Back to Journals » International Journal of Nanomedicine » Volume 15
Endocytosis and Organelle Targeting of Nanomedicines in Cancer Therapy
Authors Wang X , Qiu Y, Wang M, Zhang C, Zhang T, Zhou H, Zhao W, Zhao W, Xia G, Shao R
Received 1 August 2020
Accepted for publication 25 September 2020
Published 25 November 2020 Volume 2020:15 Pages 9447—9467
DOI https://doi.org/10.2147/IJN.S274289
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Xiaowei Wang, Yuhan Qiu, Mengyan Wang, Conghui Zhang, Tianshu Zhang, Huimin Zhou, Wenxia Zhao, Wuli Zhao, Guimin Xia, Rongguang Shao
Institute of Medicinal Biotechnology, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, People’s Republic of China
Correspondence: Wuli Zhao
Institute of Medicinal Biotechnology, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 10050, People’s Republic of China
Tel +86-10-83166673
Email [email protected]
Guimin Xia
Institute of Medicinal Biotechnology, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 10050, People’s Republic of China
Tel +86-10-63150697
Email [email protected]
Abstract: Nanomedicines (NMs) have played an increasing role in cancer therapy as carriers to efficiently deliver therapeutics into tumor cells. For this application, the uptake of NMs by tumor cells is usually a prerequisite to deliver the cargo to intracellular locations, which mainly relies on endocytosis. NMs can enter cells through a variety of endocytosis pathways. Different endocytosis pathways exhibit different intracellular trafficking routes and diverse subcellular localizations. Therefore, a comprehensive understanding of endocytosis mechanisms is necessary for increasing cellular entry efficiency and to trace the fate of NMs after internalization. This review focuses on endocytosis pathways of NMs in tumor cells, mainly including clathrin- and caveolae-mediated endocytosis pathways, involving effector molecules, expression difference of those molecules between normal and tumor cells, as well as the intracellular trafficking route of corresponding endocytosis vesicles. Then, the latest strategies for NMs to actively employ endocytosis are described, including improving tumor cellular uptake of NMs by receptor-mediated endocytosis, transporter-mediated endocytosis and enabling drug activity by changing intracellular routes. Finally, active targeting strategies towards intracellular organelles are also mentioned. This review will be helpful not only in explicating endocytosis and the trafficking process of NMs and elucidating anti-tumor mechanisms inside the cell but also in rendering new ideas for the design of highly efficacious and cancer-targeted NMs.
Keywords: nanomedicine, endocytosis pathway, clathrin, caveolae, endosome, organelle targeting
Introduction
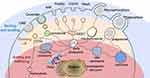
Nanomedicines (NMs) have played an increasing role in cancer therapy for the remarkable ability to increase therapeutic efficacy and decrease systematic toxicity.1–3 NMs, or nanoparticles, are nanosized drug particles, such as liposomes, micelles, polymeric NMs and polymeric-drug conjugates, delivering therapeutic entities in a controlled manner to a desired site. Large amounts of NMs for cancer therapy are undertaking research, some successful ones have come onto the market, such as doxorubicin (DOX) loaded liposome (DOXIL®), albumin-bound paclitaxel nanocomplex (nab-paclitaxel, Abraxane®) and liposomal irinotecan (Onivyde®).4 Inherent in the broad research of NMs is the recognition that the nanosized features offer unique advantages for cancer therapy. In terms of drug delivery, NMs can not only increase drug concentration in the tumor tissue, but can also improve cellular uptake and realize organelle-specific delivery of the loaded drug by adopting various nanomedicine design strategies.5,6
Tumor tissues are the primary accumulating target for NMs owing to pathophysiological differences of tumor from normal tissues. High permeability of blood vessels and impaired lymphatic drainage allows NMs ranging from 10 to 500 nm selectively leaking from vascular lumen and achieving higher accumulation in tumor compared to normal tissue, known as the enhanced permeability and retention (EPR) effect.7,8 Although recently, trans-endothelial pathway is raised up as a new mechanism to explain tumor accumulation of NMs,9 EPR effect is still a recognized approach allowing NMs to passively accumulate in tumor tissues to improve the clinical benefit while decreasing adverse effects.10,11
However, targets of many anticancer agents are localized in subcellular compartments, drug-loaded NMs are expected to not only concentrate at tumor tissues, but also translocate through cell membranes and even target subcellular organelles. Endocytosis is the primary way for NMs to gain cellular entry.2 Efficient cellular uptake of NMs is guarantee for effective intracellular drug delivery. Moreover, different endocytosis pathways lead to different intracellular trafficking fate and localization of NMs. Hence, comprehensive understanding of endocytosis pathways of NMs would be key to achieve efficient uptake and trace the fate of NMs after internalizing into tumor cells, thus to explicate the treatment efficacy and toxicological profile of the cargo transported inside the cells.
Moreover, the role of endocytosis is by no means limited to providing cellular entry pathway for NMs. With the advancement of nanotechnology, endocytosis can be exploited to achieve special delivery purposes of NMs. In some cases, endocytosis is exploited to selectively control uptake. For example, by changing particle physicochemical properties, uptake by phagocytic cells can be limited and thus to avoid clearance of NMs by reticuloendothelial system (RES); by surface-modification with specific ligands, NMs can exhibit improved cellular uptake via receptor-mediated endocytosis and even targeted delivery to specific subcellular organelles.12 Increased target selectivity leads to improved potency towards tumor cells and/or decreased toxicity towards normal cells compared to the passively targeting modes. In other applications, endocytosis is exploited to enable drug activity.13 By encapsulating into nanocarriers, effective cellular endocytosis can be realized for those drugs that require intracellular delivery to take anti-tumor effect but are easily degraded inside the cell, such as oligonucleotides and certain proteins.
Based on the above, this review focuses on the following three parts concerning endocytosis of NMs for cancer therapy:
- An overview on different endocytosis pathways. Latest reported molecules, intracellular trafficking routes and typical non-ligand NMs for cancer therapy involved in different endocytosis pathways are focused on.
- Strategies for designing efficient anti-tumor NMs by actively exploiting endocytosis.
- Strategies for designing NMs capable of efficient subcellular targeting.
We hope this review can not only be helpful in explicating endocytosis and the trafficking process of NMs as well as elucidating anti-tumor mechanisms inside the cell, but also render new ideas for the design of highly efficacious, multifunctional and cancer-targeted NMs, which is of clinical value.
Endocytosis Pathways
Endocytosis is an energy dependent process, by which cells internalize substances from their surroundings using vesicles generated from the plasma membrane. When NMs are in the extracellular environment, they enter cells through different endocytosis pathways following the fundamental steps (Figure 1). i) Binding and budding. NMs interact with the cell surface through non-specific interactions such as electrostatic and hydrophobic interactions or via specific ligand-receptor driven interactions.14 Subsequently, NMs are engulfed in the cell membrane to form invaginations, which is called budding. ii) Pinching off. The “buds” are pinched off to form different endocytic vesicles, following with infusing into early endosomes.15 iii) Sorting and intracellular trafficking. Early endosomes act as a sorting machine and can carry the cargos to specific subcellular organelles, to recycling endosomes for apical recycle recycled, to basolateral compartment for releasing.16–18 Besides, early endosomes can mature to late endosomes (also called multivesicular body). NMs that failed to escape from the endosomes face degradation in the lysosome.16–18
According to the proteins involved, endocytosis pathways are generally classified into phagocytosis and pinocytosis.19 Pinocytosis is ubiquitous in almost any eukaryotic cell and is employed by NMs when entering into the tumor cell. Pinocytosis can be further sub-classified into clathrin-mediated, caveolae-mediated, clathrin- and caveolae-independent endocytosis and macropinocytosis. However, phagocytosis typically occurred in phagocytes.
In this part, the latest insight into various pinocytosis pathways will be discussed, as summarized in Table 1. We focus on critical molecules involved and their expression changes in the tumor cell, so as to elucidate functional differences of endocytosis pathways in a tumor. Apart from that, we also concentrate to exhibit intracellular trafficking routes after cellular entry through different endocytosis pathways. What’s more, examples of non-ligand NMs that have been shown to employ specific pathways will also be summarized. What must be emphasized is that trafficking pathway of the same kind of NMs may vary depending on multiple factors, including physicochemical characteristics of nanoparticles, the peculiarities of the endocytic machinery in different cell types and so on. We are just trying to find some general rules here. As NMs are tended to clearance when engulfed via phagocytosis,20 resulting in loss of efficacy, so, lastly, strategies for NMs to evade phagocytosis will be reviewed.
 |
Table 1 Endocytosis Pathways for Nanomedicines in Tumor |
Pinocytosis
Clathrin-Mediated Endocytosis (CME)
Clathrin-mediated endocytosis (CME) is a classical route in all known mammalian cells for cellular entry of cargoes involving nutrient uptake, signal pathways controlling and membrane recycling, which is marked by clathrin-coated vesicles (CCVs). Cellular engulfment of cargoes through CME is mediated by their specific transmembrane receptors from the cell surface into CCVs.21,22 Transferrin receptor (TfR),23 low density lipoprotein receptor (LDLR)24 and epidermal growth factor receptor (EGFR),22 responsible for the cellular internalization of transferrin (Tf), low density lipoprotein (LDL) and epidermal growth factor respectively, are classic receptors involved in CME and some of which are reported to over-express in tumor cells. What’s more, these ligands can be used as markers of CME in the endocytic studies of NMs, as their trafficking relies mainly on this pathway.25
Formation of CCVs begins with recruitment of coated proteins on the cytosolic side of the plasma membrane, involving not only clathrin, but also adaptor protein 2 (AP2) and accessory proteins such as AP180 and epsin.26 Clathrin, a cytosolic trimeric protein, is the main unit of CCVs and the interaction among heavy chains of clathrin constructs the polygonal lattice structure of CCVs21 (Figure 2A27 and B 28). AP2 acts as an interaction hub which links up the transmembrane receptors and their specific cargoes and binds them to clathrin. Moreover, cargo-specific adaptors are also involved to recruit receptors to AP2. DAB2 (Disabled homologue 2) and Numb are two examples, which are in charge of LDLR and Notch, respectively. All the coated proteins function as an endocytic module, leading to cell membrane invagination and formation of a clathrin-coated pit. GTPase dynamin polymerizes at the neck of the coated pit and induces membrane scission and vesicle pinching-off. Meanwhile, actin polymerization, on one hand, takes place at the neck of the pit aiding in vesicle production,22 on the other hand offers pulling forces and defines the movement of the CCVs towards the interior of the cells.29 Different stages of CME is exhibited in Figure 2C.30 The size of CCVs is 120 nm in an average diameter and the overall lifetime of an endocytic event is 60~120 s in mammalian cells.21
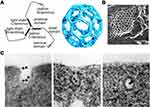 |
Figure 2 Molecular structures and process related to clathrin-mediated endocytosis. (A) Schematic diagram of clathrin molecular (left) and clathrin-coated lattice (right). Reproduced from Smith CJ et al. Clathrin coats at 21 Å resolution: a cellular assembly designed to recycle multiple membrane receptors. EMBO J (1998) 17: 4943–495. Copyright 1998 John Wiley and Sons.27 (B) Clathrin-coated lattice captured by electron microscope (scale bar = 100 nm). Modified with permission of Rockefeller University Press, from Heuser JE, Anderson RGW. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. Journal of Cell Biology, 1989; 108(2): 389–400, Copyright 1989; permission conveyed through Copyright Clearance Centre Inc.28 (C) Electron microscope graph showing different stages of clathrin-mediated endocytosis of Transferrin modified colloidal gold granules. Modified with permission of Rockefeller University Press, from Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. Journal of Cell Biology, Aug 1983; 97(2): 329–339, Copyright 1983; permission conveyed through Copyright Clearance Centre Inc.30 (scale bar = 100 nm). |
When CCVs are internalized by CME, the clathrin coat is disassembled and recycled back to the cytoplasm for the next endocytosis cycle.21 As for the uncoated-vesicles encapsulating cargoes, they fuse with the endosomes where they are sorted either to the recycling endosomes to be transported back to the plasma membrane or to more mature endosomes and later to lysosomes or multivesicular bodies, which finally lead to a trans-Golgi network.31,32
Proteins involved in CME have been reported to be perturbed in cancers, which may result in fluctuation of CME. As with core components of CME (clathrin, AP2 and EPS15), few reports are about expression level change in tumor. However, fusions of gene coding for clathrin heavy chain and EPS15 are observed in blood cancer, such as lymphomas and leukemias.22 Meanwhile, somatic mutation is found in several solid tumors, like breast, renal and lung cancers.33 Those mutations can lead to protein altering and missense of core components and finally CME defects, bringing negative effects on the entry of NMs though CME.
