Back to Journals » Clinical Ophthalmology » Volume 17
End-to-End Impact of a Cloud-Based Surgical Planning System on Efficiency in Cataract Surgery: A Time-and-Motion Study
Authors Zavodni Z, Pan LC, Mok K , Cheng H, O'Boyle D
Received 27 October 2022
Accepted for publication 14 June 2023
Published 3 July 2023 Volume 2023:17 Pages 1885—1896
DOI https://doi.org/10.2147/OPTH.S392669
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Zachary Zavodni,1 Li-Chen Pan,2 Kaitlyn Mok,2 Hang Cheng,3 Derek O’Boyle4
1The Eye Institute of Utah, Salt Lake City, UT, USA; 2Veranex Solutions, Inc., Boston, MA, USA; 3Alcon Vision, LLC, Fort Worth, TX, USA; 4Alcon Laboratories Ireland Ltd., Cork, Ireland
Correspondence: Kaitlyn Mok, Veranex Solutions, Inc, 33 Arch Street, Boston, MA, USA, Tel +1 617-865-8408, Email [email protected]
Purpose: Inefficiencies from manual data entry and non-integration exist throughout the cataract surgery workflow. The aim of this study was to evaluate the impact of SMARTCataract, an innovative cloud-based digital surgical planning platform (SPS) on efficiency in preoperative (diagnostic workup, surgery planning), intraoperative, and postoperative phases of cataract surgery. The primary objective aimed to assess time and number of manual transcription data points (TPs) required for all pre-, intra-, and postoperative devices that integrate with the SPS and surgery planning time across three patient types (post-refractive, astigmatic, conventional). The secondary objective aimed to assess the overall efficiency impact of the SPS on the surgery workflow for the three patient types by leveraging time-and-motion methods and workflow mapping.
Patients and Methods: This prospective, observational, real-world, pre- and post-cohort time-and-motion study included patients undergoing evaluation for cataract surgery and/or surgery at the study site. Assessed variables included time and TPs required for clinical activities and devices associated with traditional manual methods (pre-cohort) versus the SPS (post-cohort). Statistical analyses (t-test) were performed comparing performance time using the SPS versus traditional methods for each integrated technology and surgery planning activity.
Results: The SPS demonstrated statistically significant time savings over traditional methods in TP data input time taken across all integrated pre-, intra-, and postoperative devices (p< 0.0001). The SPS additionally demonstrated statistically significant time savings in preoperative surgery planning across post-refractive (p< 0.0001), astigmatic (p=0.0005), and conventional (p=0.0004) cataract patient groups. Overall, the SPS reduced end-to-end patient workflow time and TPs for post-refractive, astigmatic, and conventional cataract patients by averages of 13.2, 12.6, and 4.3 minutes and 184, 166, and 25 TPs per patient, respectively.
Conclusion: Through the SPS’ integration and surgery planning capabilities, substantial time efficiencies can be achieved for cataract surgery practices, clinicians, and patients compared to surgery planning with traditional manual methods.
Keywords: time efficiency, surgical planning software, real-world observational study, manual transcription
Plain Language Summary
Why was the study done?
- Cataract surgery is an important treatment for those with cataract-induced vision impairment, but issues such as manual data entry and non-integration of devices utilized in the surgical workflow create inefficiencies within the cataract surgery treatment paradigm.
What did the researchers do and find?
- In this real-world, observational study, researchers evaluated workflow efficiency by measuring time and number of transcription points (TPs) associated with surgery planning activities with SMARTCataract, a cloud-based integrative digital solution for cataract surgery planning, compared to surgery planning with standard methods.
- Cumulative end-to-end time savings and number of TPs were assessed for different patient types (post-refractive, astigmatic, conventional).
- Compared to standard methods, surgery planning with the cloud-based surgery planning solution was found to require less time and reduce TPs for all integrated technologies among post-refractive, astigmatic, and conventional patients.
What do these results mean?
- SMARTCataract offers time savings and simplified surgery planning to surgery clinics by streamlining the surgery planning process and addressing data transcription and integration issues throughout the patient care workflow.
- Enhanced workflow efficiencies can translate into higher patient volume, increased practice revenue, and improved patient experience for surgery clinics.
