibited the phosphorylation of eIF2α, significantly decreased the EtNBSe-PDT-induced autophagy. Furthermore, a previous study has suggested that 4-PBA could act as a chemical chaperone by causing a reduction in the load of mutant and misfolded proteins retained in the ER.31 In this study, when the ER stress was inhibited by 4-PBA, we observed that the activation of autophagy was also inhibited. These results were consistent with the hypothesis that adequate ER stress results in the inhibition of protein synthesis and critical adaptation responses, including autophagy that lessens protein folding in the ER. In addition, these results were consistent with the findings of Liu et al25 who reported that the inhibition of ER stress decreased the Mn-induced autophagy in neuronal cells, indicating that the autophagy was ER stress dependent.
Conclusion
We found that EtNBSe was an effective antineoplastic PDT agent with specific tissue accumulation and high phototoxicity. We found that EtNBSe-PDT could induce ER stress and autophagy, meanwhile the PERK–eIF2α signaling pathway was involved in ER stress induced by EtNBSe-PDT, which in turn further promoted autophagy in A-431 cells. These results will enrich our understanding of the mechanisms mediating EtNBSe-PDT-induced tumor cell death and the relationships between ER stress and autophagy. Further investigation is required to determine how to regulate autophagy and ER stress in order to improve the antitumor effects of EtNBSe-PDT.
Acknowledgment
This study was supported by the New Xiangya Talent Project of the Third Xiangya Hospital of Central South University (20170301).
Disclosure
The authors report no conflicts of interest in this work.
References
Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68(6):957–966. | ||
Cranmer LD, Engelhardt C, Morgan SS. Treatment of unresectable and metastatic cutaneous squamous cell carcinoma. Oncologist. 2010;15(12):1320–1328. | ||
Stratigos A, Garbe C, Lebbe C, et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51(14):1989–2007. | ||
Olesen UH, Bojesen S, Gehl J, Haedersdal M. Anticancer drugs and the regulation of Hedgehog genes GLI1 and PTCH1, a comparative study in nonmelanoma skin cancer cell lines. Anticancer Drugs. 2017;28(10):1106–1117. | ||
Kerr C, Adhikary G, Grun D, George N, Eckert RL. Combination cisplatin and sulforaphane treatment reduces proliferation, invasion and tumor formation in epidermal squamous cell carcinoma. Mol Carcinog. 2017;57(1):3–11. | ||
Apalla Z, Nashan D, Weller RB, Castellsagué X. Skin cancer: epidemiology, disease burden, pathophysiology, diagnosis, and therapeutic approaches. Dermatol Ther. 2017;7(suppl 1):5–19. | ||
Sajisevi M, Rigual NR, Bellnier DA, Seshadri M. Image-guided interstitial photodynamic therapy for squamous cell carcinomas: preclinical investigation. J Oral Maxillofac Surg Med Pathol. 2015;27(2):159–165. | ||
Kataoka H, Nishie H, Hayashi N, et al. New photodynamic therapy with next-generation photosensitizers. Ann Transl Med. 2017;5(8):183. | ||
Sun M, Zhou C, Zeng H, et al. Hiporfin-mediated photodynamic therapy in preclinical treatment of osteosarcoma. Photochem Photobiol. 2015;91(3):533–544. | ||
Garg AD, Agostinis P. ER stress, autophagy and immunogenic cell death in photodynamic therapy-induced anti-cancer immune responses. Photochem Photobiol. 2014;13(3):474–487. | ||
Li KT, Chen Q, Wang DW, et al. Mitochondrial pathway and endoplasmic reticulum stress participate in the photosensitizing effectiveness of AE-PDT in MG63 cells. Cancer Med. 2016;5(11):3186–3193. | ||
Dos Santos AF, Terra LF, Wailemann RA, et al. Methylene blue photodynamic therapy induces selective and massive cell death in human breast cancer cells. BMC Cancer. 2017;17(1):194. | ||
Rashid HO, Yadav RK, Kim HR, Chae HJ. ER stress: autophagy induction, inhibition and selection. Autophagy. 2015;11(11):1956–1977. | ||
Foley JW, Song X, Demidova TN, Jalil F, Jilal F, Hamblin MR. Synthesis and properties of benzo[a]phenoxazinium chalcogen analogues as novel broad-spectrum antimicrobial photosensitizers. J Med Chem. 2006;49(17):5291–5299. | ||
Akilov OE, Kosaka S, O’Riordan K, et al. The role of photosensitizer molecular charge and structure on the efficacy of photodynamic therapy against Leishmania parasites. Chem Biol. 2006;13(8):839–847. | ||
Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140(3):313–326. | ||
Fels DR, Koumenis C. The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol Ther. 2006;5(7):723–728. | ||
Akilov OE, Kosaka S, O’Riordan K, Hasan T. Photodynamic therapy for cutaneous leishmaniasis: the effectiveness of topical phenothiaziniums in parasite eradication and Th1 immune response stimulation. Photochem Photobiol. 2007;6(10):1067–1075. | ||
Lewis MR, Goland PP, Sloviter HA. The action of oxazine dyes on tumors in mice. Cancer Res. 1949;9(12):736–740. | ||
Lewis MR, Sloviter HA, Goland PP. In vivo staining and retardation of growth of sarcomata in mice. Anat Rec. 1946;95:89–96. | ||
Kim KH, Jin HJ, Cheong BH, et al. Optical configurations for nematic liquid crystal device switchable between reflective and transmissive modes. Appl Opt. 2010;49(25):4774–4779. | ||
Firczuk M, Gabrysiak M, Barankiewicz J, et al. GRP78-targeting subtilase cytotoxin sensitizes cancer cells to photodynamic therapy. Cell Death Dis. 2013;4:e741. | ||
Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61(4):250–281. | ||
Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. | ||
Liu C, Yan DY, Tan X, et al. Effect of the cross-talk between autophagy and endoplasmic reticulum stress on Mn-induced alpha-synuclein oligomerization. Environ Toxicol. 2018;33(3):315–324. | ||
Qiao Q, Sun C, Han C, Han N, Zhang M, Li G. Endoplasmic reticulum stress pathway PERK-eIF2alpha confers radioresistance in oropharyngeal carcinoma by activating NF-kappaB. Cancer Sci. 2017;108(7):1421–1431. | ||
Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486–541. | ||
Jang JW, Song Y, Kim SH, Kim J, Seo HR. Potential mechanisms of CD133 in cancer stem cells. Life Sci. 2017;184:25–29. | ||
Lin S, Yang L, Shi H, et al. Endoplasmic reticulum-targeting photosensitizer Hypericin confers chemo-sensitization towards oxaliplatin through inducing pro-death autophagy. Int J Biochem Cell Biol. 2017;87:54–68. | ||
Lee H, Noh JY, Oh Y, et al. IRE1 plays an essential role in ER stress-mediated aggregation of mutant huntingtin via the inhibition of autophagy flux. Hum Mol Genet. 2012;21(1):101–114. | ||
Mai CT, Le QG, Ishiwata-Kimata Y, Takagi H, Kohno K, Kimata Y. 4-Phenylbutyrate suppresses the unfolded protein response without restoring protein folding in Saccharomyces cerevisiae. FEMS Yeast Res. 2018;18(2):82–89. | ||
Sanovic R, Krammer B, Grumboeck S, et al. Time-resolved gene expression profiling of human squamous cell carcinoma cells during the apoptosis process induced by photodynamic treatment with hypericin. International Journal of Oncology. 2009;35(4):921–939. | ||
Garg AD, Krysko DV, Verfaillie T, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. Embo Journal. 2014;31(5):1062–1079. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.