Expression level of some cargo-specific adaptors changes in several solid tumors. For example, DAB1 and Numb are downregulated in ovarian, prostate, bladder, breast, colorectal and esophageal cancer.34 Down expression of those protein may lead to retention of corresponding receptors on the cell membrane surface, which will influence internalization of NMs active targeting to those receptors. However, recruitment of TfR and EGFR directly relies on AP2,21 free from influence of expression change of the cargo-specific adaptors.
CME engages the majority of the receptor mediated cellular uptake of NMs,12,35 which will be discussed in detail later in the Receptor-Mediated Active Targeting of NMs to Tumor Cells. Besides, the following non-ligand NMs can also employ CME.
NMs with positive surface charge are reported to enter cells with relatively higher efficiency due to negative charges on the cell surface and prefer CME for cellular uptake.31,36 Cationic liposomes based on 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) for gene delivery,37 cationic silica-based nanomaterials (SNTs),38 cationic chitosan NMs39 are shown to utilize CME for cellular entry.
What’s more, surface modification with cationic polymer is explored to design positively charged NMs that employ CME. Modification of NMs with cationic polymer poly-L-lysine (PLL) is a typical example, showing significant enhancements of the cellular uptake and more rapidly internalized via CME.40 Peifeng Liu41 studied the cellular uptake mechanism of PLL modified poly-(lactide-co-glycolide acid) (PLGA) based nanoparticle PEAL on three hepatoma cells: HepG2, Huh7 and PLC. Results indicated that CME was the main uptake pathways of PEAL in HepG2, Huh7 and PLC cells. What’s more, macropinocytosis was also found as an uptake pathway of PEAL in HepG2 cells, but not in Huh7 and PLC, exhibiting cellular difference. These facts indicated that nanomedicine surface modification and cell type could influence cellular internalization routes.
Although positively charged NMs exhibit higher cellular uptake efficiency, they also elicit cytotoxic effects, which can be attributed to plasma membrane depolarization caused by cationic nanoparticles.42,43 Furthermore, intracellular accumulation of positively charged NMs may result in damage of membrane-organized organelles and ultimately leading to cell death.44 Consequently, surface charge should be considered carefully when designing NMs to decrease unwanted side effects.
Diolylphosphatidylcholine (DOPC) is a conventional composition of liposome with a neutral charge, which is widely used in gene delivery.37,45 NMs composed of DOPC are also reported to enter tumor cells through CME. Keita Un46 verified that a DOPC based liposome underwent CME for the entry of HeLa and HT-29 cells and could be transported to endoplasmic reticulum (ER) and Golgi apparatus (GA) after escaping from the endosome/lysosome. Consistent results have been reported, in which DOPC-based liposome also navigated CME when interacted with bovine brain cell47 and rat liver cell.48 The above results indicate a selectivity of DOPC containing formulations towards CME, which may suggest that in this particular case, chemical composition plays a more important role in the choice of the endocytosis pathway than other factors.
Caveolae-Mediated Endocytosis
Caveolae belong to non-planar lipid rafts, a cholesterol-rich functional domain of the plasma membrane, and are responsible for endocytosis, cell signaling and membrane organization.49,50 Structurally, caveolae are 60–80 nm flask-shaped invaginations of the plasma membrane that can be identified by electron microscopy,51 as shown in Figure 3A–C.52,53 Besides, caveolae are distinguished from CCVs in the following ways. Firstly, caveolae show constant shape with consistent curvature which is relatively static on the cell surface. Whereas clathrin-coated vesicles are dynamic structures with a rapid progression from a flat clathrin lattice to an increasingly invaginated structure.54 Secondly, the coat of caveolae is less evident. Platinum-replica electron microscopy (PREM) shows a striped structure of caveolar coat in comparison with the hexagon network of clathrin coat.55
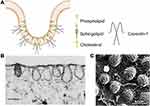 |
Figure 3 Schematic diagram and images of caveolae. (A) Schematic diagram of caveolae. (B) Thin-section electron microscopy image of fibroblast caveolae. Reproduced from Rothberg KG, Heuser JE, Donzell WC, Ying Y-S, Glenney JR, Anderson RGW. Caveolin, a protein component of caveolae membrane coats. Cell. 1992; 68(4): 673–682. Copyright 1992, with permission from Elsevier.52 (scale bar = 0.25 μm). (C) Rapid-freeze, deep-etch image of fibroblast caveolae. Reproduced with permission of Annual Reviews, Inc, from Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998; 67: 199–225, Copyright 1998; permission conveyed through Copyright Clearance Centre Inc.53 (scale bar = 0.1 μm). |
Integral membrane proteins termed caveolins (particularly caveolin-1 (Cav-1)) work together with cavins (particularly cavin-1) to generate caveolae in tumor cells. Genetic ablation of Cav-1 abolishes caveolae formation in tumor cells.56 Cav-1, ubiquitously in non-muscle cells, is a hairpin-like transmembrane protein (Figure 3A) with its amino- (N-) and carboxyl- (C-) terminal domains facing the cytosol.54 Tyrosine residue 14 (Y14) in the N-terminal domain is reversibly phosphorylated to modulate caveolae internalization and tumor cell growth suppression.57 Importantly, Cav-1 directly binds cholesterol, presumably through a specific motif within its caveolin scaffolding domain (CSD, residues 82–101), which is critical for caveolae stability.54 Thus, inhibitors causing cholesterol depletion, such as nystatin,58 methyl-beta-cyclodextrin (mβCD) and filipin (causing)31,59 can disrupt caveolar structures and are used as specific inhibitors of caveolae-mediated endocytosis in elucidating endocytosis of NMs. Actually, caveolins include three isoforms, besides Cav-1, Cav-2 is coexpressed with Cav-1, acting as scaffolding protein within caveolae60 while Cav-3 specifically expresses in muscle cells. As for cavin-1, it may induce membrane curvature. In the absence of cavin-1, Cav-1 can form functional non-caveolar domains. What’s more, caveolae-mediated endocytosis is dynamin-dependent, which is responsible for vesicle scission and budding-off.31 Budding and internalization of caveolae can be stimulated by several agents.
As Cav-1 plays a dominant role for the formation of caveolae in tumor, the expression of Cav-1 may indicate the distribution of caveolae in tumor cells. However, there is no universal rules for change in Cav-1 expression between cancer cells and their normal counterparts, and Cav-1 expression depends on tumor cell type and disease stage. As concluded in related reviews, Cav-1 is downregulated in breast cancer, GC, hepatic cancer, colon and ovarian carcinoma cells; while upregulated in esophagus, pancreatic, renal, prostate and colorectal cancer.61,62 What’s more, Cav-1 expression varies during carcinoma progression: low in early stages, where its role of curbing proliferation predominates, but high in advanced stages, where it correlates with invasive phenotypes and therapeutic resistance.57 NMs employing caveolae-mediated endocytosis may achieve improved tumor accumulation in those Cav-1 upregulated cancers to obtain better therapeutic efficacy.
Compared with tumor tissue, caveolae are abundant in terminally differentiated cells, like endothelial cells.31,63,64 Interestingly, nearly 90% of all cancers including breast, lung, prostrate and colon cancer originate from normal epithelial cells.25 Different location of caveolae between normal and cancer epithelial cells has been reported. In endothelial cells forming a monolayer in situ, a narrow parajunctional strip of plasma membrane was found to be devoid of caveolae by freeze-fracture studies.65 Strikingly, in another research by Gaurav Sahay,25 an absence of caveolae-mediated endocytosis route was observed at the apical side of confluent normal epithelial cells and thus a DOX-loaded micelle sequestered in tight junction (TJ) regions of the cell membrane without entering the normal cells. These facts remind us that the differential location of this endocytosis route between normal and cancer cells may be taken advantage of for an efficient delivery of NMs to the epithelial cancer cells.
The intracellular destinations of the endocytic caveolar vesicles vary in different cells. In endothelial cells caveolae are able to perform trans-endothelial transport, which can be utilized for the trans-vascular endothelial cell delivery in tumor tissues.66,67 While in tumor cells, the endocytic caveolar vesicles are initially fused with early endosome or caveosomes. The neutral pH of caveosomes can be considered as a means to avoid the acidic hydrolytic environment of lysosomes, which has attracted tremendous attention in the cellular delivery of proteins and DNA by NMs.68,69 The sorting of caveosomes to the GA and ER may also be exploited for the targeting delivery of NMs to these subcellular compartments.31
Albumin-bound NMs can take advantage of caveolae-mediated endocytosis. They can bind to the glycoprotein 60 (gp60) receptor localized in caveolae that facilitates the endothelial caveolae-mediated transcytosis.70 Another albumin binding protein SPARC is reported to overexpress on the cancer cell surface and can bind to the albumin-NMs, leading to the uptake of the carrier into tumor cells by endocytosis.71 One typical example is nab-paclitaxel, albumin-bound form of paclitaxel approved by FDA for metastatic or relapsed breast cancer.72 Caveolae-mediated endocytosis of nab-paclitaxel was proved in pancreatic cancer cells by Moumita Chatterjee.56 The report indicated that Cav-1 expression level was different in diverse pancreatic cancer cells and the expression levels were correlated positively with sensitivity and cellular resistance to nab-paclitaxel. These findings suggested Cav-1 as a predictive biomarker for the response to albumin-bound paclitaxel nanocomplex and other albumin-based cancer therapeutic drugs.