Introduction
Cataract surgery is an increasingly common treatment for one of the leading causes of preventable blindness in the world.1 In the US, over 20.5 million people over the age of 40 have at least one cataract, approximately 3.7 million surgeries are performed each year, and projections estimate an increase in cataract prevalence to 38.7 million by 2030.2,3 To continue delivering expeditious treatment to a large and still-growing cataract population, cataract surgery clinics may wish to seek time-saving digital solutions to enhance efficiency in the existing care paradigm. The workflow paradigm can be summarized into four phases: preoperative assessment, preoperative surgery planning, intraoperative procedure, and postoperative evaluation.
Preoperative assessment involves several ophthalmic measurements and intraocular lens (IOL) power calculations in preparation for surgery. According to each patient’s clinical needs, preoperative assessment and surgery planning may involve devices such as Atlas 9000 (“corneal topographer”) [Atlas 9000; Carl Zeiss Meditec AG, Jena, Germany], IOLMaster 700 (“swept source optical coherence tomography [SS-OCT] biometer”) [IOLMaster 700; Carl Zeiss Meditec, Inc., Dublin, CA, USA], Lenstar LS 900 (“optical low-coherence reflectometry [OLCR] biometer”) [Lenstar LS 900; Haag-Streit USA, Inc., Mason, OH, USA], an optical coherence tomographer (OCT) [CIRRUS HD-OCT 5000; Carl Zeiss Meditec, Inc., Dublin, CA, USA], or Pentacam (“corneal tomographer”) [Pentacam; OCULUS Inc., Wetzlar, Germany]. Some patients with (in either one or both eyes) a past corneal refractive surgery or current astigmatism may undergo additional preoperative testing to support surgery planning compared to conventional patients who are neither post-refractive nor astigmatic. In addition, the IOL power calculation process can be automated, though devices capable of automatic calculation may be limited; instead, online IOL power calculation tools that require digital interface for data entry (eg Barrett True-K, Barrett Toric, and Barrett Universal formulas) may be used to calculate IOL power in post-refractive, astigmatic, and conventional eye types.
As part of the intraoperative phase, wavefront aberrometry is utilized for post-refractive and astigmatic patients. One such technology is the Optiwave Refractive Analysis (ORA) SYSTEM intraoperative wavefront aberrometer (“intraoperative aberrometer”) [ORA SYSTEM; Alcon, Inc., Fort Worth, TX, USA]. The intraoperative aberrometer allows surgeons to evaluate refractive findings, refine IOL power, measure cylinder power, and determine IOL alignment in real-time to provide optimal refractive outcomes in cataract surgery.4 Powering the intraoperative aberrometer is the ORA SYSTEM AnalyzOR database (“intraoperative aberrometer cloud-based database”) [ORA SYSTEM AnalyzOR; Alcon, Inc., Fort Worth, TX, USA].
Previous research on the cataract surgery workflow has highlighted several time-related inefficiencies in its current practice: technicians spend valuable time transcribing patient information into individual tools and devices where they could be performing more meaningful technical tasks; manual transcription of data points creates potential for errors; errors in transcription, when corrected, implicate additional time expenditure; increasing complexity of diagnostic testing further prolongs the patient care workflow.5–9 Indeed, it has been reported that data entry errors occur in transferring preoperative device measurements data points from one location to another.10
SMARTCataract (“surgical planning software [SPS]”), a comprehensive digital health solution, supports management of the cataract surgery workflow through its diagnostic and cataract surgery device integration capabilities through a cloud-based infrastructure, in addition to offering a digitally enabled cataract planning solution. Employing time-and-motion techniques, the aim of this study was to assess and quantify the time efficiency associated with use of the SPS compared to the standard of care (SoC) across all phases of the cataract patient workflow.
The primary objective aimed to quantify the integration and surgery planning capabilities of the SPS compared to SoC. Endpoints of analysis included 1) time required to operate all pre-, intra-, and postoperative devices that integrate with the SPS, 2) time to plan surgery by patient type (post-refractive, astigmatic, conventional), and 3) number of data transcription points (TPs) required for each process. The secondary objective was to assess overall time taken by patient type and expressed as per-patient (ie two eyes) findings. The cumulative end-to-end time savings across the different patient types were interpreted as surrogate markers of improved clinical efficiency.