Negatively charged NMs are more likely to utilize caveolae-mediated endocytosis. One typical example is Doxil, the first FDA-approved liposomal form of DOX with slightly negative charge (−2.6 mV), which is used to treat patients with metastatic ovarian cancer, metastatic breast cancer and multiple myeloma.73 Gaurav Sahay25 verified a caveolae-mediated pathway to enter epithelial cancer cell MCF-7/ADR for this liposome. Notably, it was routed to lysosomes, instead of bypassing the lysosome, where the drug is apparently released. Moreover, anionic poly(amidoamine) (PAMAM) dendrimers entering A549 lung epithelial cells,74 negatively charged quantum dot (QD) NMs entering human epidermal keratinocytes (HEKs)75 also appeared to be mainly through caveolae mediated endocytosis.
Although the cell plasma membrane is typically overall negatively charged, NMs with negative surface charges can also efficiently overcome the anionic cell plasma membrane and accumulate within cells.25,74,75 This suggests that interaction between nanoparticle and cell surface in cellular uptake is more complicated than the simplified notion of electrostatic interactions, which may be related to the formation of a serum protein corona around the nanoparticle surface and still needs further study.76,77
Macropinocytosis
Macropinocytosis is a kind of clathrin-, caveolae- and dynamin-independent transient endocytosis, initiated spontaneously or in response to growth factor receptor stimulation,78 which leads to assembly of the actin cytoskeleton and triggers extensions of membrane ruffles. Ruffles curve into open, crater-like “cups”79 and close at their distal margins to engulf extracellular fluid. Combined with membrane fusion and fission, intracellular vacuole forms, which is also termed as macropinosome with a diameter of 0.5–10 µm,80 larger than other vesicles formed during pinocytosis.
Macropinocytosis is possible for almost any cell and can internalize large particles of submicron and micron size in cells. In most cases this pathway may serve as a non-specific entry point.31 As a consequence, macropinocytosis often occurs in conjunction with clathrin-41 and caveolae-mediated endocytosis.81
Clathrin- and Caveolae-Independent Endocytosis
These endocytosis pathways occur in cells devoid of both CME and caveolae and are sub-classified as Arf6-dependent, flotillin-dependent, Cdc42-dependent and RhoA-dependent endocytosis based on the small G-proteins involved.82 These pathways are cholesterol-dependent, while the dynamin dependence is controversial.51,82 Endocytosis vesicles in these kinds of pathways have an average diameter of 90 nm and cargoes encapsulated in are reported to be delivered to clathrin-independent carrier (CLIC) or GPI-anchored protein-enriched early endosomal compartments (GEEC),83 followed by the transfer to late endosomes and lysosomes. In addition, the cargo can be routed to the trans-Golgi network or recycled back to the plasma membrane.12
NMs entering cells via pathways mentioned above are not commonly reported. DNA polyplexes formed through DNA complexation with PAMAM can achieve efficient gene expression through flotillin-dependent endocytosis.82 Amorphous silica NMs (aSNPs) were shown to colocalize with flotillin-bearing endocytic vesicles in lung epithelial and endothelial cells.84 What’s more, the knockdown of flotillin resulted in a decreased uptake of aSNPs suggesting that their uptake was flotillin-dependent.
Strategies for NMs to Avoid Uptake by Phagocytes
Phagocytosis performs predominantly in phagocytes, as mentioned above, such as macrophages, neutrophils, monocytes and dendritic cells. Phagocytosis is a receptor-mediated process and is characterized by the large endocytosed vesicles (0.5–10 μm in diameter), known as phagosomes.85 In mammalian organisms, phagocytosis is responsible for engulfing the disabled particles, senescent cells and infectious microorganisms as a response of innate and adaptive immunity.12,86 NMs intended to accumulate in tumor cells need to avoid uptake by phagocytes, to avoid being eliminated by phagocytes mediated by immune response. Therefore, designing of immune tolerant NMs is essential to ensure effective drug delivery to tumor cells and the efforts are concentrated on the surface modification and shape design.
Surface Modification
Yaqing Qie20 studied the strategy of surface modification with polyethylene glycol (PEG) and a specific biomolecule CD47 to evade phagocytosis in macrophage. The results turned out that modification with PEG (PEGylation) reduced nanoparticle uptake by all types of macrophages, which may stem from the ability of PEG to reduce the adsorption of a wide range of soluble proteins such as complements, glycosylated proteins, and lipoproteins.87 CD47, an integrin-associated transmembrane protein, was previously shown to be up-regulated in multiple cancer cells to evade phagocytic clearance.88 Similar to PEGylated samples, NMs coated with CD47 also significantly reduced phagocytosis across all macrophage populations.
Although PEGylation is effective to avoid phagocytic clearance, immunogenicity of PEG is a potential limitation which may result in increased clearance and reduced efficacy of PEGylated NMs after repeated administration, known as accelerated blood clearance phenomenon (ABC phenomenon).89–91 Therefore, alternative materials are developed, such as poly(glycerols) (PGs), poly(oxazolines) (POZ) and poly(carboxybetaine) (pCB), which are low immunogenic without compromising stealth behavior. Huan Xu92 proved that PEOz surface-decorated liposomes exhibited excellent long circulating properties in vivo and PEOz might be a promising biomaterial for the modification of liposomes. Besides synthetic polymers, natural cell membranes to coat NMs are also promising to achieve the aforementioned goals. Yanhua Tian93 and Milad Riazifar94 proved that exosome membrane (EM) coated NMs were resistant to phagocytosis and could significantly prolong blood circulation time, owing to the high expression level of CD47 on the membranes. Although bio-originated surface design is inspiring to achieve phagocytosis evasion, further consideration is still needed for the heterogeneous nature of bio-originated materials and activity guarantee of these materials during complex formulation progress.
Shape Design
For shape design, particle shape has significant influence on phagocytosis, which mainly relates to the aspect ratio of particles. It was proved that elongated particles with higher aspect ratio were less prone to phagocytosis.95–97 The round or spherical nanoparticles with minimum aspect ratio are more prone to phagocytosis than other shaped nanoparticles. Worm-like nanoparticles95 showed 6 to 20 times less phagocytosis than spherical particles of the equal volume. For needle-like particles, they can be essentially considered as high aspect ratio ellipses, which may exhibit similar phagocytosis characteristic as worm-like nanoparticles.95 Julie A. Champion96 prepared irregular nanoparticles in the shape of UFOs (sphere radius 1.5 μm, ring radius 4 μm), which were also shown to be less prone to phagocytosis. Role of distinct shapes of nanoparticles in phagocytosis were supplemented in Shape Design shown in blue words. Particles with high aspect ratio can generate multiple local particle shapes at the initial point of cell attachment. Once the local particle shapes are in decreased curvature, phagocytosis may not occur due to the failure to create the required actin structure. The decreased phagocytosis of particles with high aspect ratio benefits from the decreased curvature regions around the particle surface.
Effect Factors of the Endocytosis Pathways
Some studies suggested that not only structure and physicochemical characteristics of nanoparticles, like size,98,99 shape,95 charge, elasticity, surface modification,20,41,100 but also the peculiarities of the endocytic machinery in different types of cells41 could affect the trafficking pathways of the NMs. Just as reports showed that particle size was a decisive parameter in determining efficiency of gene transfer,68,98,99 in which small PEI polyplexes with diameter smaller than 200 nm were taken up predominantly via clathrin-mediated endocytosis, while large PEI polyplexes (>500 nm) entered cells almost exclusively via the caveolae-mediated pathway. Some worm-like particles with high aspect ratios were less prone to phagocytosis than spherical particles.95 Charge of nanoparticles may also affect endocytosis pathway. NMs with positive surface charge were reported to prefer CME for cellular uptake,31 such as DOTAP based liposome for gene delivery,37 cationic silica-based nanomaterials (SNTs)38 and cationic chitosan NMs,39 while negatively charged NMs were more likely to utilize caveolae-mediated endocytosis, with one typical example as Doxil.25 For the effect of cell types, PLGA based nanoparticle employed different endocytosis pathways to enter three different kinds of hepatoma cells.41 For more systematical analysis, the author can refer to published reviews.13,19,101
Strategies to Actively Exploit Endocytosis for Antitumor NMs
Receptor-Mediated Active Targeting of NMs to Tumor Cells
To increase the affinity to tumor cells, active targeting NMs are prepared by surface-modification with specific ligands to receptors over-expressed on the surface of tumor-related cells. Specific receptor-ligand interaction assists NMs to bind with the tumor-related receptors, leading to improved endocytosis amount in the tumor cell.
Commonly Targeted Receptors in Tumor Cells and Corresponding Examples of NMs
Receptor-ligand pairs commonly used for cancer cell targeting are overviewed in Table 2. Two common kinds of receptors are being explored.102 The first is internalization-prone receptors over-expressed on the surface of tumor cells, like TfR, EGFR, folate receptor (FR) and prostate-specific membrane antigen (PSMA). Active targeting to this kind of receptor directly enhances intracellular uptake of NMs through receptor-mediated endocytosis, mainly navigate CME as stated above, and thus improves anti-tumor efficacy. While the second kind of receptors are those existing in the tumor microenvironment, especially on the capillary endothelial cell surface, such as vascular endothelial growth factor receptor (VEGFR) and αvβ3 integrin receptor. Active targeting of this kind of receptor, together with passive targeting based on the EPR effect, increases accumulation in the interstitial spaces of the tumor that are eventually endocytosed by cancer cells.
 |
Table 2 Summary of Receptor-Ligand Pairs Used for Cancer Cell Targeting |
TfR expresses 100-fold higher in cancer cells than the regular expression of normal cells,12 making it one of the most attractive targets. MBP-426 is a liposome conjugated to Tf loaded with oxaliplatin. Preclinical study of MBP-426, compared to the corresponding ligand-lacking formulation, showed increased Tf-specific cell internalization in Tf-overexpressing Colon 26 cells,103 2.5 times higher concentration in the tumor of oxaliplatin 72 h after administration and superior tumor suppression in tumor-bearing mice.104 MBP-426 is currently undergoing Phase II trial for second line treatment of gastroesophageal or esophageal adenocarcinoma.105 Anti-EGFR or anti-HER-2 monoclonal antibody-grafted nanopreparations, mainly as immunoliposomes have been studied as anticancer therapeutics. Anti-EGFR-IL-DOX, conjugated with Fab’ (fragments antigen-binding) fragments of anti-EGFR monoclonal antibody (mAb) cetuximab and loaded with DOX, is a promising immunoliposomes targeted to EGFR. In vitro studies demonstrated about 30-fold more EGFR-positive cell internalization and 29-fold more effectiveness of anti-EGFR ILs compared to the ligand-lacking counterpart.106 Anti-EGFR-IL-DOX is now in phase II trial as a first-line therapy in patients with advanced triple negative, EGFR positive breast cancer.107
Obstacles to Achieve Active Targeting of NMs
Active targeting of NMs may be more complicated than it seems for the following reasons:
Firstly, the increased level of cell uptake is associated with ligand density. Drew R. Elias108 created NMs labeled with HER2/neu targeting affibodies at different ligand densities, among which an intermediate ligand density provided statistically significant improvements in cell binding in comparison with higher and lower ligand densities. The same phenomenon conserved with small targeting molecule-folic acid. The decrease of uptake in the high ligand density may be caused by nanoparticle competition, steric hindrance,109 suggesting a necessity to optimize ligand density when preparing active-targeting NMs.
Secondly, ligand modification may change intracellular itineraries of NMs. Qiang Zhang’s group17 prepared three kinds of polypeptide ligand (FcBP, 7pep and c(RGDfK)) modified PEG-PCL micelles and studied how the decorations influenced the intracellular trafficking. Results showed that 7pep decorated micelles were recycled to apical plasma membrane in a ligand dependent way. c(RGDfK) decorated micelles were transferred through the Golgi complex to basolateral plasma membrane. While FcBP decorated micelles took both the recycling pathway and transcytosis but bypass the Golgi complex. Thus, in the design of active targeting nanocarriers receptor character should be considered.
Thirdly, non-specific surface interactions exist between ligand-modified NMs with cells and organelles due to the hydrophilicity and exogenous nature of NMs. One remedy could be raised as to modify the surface of the NMs with “inert” polymer with minimal cellular interactions. Besides, choosing one or multiple targeting ligand(s) with higher affinity allowing for specific binding with their receptor may be another solution.31
Actually, obstacles to achieve efficient ligand modification for active targeting are not limited to the above. How to define the extent of over-expressing of a receptor on the tumor cell? How to deal with the tumor heterogeneity? How to realize equal effectiveness between in vitro and in vivo therapy? These questions still remain to be answered in improving tumor cell endocytosis by tumor-targeting.110
Transporter-Mediated Cellular Uptake for Antitumor NMs
Except for cell-surface receptors, transporters in the plasma membrane are also exploited as targets for the delivery of anti-cancer NMs. Plasma membrane transporters are generally classified into two major families: ATP-binding cassette (ABC) transporters and solute carriers (SLC) with the following characteristics.111 First of all, they are in charge of providing nutrients to all mammalian cells and this transportation is substrate selective. Secondly, most transporters have a site-specific expression in different tissues. Moreover, expression of transporters may also upregulate in selective cells under pathological conditions, such as cancer. Unique function and expression characteristics make plasma membrane transporters ideal targets to facilitate NMs delivery to selective cells, thus to improve cellular accumulation in targeted cells, increase therapeutic efficacy and decrease off-target side effects.112 As for anti-cancer NMs, plasma membrane transporters are helpful in two aspects. Firstly, they can mediate selective delivery to cancer cell and improve cellular accumulation. Secondly, they can enhance permeation across biological barriers such as the blood–brain barrier (BBB) before NMs encounter tumor cells.
Transporter-Targeted NMs for Increased Tumor Cellular Internalization
Tumor cells have an increased demand for nutrients to support their malignant proliferation, including glucose, amino acids and vitamins.111 To meet the increased demands, corresponding plasma membrane transporters are upregulated in tumor cells and can be used for NMs to realize cancer cell targeting, as summarized in Table 3. By conjugating selective substrates to nanoparticles, the corresponding transporter-targeted NMs can be prepared. Longfa Kou113 developed both OCTN2 and ATB0,+-targeted nanoparticles by conjugating L-carnitine to PLGA nanoparticles (LC-PLGA NPs). Results indicated that the cellular endocytosis of LC-PLGA NPs was both OCTN2 and ATB0,+-mediated and was increased compared to unmodified nanoparticles in colon cancer cell line Caco-2, in which cell both transporters were overexpressed. Additionally, an endocytosis inhibiting study showed that OCTN2-assisted entry of LC-PLGA NPs occurred via CME and caveolae-mediated endocytosis.114 Lin Li115 prepared LAT1-targeted PLGA nanoparticles by surface decorating of glutamate, which exhibited better tumor accumulation and antitumor effects compared to the undecorated ones. The above results suggested that the overexpressed transporters on cancer cells were potential targets for the rational design of active-targeting NMs.
 |
Table 3 Transporters Used for Cancer Cell Targeting |
Transporter-Targeted NMs to Improve Transfer Across Biological Barriers Related to Tumor Tissue
Tumor cells buried under biological barriers present an additional problem in effective drug delivery. The anti-cancer NMs need to cross the biological barrier before encountering tumor cells. For example. NMs targeted to the brain tumor cells have to cross the BBB firstly. Selective transporters are expressed at high levels in endothelial cells that constitute BBB, such as OCTN2, LAT1 and ChT1 (choline transporter 1, SLC5A7, in charge of choline transport).111 By taking advantage of the specific expression of OCTN2 on both brain capillary endothelial cells and glioma cells, Longfa Kou116 prepared L-carnitine conjugated PLGA nanoparticles (LC-PLGA NPs), which enhanced the uptake of LC-PLGA NPs in both the BBB endothelial cell line hCMEC/D3 and the glioma cell line T98G. Furthermore, in vivo mouse studies showed that LC-PLGA NPs achieved high accumulation in brain as well as improved anti-glioma efficacy. A similar achievement was reported by Lin Li,117 in which Docetaxel-loaded glutamate conjugated LAT1-targeting liposomes (DTX-TGL) were applied to enhance BBB penetration and glioma therapy, proved by both in vitro and in vivo studies in C6 glioma cells.
Differences exist between receptors and transporters in the plasma membrane. First of all, the substrates of transporters are small molecules with no or little immunogenicity and steric hindrance. Whereas most of the ligands for receptors are macromolecules (eg, LDL, transferrin).12,113 Lack of immunogenicity of the substrates offers advantages in preparing active-targeting NMs, which may decrease clearance by phagocytes in vivo. Secondly, transporters usually have broad substrate selectivity while the ligands for receptors are much more specific.111 The difference of the specificity may be a double-edged sword. On one side, broad substrate selectivity provides multiple choices for surface modification of the nanoparticles. Therefore, more transporters can be selected for NMs to target, which is advantageous. On the other side, due to the broad selectivity, NMs decorated with certain substrates may compete with other substrates when interacting with the corresponding transporter, which may attenuate the targeting advantages. Therefore, selecting of the substrate with high affinity to the transporter is important when preparing transporter-targeted NMs.
NMs Ensure Efficient Endocytosis of Gene Therapeutics into Cancer Cell
NMs are Necessary for Cellular Endocytosis of Gene Therapeutics
Gene therapeutics have long been a hotspot and are playing an increasingly important role in cancer treatment. About 65% of all gene therapy trials worldwide are aimed at the treatment of solid or hematological malignancies,118 which made cancer gene therapy a dominant area in both basic and clinical research. Gene therapeutics, including plasmid DNA, antisense oligonucleotides and RNA interference (RNAi), etc., take action at genetic roots by counteracting or replacing malfunctioning genes that are involved in cancer-related pathways within the cells.119 Therefore, targets of gene therapeutics are inside the cells and therapeutic efficacy is reliant on effective cellular internalization.
However, hurdles exist in endocytosis of naked gene-based drugs.120 Their hydrophilic nature, large molecular size and negative charge prevent them from crossing cell membranes. Moreover, inside the cell, nucleic acids failed to escape from endosomes facing degradation in lysosome. To enable effective gene therapy, carriers are needed for gene therapy to realize cellular entry and protect them from degradation. Although virus-based carriers, like the lentiviral system, are used with high transfection efficiency, security of viral vectors is not guaranteed due to strong immunogenicity and mutagenesis caused by random integration of viral DNA.121 In contrast, NMs, as non-viral carriers with low immunogenicity and safety profile, are more extensively applied.122
NMs based on cationic lipids, polymers and dendrimers are often used in gene therapy for cancer.123 They contribute to enabling anti-tumor effectiveness of gene therapeutics in two ways. Firstly, NMs enhance tumor cellular uptake employing various endocytosis pathways. NMs based on cationic lipids are more tended to endocytosis through CME.68 While NMs based on cationic polymers employ caveolae-mediated endocytosis or CME, depending on the properties of the polymer.31 Secondly, NMs can protect nucleic acids from degradation in cancer cells by changing the intracellular route and bypassing lysosome. In previous studies, caveolae-mediated endocytosis was reported to play an important role in achieving endosome escape by fusing endocytic vesicles with neutral caveosomes.68,124 While recent researches place more emphasis on different endosome escaping mechanisms including membrane destabilization and proton sponge, depending on specific nanomaterial with different physicochemical properties.
Cationic NMs for Gene Endocytosis
Cationic polymers complexed with nucleic acid (polyplexes) have been used for efficient gene transfection showing the ability of endosome escaping, among which polyethyleneimine (PEI) and PAMAM are widely studied.37,125–127 THe amine in these polymers can absorb protons and drive the osmotic swelling and rupture of endosomes, finally leading to release of internalized NMs, which is commonly known as the proton sponge effect.15 Polymeric NMs based on polyethyleneimine-block-polylactic acid (PEI-PLA) for delivery of small interfering RNA (siRNA),127 nuclear localization sequences (NLS) decorated PEI/NLS/pDNA for delivery of plasmid DNA (pDNA)126 are good examples.
It was postulated that a high PEI concentration, highly branched architecture and cationic polymer rigidity is important in facilitating endosomal escape.14 Nevertheless, cytotoxicity arising from exposure to cationic materials still remain challenging and hinder the application of PEI in vivo. To solve the problem, PEI was employed as a surface decorating material on distearoyl phosphoethanolamine-polyethyleneimine (DSPE-PEI) based liposome. The prepared liposome could be disrupted to transiently release cargos in response to high H+ levels in tumor cells, which served as a pten gene carrier to accomplish superior plasmid delivery, endosome escape and effcient transfection.128
Another efficient gene transfer carrier is based on cationic lipids, which can facilitate electrostatic interactions with anionic oligonucleotides to form lipoplexes. In addition, these lipids can mediate electrostatic interaction between lipoplexes and the endosomal membrane and facilitate endosomal release of oligonucleotides prior to endosome/lysosome fusion through membrane destabilization.129 Xiuxiu Cong130 successfully designed plasmid DNA-encapsulated cationic lipid liposome formulated with DOTAP, which significantly increased the tumor cell death and improve the antitumor immunity through enhancing the immunogenicity of dying tumor cells.
Helper lipids, such as dioleoyl phosphatidylethanolamine (DOPE), cholesterol or dioleoyl phosphatidyl choline (DOPC), usually neutrally charged, are often employed with cationic lipids in order to gain high transfection efficiency. DOPE can realize conformation transformation to an inverse hexagonal phase in the acidic pH of endosome, promoting endosomal release upon formation of pores stabilized by the hexagonal phase forming lipids.37 Cholesterol is also commonly used in lipoplexes to stabilize lipid bilayers and promote membrane fusion. When present at high percentages, cholesterol seems to enhance the activity of cationic lipids and promote gene transfer.129 Lipoplexes containing both cholesterol and DOPE, demonstrated a cholesterol-dependent increase in DNA transfection efficiency at 40mol% cholesterol compared to lipoplexes devoid of cholesterol.131
Organelle Targeting Strategies for NMs
After endocytosis of tumor cell, an increasing number of NMs are designed to precisely deliver drugs to specific intracellular compartments, such as cytoplasm, nucleus, mitochondria and other cellular organelles. Although various endocytosis vesicles may deliver the cargos to subcellular organelles, this intracellular delivery is an uncontrolled form. In comparison, functional modification with appropriate signals on the nanoparticle surface without compromising its functional moiety may realize intracellular targeting in a controlled method.35 Therefore, nanoparticle modifications targeting different subcellular organelles are introduced here, as summarized in Table 4.
 |
Table 4 Organelle Targeting Nanomedicines Mentioned in This Article |
Lysosomes
Lysosomes are considered as digesting components of the cell because of the presence of various hydrolases.132 NMs are employed to aid endosomal escaping and protect the therapeutic drug from degradation by lysosomes in case the endosome or lysosome is not the final therapeutic target. But in certain cases,25,133 like Doxil as mentioned above, the entry through this pathway may destine to the lysosomes and employ lysosomal pH as a trigger for release of a cytotoxic drug within the cancer cells. So, the acidic environment in lysosome may be a double-edged sword and things depend not only on the physicochemical property of NMs but also on the drugs encapsulated within.
However, lysosome is more than a digesting machine. Recent studies revealed the important role of lysosomes in cancer cell-death triggered by the release of lysosomal enzymes, like cathepsins.134,135 It was observed that the disruption of the lysosomal membrane often led to the release of cathepsins through a process of lysosome membrane permeabilization (LMP), which can be induced by various mechanisms, involving ROS generation and utilization of lysosomotropic agents.136 Ceramide, a precursor of sphingosine produced by the lysosomal enzyme, acid ceramidase, is a promising molecule for the induction of LMP.137 Intracellular delivery of ceramides via transferrin-functionalized liposomes induced increased apoptosis in vitro in HeLa and in vivo in A2780-ovarian carcinoma xenograft mouse model.138
Cytoplasm
Cytoplasm is where many physiological processes take place, like signaling, transport and metabolism. Thus, cytoplasm has been demonstrated to be the therapeutic target for cancer. Targeting the cytoplasm is also a method for reaching the nuclear compartment. As reported by Sudipta Basu,139 a hexadentate-PLGA polymer chemically conjugated to a selective MAPK inhibitor was taken up by cancer cells and could release the active agent in the cytoplasm to inhibit proliferation and induce apoptosis in vitro.
Endosomal escape is an access for NMs to the cytoplasm. Apart from which, the direct transport of cargo across the plasma membrane can be achieved by surface modification of CPP. As a kind of short peptide that can carry macromolecules into cells, CPP is capable of crossing the biological membrane barriers of the cell membrane and this process is not relying on classical endocytosis. TAT140 and iRGD141 are two popular examples of CPP. Notably, the ability of iRGD to directly translocate across the plasma membrane is greatly lost when conjugated with a cargo. As a consequence, iRGD usually employs co-administration via systemic injection to improve the therapeutic index of drugs including NMs (nab-paclitaxel and Doxil).141 What’s more, CPP can be further decorated by functional molecules to target specific organelles. Meyer GA combined nuclear localization signal (NLS) with TAT, which may localize in the nucleus.142 The potential to be reconstructed renders CPP broad application space in clinics for intracellular targeting therapy.
Endoplasmic Reticulum (ER) and Golgi Apparatus (GA)
Dilation of ER and ER stress are reported to result in paraptosis, a vacuolization-associated cell death in cancer cells.143 Paraptosis can be induced by gambogic acid, an anticancer drug with xanthone structure, which makes ER a potential therapeutic target.
Surface modification of ER signal peptide or ER-retrieval sequence can be used for active targeting to ER. An intracellularly targeted delivery system based on PLGA NMs decorated with a peptide containing specific ER-targeting moieties (KKXX signal) are reported to uptake by dendritic cell and efficiently accumulated on ER.144 Another lecture reported that PLGA based NMs are observed to accumulate predominantly in GA in the case of human epithelial cells like Caco-2.145
Mitochondria
Mitochondria are bilayered film-coated (inner and outer mitochondrial membranes) semi-autonomous organelle, with the mitochondrial DNA enclosed in the inner membrane. Referred to as the powerhouses of the cell, mitochondria have recently emerged as one of the major targets in cancer therapy, because of their central role in cellular differentiation, metabolism, signaling, key modulator of programmed cell death and being the alternative home of cellular genomic materials.146,147
Lipophilic cations are generally known to target the mitochondria, primarily because of the high membrane potential (negative inside) owing to the high-density phospholipids in the inner membrane.12 Triphenylphosphonium (TPP), fulfilling the prerequisite of positive charge and the lipophilicity, has been shown to selectively reach the inner mitochondrial membrane. TPP can not only conjugate to small molecule-based drugs as a delivering vector to mitochondria, but can also be incorporated in the lipid for nanoparticle construction by covalently conjugating with stearyl moieties. Along this line, a phosphatidylcholine (PC)-based TPP-coated positively charged NMs comprised of α-tocopheryl succinate (TOS, inhibitor of complex II in electron transport chain) and obatoclax (Obt, inhibitor of Bcl-2) were engineered.148 The TOS-TPP-Obt-NPs entered into acidic lysosomes via macropinocytosis, followed by lysosomal escape and finally homed into the mitochondria over a period of 24 h, leading to mitochondrial mediated cellular apoptosis in HeLa cells.
Arginine-rich peptide octaarginine (R8), which preferentially interacts with the inner membrane of mitochondria, also has been reported as a mitochondrial targeting device of NMs.149 What’s more, inorganic NMs, such as cuprous oxide NMs (Cu2O NPs),150 can be taken up by mitochondria, which damaged their membranes and thereby inducing apoptosis in tumor cells.
Nucleus
The nucleus, a double lipid bilayer wrapped organelle, has been a focus of targeted drug and DNA (DNA as a drug for gene therapy) delivery. For most instances, the NMs deliver the drugs into the cell and the drug molecules diffuse through the cytosol to reach the nuclear target. Conjugation of NLS to the NMs has been demonstrated to direct the cargo to the nuclear target. PLGA based NMs151 and cargo-loaded mesoporous silica NMs (MSNs)152 are all reported to be decorated with NLS for an efficient targeting of the nucleus.
Summary and Perspectives
Rapid development of NMs for cancer therapy relies on an integrative effort of physiochemistry, pharmaceutics and pharmacology, among which endocytosis of NMs is at the interface of biology and material science. Comprehensive understanding of cellular entry and intracellular trafficking is critical for design and targeting delivery of NMs. In this review, we summarized the characteristics of different endocytosis pathways, mainly focusing on specific effector molecules involved and possible intracellular trafficking routes mediated by definitive endocytic routes. In addition, NMs used for gene delivery based on endosome escaping, latest active targeting strategies towards tumor cells as well as intracellular organelles were introduced.
Although impressive achievements have been achieved, endocytosis and intracellular targeting for cancer cells is still in its fledgeling stages. Little difference has been explicated on the endocytosis pathway between cancer cell and normal cell. Almost all the consensus comes from in vitro studies, little in vivo research has been reported, which may be more complicated. Challenges still remain and deeper studies are still needed in the future.
With the development of precision therapy, the abundance of targeting strategies for cancer therapy have been exploited. However, few of them have been applied in clinics. Unsatisfied therapeutic effectiveness and unexpected side effects are still a worry. By summarizing different endocytosis pathways, we hope it will be helpful to render new ideas for nanomedicine design. What’s more, understanding of the definite endocytosis pathway one nanomedicine prefers and the effector proteins involved may facilitate in searching endocytosis-relevant clinical biomarkers to select patients most likely to benefit from nanomedicine therapy, which is of great clinical value.
Acknowledgments
This work was financially supported by the National Key Research and Development Program of China (2016YFA0201504), NSFC (81673383, 81603063, 81102464, 81803479,) and CIFMS (2016-I2-M-3-013).
Disclosure
The authors report no conflicts of interest in this work.
References
1. van der Meel R, Sulheim E, Shi Y, Kiessling F, Mulder WJM, Lammers T. Smart cancer nanomedicine. Nat Nanotechnol. 2019;14(11):1007–1017. doi:10.1038/s41565-019-0567-y
2. Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17(1):20–37.
3. Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–760. doi:10.1038/nnano.2007.387
4. Pelaz B, Alexiou C, Alvarez-Puebla RA, et al. Diverse applications of nanomedicine. ACS Nano. 2017;11(3):2313–2381. doi:10.1021/acsnano.6b06040
5. Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J. Analysis of nanoparticle delivery to tumours. Nature Reviews Materials. 2016;1(5):16014. doi:10.1038/natrevmats.2016.14
6. Chen H, Zhang W, Zhu G, Xie J, Chen X. Rethinking cancer nanotheranostics. Nat Rev Mater. 2017;2.
7. Fang J, Islam W, Maeda H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv Drug Deliv Rev. 2020. doi:10.1016/j.addr.2020.06.005
8. Prabhakar U, Maeda H, Jain RK, et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73(8):2412–2417. doi:10.1158/0008-5472.CAN-12-4561
9. Sindhwani S, Syed AM, Ngai J, Kingston BR, Chan WCW. The entry of nanoparticles into solid tumours. Nat Mater. 2020;51.
10. Hoffman AS, Lai JJ. Three significant highlights of controlled drug delivery over the past 55 years: pEGylation, ADCs, and EPR. Adv Drug Deliv Rev. 2020. doi:10.1016/j.addr.2020.05.013
11. Sakurai A. Harashima. targeting tumor endothelial cells with nanoparticles. Int J Mol Sci. 2019;20(23):5819. doi:10.3390/ijms20235819
12. Yameen B, Choi WI, Vilos C, Swami A, Shi J, Farokhzad OC. Insight into nanoparticle cellular uptake and intracellular targeting. J Controlled Release. 2014;190:485–499. doi:10.1016/j.jconrel.2014.06.038
13. Akinc A, Battaglia G. Exploiting endocytosis for nanomedicines. Cold Spring Harb Perspect Biol. 2013;5(11):a016980–a016980. doi:10.1101/cshperspect.a016980
14. Patel S, Kim J, Herrera M, Mukherjee A, Kabanov AV, Sahay G. Brief update on endocytosis of nanomedicines. Adv Drug Deliv Rev. 2019;144:90–111. doi:10.1016/j.addr.2019.08.004
15. Smith SA, Selby LI, Johnston APR, Such GK. The endosomal escape of nanoparticles: toward more efficient cellular delivery. Bioconjug Chem. 2019;30(2):263–272. doi:10.1021/acs.bioconjchem.8b00732
16. Yang D, Liu D, Deng H, et al. Transferrin functionization elevates transcytosis of nanogranules across epithelium by triggering polarity-associated transport flow and positive cellular feedback loop. ACS Nano. 2019;13(5):5058–5076. doi:10.1021/acsnano.8b07231
17. Song X, Li R, Deng H, et al. Receptor mediated transcytosis in biological barrier: the influence of receptor character and their ligand density on the transmembrane pathway of active-targeting nanocarriers. Biomaterials. 2018;180:78–90. doi:10.1016/j.biomaterials.2018.07.006
18. Inpanathan S, Botelho RJ. The lysosome signaling platform: adapting with the times. Front Cell Dev Biol. 2019;7:113.
19. Manzanares D, Cena V. Endocytosis: the nanoparticle and submicron nanocompounds gateway into the cell. Pharmaceutics. 2020;12:4. doi:10.3390/pharmaceutics12040371
20. Qie Y, Yuan H, von Roemeling CA, et al. Surface modification of nanoparticles enables selective evasion of phagocytic clearance by distinct macrophage phenotypes. Sci Rep. 2016;6:26269. doi:10.1038/srep26269
21. Kaksonen M, Roux A. Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2018;19(5):313–326. doi:10.1038/nrm.2017.132
22. McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12(8):517–533. doi:10.1038/nrm3151
23. Sochacki KA, Dickey AM, Strub MP, Taraska JW. Endocytic proteins are partitioned at the edge of the clathrin lattice in mammalian cells. Nat Cell Biol. 2017;19(4):352–361. doi:10.1038/ncb3498
24. Mulkearns EE, Cooper JA. FCH domain only-2 organizes clathrin-coated structures and interacts with Disabled-2 for low-density lipoprotein receptor endocytosis. Mol Biol Cell. 2012;23(7):1330–1342. doi:10.1091/mbc.e11-09-0812
25. Sahay G, Kim JO, Kabanov AV, Bronich TK. The exploitation of differential endocytic pathways in normal and tumor cells in the selective targeting of nanoparticulate chemotherapeutic agents.Biomaterials2010;31(5):923–933. doi:10.1016/j.biomaterials.2009.09.101
26. Haucke V, Kozlov MM. Membrane remodeling in clathrin-mediated endocytosis. J Cell Sci. 2018;131:17. doi:10.1242/jcs.216812
27. Smith CJ, Grigorieff N, Pearse BM. Clathrin coats at 21 Å resolution: a cellular assembly designed to recycle multiple membrane receptors. EMBO J. 1998;17(17):4943–4953. doi:10.1093/emboj/17.17.4943.
28. Heuser JE, Anderson RGW. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biology. 1989;108(2):389–400. doi:10.1083/jcb.108.2.389
29. Hohendahl A, Talledge N, Galli V, Shen PS, Roux A. Structural inhibition of dynamin-mediated membrane fission by endophilin. eLife Sciences. 2017;6:e26856. doi:10.7554/eLife.26856
30. Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97(2):329–339. doi:10.1083/jcb.97.2.329
31. Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010;145(3):182–195. doi:10.1016/j.jconrel.2010.01.036
32. Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10(9):597–608. doi:10.1038/nrm2755
33. Kan Z, Jaiswal BS, Stinson J, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466(7308):869–873. doi:10.1038/nature09208
34. Schmid EM, McMahon HT. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448(7156):883–888. doi:10.1038/nature06031
35. Rajendran L, Kn?lker H-J, Simons K. Subcellular targeting strategies for drug design and delivery. Nat Rev Drug Discov. 2010;9(1):29–42. doi:10.1038/nrd2897
36. Asati A, Santra S, Kaittanis C, Perez JM. Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano. 2010;4(9):5321–5331. doi:10.1021/nn100816s
37. Rezaee M, Oskuee RK, Nassirli H, Malaekeh-Nikouei B. Progress in the development of lipopolyplexes as efficient non-viral gene delivery systems. J Control Release. 2016;236:1–14. doi:10.1016/j.jconrel.2016.06.023
38. Radaic A, de Jesus MB. Solid lipid nanoparticles release DNA upon endosomal acidification in human embryonic kidney cells. Nanotechnology. 2018;29(31):315102. doi:10.1088/1361-6528/aac447
39. Jiang LQ, Wang TY, Webster TJ, et al. Intracellular disposition of chitosan nanoparticles in macrophages: intracellular uptake, exocytosis, and intercellular transport. Int J Nanomedicine. 2017;12:6383–6398. doi:10.2147/IJN.S142060
40. Vasir JK, Labhasetwar V. Quantification of the force of nanoparticle-cell membrane interactions and its influence on intracellular trafficking of nanoparticles. Biomaterials. 2008;29(31):4244–4252. doi:10.1016/j.biomaterials.2008.07.020
41. Liu P, Sun Y, Wang Q, Sun Y, Li H, Duan Y. Intracellular trafficking and cellular uptake mechanism of mPEG-PLGA-PLL and mPEG-PLGA-PLL-Gal nanoparticles for targeted delivery to hepatomas. Biomaterials. 2014;35(2):760–770. doi:10.1016/j.biomaterials.2013.10.020
42. Lin J, Zhang H, Chen Z, Zheng Y. Penetration of lipid membranes by gold nanoparticles: insights into cellular uptake, cytotoxicity, and their relationship. ACS Nano. 2010;4(9):5421–5429. doi:10.1021/nn1010792
43. Arvizo RR, Miranda OR, Thompson MA, et al. Effect of nanoparticle surface charge at the plasma membrane and beyond. Nano Lett. 2010;10(7):2543–2548. doi:10.1021/nl101140t
44. Wang F, Bexiga MG, Anguissola S, et al. Time resolved study of cell death mechanisms induced by amine-modified polystyrene nanoparticles. Nanoscale. 2013;5(22):10868–10876. doi:10.1039/c3nr03249c
45. Hadinoto K, Sundaresan A, Cheow WS. Lipid–polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. European J Pharmaceutics Biopharmaceutics Official J Arbeitsgemeinschaft Für Pharmazeutische Verfahrenstechnik E V. 2013;85(3):427–443.
46. Un K, Sakai-Kato K, Oshima Y, Kawanishi T, Okuda H. Intracellular trafficking mechanism, from intracellular uptake to extracellular efflux, for phospholipid/cholesterol liposomes. Biomaterials. 2012;33(32):8131–8141. doi:10.1016/j.biomaterials.2012.07.030
47. Lawaczeck R, Gervais M, Nandi PK, Nicolau C. Fusion of negatively charged liposomes with clathrin-uncoated vesicles. Biochim Biophys Acta. 1987;903(1):112–122. doi:10.1016/0005-2736(87)90161-1
48. Blumenthal R, Henkart M, Steer CJ. Clathrin-induced pH-dependent fusion of phosphatidylcholine vesicles. J Biological Chemistry. 1983;258(5):3409–3415.
49. Lingwood D, Kai S. Lipid rafts as a membrane-organizing principle. Science. 2015;327(jan.1):46–50. doi:10.1126/science.1174621
50. Simons K, Sampaio JL. Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol. 2011;3(10):a004697. doi:10.1101/cshperspect.a004697
51. Thottacherry JJ, Sathe M, Prabhakara C, Mayor S. Spoiled for choice: diverse endocytic pathways function at the cell surface. Annu Rev Cell Dev Biol. 2019;35:55–84. doi:10.1146/annurev-cellbio-100617-062710
52. Rothberg KG, Heuser JE, Donzell WC, Ying Y-S, Glenney JR, Anderson RGW. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68(4):673–682. doi:10.1016/0092-8674(92)90143-Z
53. Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi:10.1146/annurev.biochem.67.1.199
54. Parton RG. Caveolae: structure, function, and relationship to disease. Annu Rev Cell Dev Biol. 2018;34:111–136. doi:10.1146/annurev-cellbio-100617-062737
55. Parton RG, Del Pozo MA, Vassilopoulos S, et al. Caveolae: the FAQs. Traffic. 2020;21(1):181–185. doi:10.1111/tra.12689
56. Chatterjee M, Ben-Josef E, Robb R, et al. Caveolae-mediated endocytosis is critical for albumin cellular uptake and response to albumin-bound chemotherapy. Cancer Res. 2017;
57. Lolo FN, Jimenez-Jimenez V, Sanchez-Alvarez M, Del Pozo MA. Tumor-stroma biomechanical crosstalk: a perspective on the role of caveolin-1 in tumor progression. Cancer Metastasis Rev. 2020;39(2):485–503.
58. Chen Y, Wang S, Lu X, Zhang H, Fu Y, Luo Y. Cholesterol sequestration by nystatin enhances the uptake and activity of endostatin in endothelium via regulating distinct endocytic pathways. Blood. 2011;117(23):6392–6403. doi:10.1182/blood-2010-12-322867
59. Guo S, Zhang X, Zheng M, et al. Selectivity of commonly used inhibitors of clathrin-mediated and caveolae-dependent endocytosis of G protein-coupled receptors. Biochim Biophys Acta. 2015;1848(10 Pt A):2101–2110. doi:10.1016/j.bbamem.2015.05.024
60. Canton I, Battaglia G. Endocytosis at the nanoscale. Chem Soc Rev. 2012;41(7):2718–2739.
61. Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Caveolae and signalling in cancer. Nat Rev Cancer. 2015;15(4):225–237. doi:10.1038/nrc3915
62. Chen D, Che G. Value of caveolin-1 in cancer progression and prognosis: emphasis on cancer-associated fibroblasts, human cancer cells and mechanism of caveolin-1 expression (Review). Oncol Lett. 2014;8(4):1409–1421. doi:10.3892/ol.2014.2385
63. Parton RG, Del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14(2):98–112.
64. Sotgia F, Martinez-Outschoorn UE, Howell A, Pestell RG, Pavlides S, Lisanti MP. Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu Rev Pathol. 2012;7:423–467. doi:10.1146/annurev-pathol-011811-120856
65. Stan RV. Structure of caveolae. Biochim Biophys Acta. 2005;1746(3):334–348. doi:10.1016/j.bbamcr.2005.08.008
66. Sindhwani S, Syed AM, Ngai J, et al. The entry of nanoparticles into solid tumours. Nat Mater. 2020;19(5):566–575. doi:10.1038/s41563-019-0566-2
67. Liu X, Jiang J, Meng H. Transcytosis - An effective targeting strategy that is complementary to “EPR effect” for pancreatic cancer nano drug delivery. Theranostics. 2019;9(26):8018–8025. doi:10.7150/thno.38587
68. Rejman J, Conese M, Hoekstra D. Gene transfer by means of lipo- and polyplexes: role of clathrin and caveolae-mediated endocytosis. J Liposome Res. 2006;16(3):237–247. doi:10.1080/08982100600848819
69. Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8(3):185–194. doi:10.1038/nrm2122
70. Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Controlled Release. 2008;132(3):171–183. doi:10.1016/j.jconrel.2008.05.010
71. Desai NP, Trieu V, Hwang LY, Wu R, Soon-Shiong P, Gradishar WJ. Improved effectiveness of nanoparticle albumin-bound (nab) paclitaxel versus polysorbate-based docetaxel in multiple xenografts as a function of HER2 and SPARC status. Anticancer Drugs. 2008;19(9):899–909. doi:10.1097/CAD.0b013e32830f9046
72. Morenoaspitia A, Perez EA. Nanoparticle albumin-bound paclitaxel (ABI-007): a newer taxane alternative in breast cancer. Future Oncol.2005;1(6):755–762. doi:10.2217/14796694.1.6.755
73. Barenholz Y. Doxil(R)–the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–134. doi:10.1016/j.jconrel.2012.03.020
74. Perumal OP, Inapagolla R, Kannan S, Kannan RM. The effect of surface functionality on cellular trafficking of dendrimers. Biomaterials. 2008;29(24–25):3469–3476. doi:10.1016/j.biomaterials.2008.04.038
75. Zhang LW, Monteiro-Riviere NA. Mechanisms of quantum dot nanoparticle cellular uptake. Toxicol Sci. 2009;110(1):138–155. doi:10.1093/toxsci/kfp087
76. Walkey CD, Olsen JB, Song F, et al. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano. 2014;8(3):2439–2455. doi:10.1021/nn406018q
77. Wang M, Gustafsson OJR, Siddiqui G, et al. Human plasma proteome association and cytotoxicity of nano-graphene oxide grafted with stealth polyethylene glycol and poly(2-ethyl-2-oxazoline). Nanoscale. 2018;10(23):10863–10875. doi:10.1039/C8NR00835C
78. Kou L, Sun J, Zhai Y, He Z. The endocytosis and intracellular fate of nanomedicines: implication for rational design. Asian J Pharmaceutical Sciences. 2013;8(1):1–10. doi:10.1016/j.ajps.2013.07.001
79. Swanson JA. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol. 2008;9(8):639–649. doi:10.1038/nrm2447
80. Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10.
81. Pe?Aloza JP, Márquez-Miranda V, Caba?a-Brunod M, et al. Intracellular trafficking and cellular uptake mechanism of PHBV nanoparticles for targeted delivery in epithelial cell lines. J Nanobiotechnology. 2017;15(1):1. doi:10.1186/s12951-016-0241-6
82. Vercauteren D, Piest M, LJvd A, et al. Flotillin-dependent endocytosis and a phagocytosis-like mechanism for cellular internalization of disulfide-based poly(amido amine)/DNA polyplexes. Biomaterials. 2011;32(11):3072–3084. doi:10.1016/j.biomaterials.2010.12.045
83. Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78(1):857–902. doi:10.1146/annurev.biochem.78.081307.110540
84. Kasper J, Hermanns MI, Bantz C. Interactions of silica nanoparticles with lung epithelial cells and the association to flotillins. Arch Toxicol. 2013;87(6):1053–1065. doi:10.1007/s00204-012-0876-5
85. Fairn GD, Grinstein S. How nascent phagosomes mature to become phagolysosomes. Trends Immunol. 2012;33(8):397–405. doi:10.1016/j.it.2012.03.003
86. Huynh KK, Kay JG, Stow JL, Grinstein S. Fusion, fission, and secretion during phagocytosis. Physiology. 2007;22:366–372.
87. A A D, Shegokar R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv. 2016;13(9):1257–1275. doi:10.1080/17425247.2016.1182485
88. Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX, Weissman IL. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer. 2019;19(10):568–586.
89. Mohamed M, Abu Lila AS, Shimizu T, et al. PEGylated liposomes: immunological responses. Sci Technol Adv Mater. 2019;20(1):710–724. doi:10.1080/14686996.2019.1627174
90. Wang F, Ye X, Wu Y, et al. Time interval of two injections and first-dose dependent of accelerated blood clearance phenomenon induced by PEGylated liposomal gambogenic acid: the contribution of PEG-specific IgM. J Pharm Sci. 2019;108(1):641–651. doi:10.1016/j.xphs.2018.10.027
91. Hoang Thi TT, Pilkington EH, Nguyen DH, Lee JS, Park KD, Truong NP. The importance of poly(ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers. 2020;12:2. doi:10.3390/polym12020298
92. Xu H, Zhang W, Li Y, et al. The bifunctional liposomes constructed by poly(2-ethyl-oxazoline)-cholesteryl methyl carbonate: an effectual approach to enhance liposomal circulation time, pH-sensitivity and endosomal escape. Pharm Res. 2014;31(11):3038–3050. doi:10.1007/s11095-014-1397-0
93. Tian Y, Li S, Song J, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–2390. doi:10.1016/j.biomaterials.2013.11.083
94. Riazifar M, Mohammadi MR, Pone EJ, et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano. 2019;13(6):6670–6688. doi:10.1021/acsnano.9b01004
95. Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2009;26(1):244–249. doi:10.1007/s11095-008-9626-z
96. Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103(13):4930–4934. doi:10.1073/pnas.0600997103
97. Mathaes R, Winter G, Besheer A, Engert J. Influence of particle geometry and PEGylation on phagocytosis of particulate carriers. Int J Pharm. 2014;465(1–2):159–164. doi:10.1016/j.ijpharm.2014.02.037
98. Rejman J, Oberle ZI, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem J. 2004;377(1):159–169. doi:10.1042/BJ20031253
99. Ogris M, Steinlein P, Carotta S, Brunner S, Wagner E. DNA/polyethylenimine transfection particles: influence of ligands, polymer size, and PEGylation on internalization and gene expression. AAPS PharmSci. 2001;3(3):E21. doi:10.1208/ps030321
100. Xia Y, Zhong J, Zhao M, et al. Galactose-modified selenium nanoparticles for targeted delivery of doxorubicin to hepatocellular carcinoma. Drug Deliv. 2019;26(1):1–11. doi:10.1080/10717544.2018.1556359
101. Donahue ND, Acar H, Wilhelm S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv Drug Deliv Rev. 2019;143:68–96. doi:10.1016/j.addr.2019.04.008
102. Biswas S, Torchilin VP. Nanopreparations for organelle-specific delivery in cancer. Adv Drug Deliv Rev. 2014;66:26–41. doi:10.1016/j.addr.2013.11.004
103. Ishida O, Maruyama K, Tanahashi H, et al. Liposomes bearing polyethyleneglycol-coupled transferrin with intracellular targeting property to the solid tumors in vivo. Pharm Res. 2001;18(7):1042–1048. doi:10.1023/A:1010960900254
104. Suzuki R, Takizawa T, Kuwata Y, et al. Effective anti-tumor activity of oxaliplatin encapsulated in transferrin-PEG-liposome. Int J Pharm. 2008;346(1–2):143–150. doi:10.1016/j.ijpharm.2007.06.010
105. Wei Y, Gu X, Cheng L, Meng F, Storm G, Zhong Z. Low-toxicity transferrin-guided polymersomal doxorubicin for potent chemotherapy of orthotopic hepatocellular carcinoma in vivo. Acta Biomater. 2019;92:196–204.
106. Mamot C, Drummond DC, Greiser U, et al. Epidermal growth factor receptor (EGFR)-targeted immunoliposomes mediate specific and efficient drug delivery to EGFR- and EGFRvIII-overexpressing tumor cells. Cancer Res. 2003;63(12):3154–3161.
107. Anti-EGFR-immunoliposomes Loaded With Doxorubicin in Patients With Advanced Triple Negative EGFR Positive Breast Cancer. NLM Identifier: NCT02833766 (02832016). Available from: http://www.clinicaltrials.gov/ct2/show/NCT02833766.
108. Elias DR, Poloukhtine A, Popik V, Tsourkas A. Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nanomedicine. 2013;9(2):194–201.
109. Alkilany AM, Zhu L, Weller H, et al. Ligand density on nanoparticles: A parameter with critical impact on nanomedicine. Adv Drug Deliv Rev. 2019;143:22–36. doi:10.1016/j.addr.2019.05.010
110. You HB, Park K. Targeted drug delivery to tumors. Myths, Reality Possibility. 2011;153(3):198–205.
111. Kou L, Bhutia YD, Yao Q, He Z, Sun J, Ganapathy V. Transporter-guided delivery of nanoparticles to improve drug permeation across cellular barriers and drug exposure to selective cell types. Front Pharmacol. 2018;9:27. doi:10.3389/fphar.2018.00027
112. Lin L, Yee SW, Kim RB, Giacomini KM. SLC transporters as therapeutic targets: emerging opportunities. Nat Rev Drug Discov. 2015;14(8):543–560.
113. Kou L, Yao Q, Sivaprakasam S, et al. Dual targeting of l-carnitine-conjugated nanoparticles to OCTN2 and ATB(0,+) to deliver chemotherapeutic agents for colon cancer therapy. Drug Deliv. 2017;24(1):1338–1349. doi:10.1080/10717544.2017.1377316
114. Kou L, Yao Q, Sun M, et al. Cotransporting ion is a trigger for cellular endocytosis of transporter-targeting nanoparticles: a case study of high-efficiency SLC22A5 (OCTN2)-mediated carnitine-conjugated nanoparticles for oral delivery of therapeutic drugs. Adv Healthc Mater. 2017;6:17. doi:10.1002/adhm.201700165
115. Li L, Di X, Wu M, et al. Targeting tumor highly-expressed LAT1 transporter with amino acid-modified nanoparticles: toward a novel active targeting strategy in breast cancer therapy. Nanomedicine. 2017;13(3):987–998.
116. Kou L, Hou Y, Yao Q, et al. L-Carnitine-conjugated nanoparticles to promote permeation across blood-brain barrier and to target glioma cells for drug delivery via the novel organic cation/carnitine transporter OCTN2. Artif Cells Nanomed Biotechnol. 2018;46(8):1605–1616.
117. Li L, Di X, Zhang S, et al. Large amino acid transporter 1 mediated glutamate modified docetaxel-loaded liposomes for glioma targeting. Colloids Surf B Biointerfaces. 2016;141:260–267. doi:10.1016/j.colsurfb.2016.01.041
118. Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR. Gene therapy clinical trials worldwide to 2017: an update. J Gene Med. 2018;20(5):e3015.
119. Naldini L. Gene therapy returns to centre stage. Nature. 2015;526(7573):351–360. doi:10.1038/nature15818
120. Amreddy N, Babu A, Muralidharan R, et al. Recent advances in nanoparticle-based cancer drug and gene delivery. Adv Cancer Res. 2018;137:115–170.
121. Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4(5):346–358. doi:10.1038/nrg1066
122. Lin G, Li L, Panwar N, et al. Non-viral gene therapy using multifunctional nanoparticles: status, challenges, and opportunities. Coord Chem Rev. 2018;374:
123. Buck J, Grossen P, Cullis PR, Huwyler J, Witzigmann D. Lipid-based DNA therapeutics: hallmarks of non-viral gene delivery. ACS Nano. 2019;13(4):3754–3782. doi:10.1021/acsnano.8b07858
124. Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Molecular Therapy J American Society Gene Therapy. 2005;12(3):468–474. doi:10.1016/j.ymthe.2005.03.038
125. Patil ML, Zhang M, Taratula O, Garbuzenko OB, He H, Minko T. Internally cationic polyamidoamine PAMAM-OH dendrimers for siRNA delivery: effect of the degree of quaternization and cancer targeting. Biomacromolecules. 2009;10(2):258–266. doi:10.1021/bm8009973
126. Zhao M, Li J, Ji H, Chen D, Hu H. A versatile endosome acidity-induced sheddable gene delivery system: increased tumor targeting and enhanced transfection efficiency. Int J Nanomedicine. 2019;14:6519–6538. doi:10.2147/IJN.S215250
127. Jin M, Guangming J, Lin K, Liqing C, Zhonggao G, Wei H. Smart polymeric nanoparticles with pH-responsive and PEG-detachable properties for co-delivering paclitaxel and survivin siRNA to enhance antitumor outcomes. Int J Nanomedicine. 2018;13:2405–2426. doi:10.2147/IJN.S161426
128. Wang Y, Wang R, Wu S, et al. Self-responsive co-delivery system for remodeling tumor intracellular microenvironment to promote PTEN-mediated anti-tumor therapy. Nanoscale. 2020;12(17):9392–9403.
129. Cheng X, Lee RJ. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv Drug Deliv Rev. 2016;99(Pt A):129–137. doi:10.1016/j.addr.2016.01.022
130. Cong X, Tian H, Liu S, et al. Cationic Liposome/DNA complexes mediate antitumor immunotherapy by promoting immunogenic tumor cell death and dendritic cell activation. ACS Appl Mater Interfaces. 2020;12:28047–28056. doi:10.1021/acsami.0c08112
131. Pozzi D, Marchini C, Cardarelli F, et al. Transfection efficiency boost of cholesterol-containing lipoplexes. Biochim Biophys Acta. 2012;1818(9):2335–2343. doi:10.1016/j.bbamem.2012.05.017
132. Piao S, Amaravadi RK. Targeting the lysosome in cancer. Ann N Y Acad Sci. 2015;1371:1.
133. Ning T, Du G, Nan W, Liu C, Hang H, Wei L. Improving penetration in tumors with nanoassemblies of phospholipids and doxorubicin. J Natl Cancer Inst. 2007;(13):13.
134. Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25(3):486–541.
135. Yamaguchi N, Tomaru U, Kiuchi T, et al. Expression of cathepsins B, D and K in thymic epithelial tumours. J Clin Pathol. 2020. doi:10.1136/jclinpath-2020-206551
136. Liu CG, Han YH, Kankala RK, Wang SB, Chen AZ. Subcellular performance of nanoparticles in cancer therapy. Int J Nanomedicine. 2020;15:675–704. doi:10.2147/IJN.S226186
137. Johansson AC, Appelqvist H, Nilsson C, Kagedal K, Roberg K, Ollinger K. Regulation of apoptosis-associated lysosomal membrane permeabilization. Apoptosis. 2010;15(5):527–540. doi:10.1007/s10495-009-0452-5
138. Koshkaryev A, Piroyan A, Torchilin VP. Increased apoptosis in cancer cells in vitro and in vivo by ceramides in transferrin-modified liposomes. Cancer Biol Ther. 2012;13(1):50–60. doi:10.4161/cbt.13.1.18871
139. Basu S, Harfouche R, Soni S, Chimote G, Mashelkar RA, Sengupta S. Nanoparticle-mediated targeting of MAPK signaling predisposes tumor to chemotherapy. Proc Natl Acad Sci U S A. 2009;106(19):7957–7961. doi:10.1073/pnas.0902857106
140. Sethuraman VA, Bae YH. TAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumors. J Controlled Release. 2007;118(2):216–224. doi:10.1016/j.jconrel.2006.12.008
141. Sugahara KN, Teesalu T, Karmali PP, et al. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science. 2010;328(5981):
142. Meyer GA, Radsak KD. Identification of a novel signal sequence that targets transmembrane proteins to the nuclear envelope inner membrane. J Biological Chemistry. 2000;275(6):3857–3866. doi:10.1074/jbc.275.6.3857
143. Seo MJ, Lee DM, Kim IY, et al. Gambogic acid triggers vacuolization-associated cell death in cancer cells via disruption of thiol proteostasis. Cell Death Dis. 2019;10(3):187. doi:10.1038/s41419-019-1360-4.
144. Hadas S-E, Diana L, David S. Intracellular targeting of PLGA nanoparticles encapsulating antigenic peptide to the endoplasmic reticulum of dendritic cells and its effect on antigen cross-presentation in vitro. Mol Pharm. 2011;8(4):1266–1275. doi:10.1021/mp200198c
145. Cartiera MS, Johnson KM, Rajendran V, Caplan MJ, Saltzman WM. The uptake and intracellular fate of PLGA nanoparticles in epithelial cells. Biomaterials. 2009;30(14):2790–2798. doi:10.1016/j.biomaterials.2009.01.057
146. Hockenbery DM. Targeting mitochondria for cancer therapy. Environ Mol Mutagen. 2010;51(5):476–489. doi:10.1002/em.20552
147. Wongrakpanich A, Geary SM, Joiner MLA, Anderson ME, Salem AK. Mitochondria-targeting particles. Nanomedicine. 2014;9(16):2531–2543. doi:10.2217/nnm.14.161
148. Mallick A, More P, Syed MMK, Basu S. Nanoparticle mediated mitochondrial damage induces apoptosis in cancer. ACS Appl Mater Interfaces. 2016;
149. Yamada Y, Harashima H. Delivery of bioactive molecules to the mitochondrial genome using a membrane-fusing, liposome-based carrier, DF-MITO-Porter. Biomaterials. 2012;33(5):1589–1595. doi:10.1016/j.biomaterials.2011.10.082
150. Hu Y, Wang Y. Cuprous oxide nanoparticles selectively induce apoptosis of tumor cells. Int J Nanomedicine. 2012;2641. doi:10.2147/IJN.S31133
151. Cheng FY, Wang SP-H, Su C-H, et al. Stabilizer-free poly(lactide-co-glycolide) nanoparticles for multimodal biomedical probes. Biomaterials. 2008;29(13):2104–2112. doi:10.1016/j.biomaterials.2008.01.010
152. Chandran M, Srinivasan V, Soundarapandian K. Cancer therapeutic proficiency of dual-targeted mesoporous silica nanocomposite endorses combination drug delivery. ACS Omega. 2017;2(11):7959–7975. doi:10.1021/acsomega.7b00978
153. Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The transferrin receptor part I: biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol. 2006;121(2):144–158. doi:10.1016/j.clim.2006.06.010
154. Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77(6):400–410. doi:10.1159/000279388
155. Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284(1):2–13. doi:10.1016/S0014-4827(02)00105-2
156. Muller C, Schibli R. Prospects in folate receptor-targeted radionuclide therapy. Front Oncol. 2013;3:249. doi:10.3389/fonc.2013.00249
157. Dostalova S, Cerna T, Hynek D, et al. Site-directed conjugation of antibodies to apoferritin nanocarrier for targeted drug delivery to prostate cancer cells. ACS Appl Mater Interfaces. 2016;8(23):14430–14441. doi:10.1021/acsami.6b04286
158. Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc Natl Acad Sci U S A. 2011;108(5):1850–1855. doi:10.1073/pnas.1011379108
159. Yang L, Mao H, Cao Z, et al. Molecular imaging of pancreatic cancer in an animal model using targeted multifunctional nanoparticles. Gastroenterology. 2009;136(5):1514–1525 e1512. doi:10.1053/j.gastro.2009.01.006
160. Backer MV, Gaynutdinov TI, Patel V, et al. Vascular endothelial growth factor selectively targets boronated dendrimers to tumor vasculature. Mol Cancer Ther. 2005;4(9):1423–1429. doi:10.1158/1535-7163.MCT-05-0161
161. Chen J, Wu H, Han D, Xie C. Using anti-VEGF McAb and magnetic nanoparticles as double-targeting vector for the radioimmunotherapy of liver cancer. Cancer Lett. 2006;231(2):169–175. doi:10.1016/j.canlet.2005.01.024
162. Tucker GC. Integrins: molecular targets in cancer therapy. Curr Oncol Rep. 2006;8(2):96–103. doi:10.1007/s11912-006-0043-3
163. Graf N, Bielenberg DR, Kolishetti N, et al. alpha(V)beta(3) integrin-targeted PLGA-PEG nanoparticles for enhanced anti-tumor efficacy of a Pt(IV) prodrug. ACS Nano. 2012;6(5):4530–4539. doi:10.1021/nn301148e
164. Luo Q, Gong P, Sun M, et al. Transporter occluded-state conformation-induced endocytosis: amino acid transporter ATB(0,+)-mediated tumor targeting of liposomes for docetaxel delivery for hepatocarcinoma therapy. J Control Release. 2016;243:370–380. doi:10.1016/j.jconrel.2016.10.031
165. Luo Q, Yang B, Tao W, et al. ATB(0,+) transporter-mediated targeting delivery to human lung cancer cells via aspartate-modified docetaxel-loading stealth liposomes. Biomater Sci. 2017;5(2):295–304. doi:10.1039/C6BM00788K
166. Coothankandaswamy V, Cao S, Xu Y, et al. Amino acid transporter SLC6A14 is a novel and effective drug target for pancreatic cancer. Br J Pharmacol. 2016;173(23):3292–3306. doi:10.1111/bph.13616
167. Hafliger P, Charles RP. The L-Type amino acid transporter lat1-an emerging target in cancer. Int J Mol Sci. 2019;20:10. doi:10.3390/ijms20102428
168. Zhang Y, Wang J. Targeting uptake transporters for cancer imaging and treatment. Acta Pharm Sin B. 2020;10(1):79–90. doi:10.1016/j.apsb.2019.12.005
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.