Materials and Methods
Study Design
A real-world, prospective, observational, pre- and post-cohort, time-and-motion study was conducted with patients undergoing cataract evaluation and surgery at a single study site (Figure 1). The pre-cohort arm (before adoption of the SPS) was defined as the SoC by assessment from an interdisciplinary team of healthcare professionals comprising technicians, nurses, and one ophthalmologist. The post-cohort arm (adoption of the SPS) was assessed by the same interdisciplinary team.
 |
Figure 1 Pre- and post-cohort study design. Abbreviations: SoC, standard of care; SPS, surgical planning software. |
This study was conducted in accordance with the principles of the Declaration of Helsinki and good clinical practice. All study measures collected for this study were strictly observational. All collected data were de-identified; no protected health information was captured or recorded. The study received a Non-Human Subjects Research Determination from Sterling Institutional Review Board (Atlanta, GA) and informed consent was waived.
Study Site and Participants
Patients scheduled to undergo evaluation for cataract surgery and receive cataract surgery at a single private practice (Salt Lake City, UT) were included in the study. Participant inclusion criteria are discussed in Box 1.
 |
Box 1 Participant Inclusion Criteria |
Data Collection Procedure and Measurement Definitions
Data were collected in real-time over three one-week periods (August 2021, September 2021, March 2022) by a team of three researchers. Researchers dedicated one day at the start of data collection to validate workflow and shadow practice staff. To evaluate the outcomes of interest, each researcher was assigned to collect data for a predefined set of clinical activities within one of four phases of the workflow (preoperative, intraoperative, postoperative, surgery planning). Predefined clinical activities established start and end time parameters for data collection (Table 1). Researchers exercised careful attention while observing practice staff perform discrete clinical activities. Hardcopy data collection forms were used to record data. In all instances, data were collected using a standard stopwatch.
 |
Table 1 Data Measurement Definitions (SoC vs SPS), by Clinical Activity for Each Phase |
Practice staff were instructed to work as normal while researchers collected data. Technicians recruited in this study possessed a range of professional experience in ophthalmology in general and with the study site in particular. A full list of the recruited technicians and their years of clinical experience is presented in Table 2.
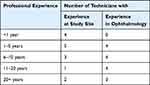 |
Table 2 Technicians, by Professional Years of Experience in Ophthalmology and at Study Site |
Data Analysis
To account for heterogeneity associated with collecting real-world data, a standard method was systematically used to address missing data. The cataract preoperative evaluation involves several individual tasks associated with a given diagnostic test or device. In the clinic, patients were followed throughout the preoperative visit and appropriate measurements were collected based on the patient’s workup and management needs.
- When the sample size for a clinical activity in the SoC arm was less than 40 data points and the missing rate was less than 30% of the overall sample size, the mean imputation technique was employed to replace missing values with the mean value.
- When the sample size for a clinical activity in the SoC arm was greater than 40 data points, the representative sampling technique was employed, and data points were randomly selected from the pool.
Time data for each device and clinical activity, as well as number of TPs, were assessed for both SoC and SPS study arms and summarized using descriptive statistics (number of observations, mean, standard deviation [SD]).
An a-priori sample size calculation was conducted to assess the primary objective for the preoperative, intraoperative, and surgery planning steps. Based on a medium effect size and 95% statistical power, a desired total sample size of 40 and 15 patients per arm were determined for the pre- and intraoperative workflow phases, respectively. For the surgery planning step, patients were classified by patient type (post-refractive, astigmatic, and conventional). Based on a medium effect size and 95% statistical power, a desired sample size of 10 patients per arm was determined for each patient group. To assess the associated time efficiency impact of the pre- and post-cohorts, statistical analyses were conducted to assess the primary objective comparing time spent by each device that integrates with the SPS as well as clinical processes obviated by the SPS. Independent sample t-tests were utilized to assess the time difference in the means for each device that integrates with the SPS compared to the SoC and to assess surgery planning time using the SPS compared to the SoC, stratified by patient type. All statistical analyses were performed using SAS (“statistical analysis software”) version 9.3. Statistical significance was considered at p<0.05.
For the secondary objective, as individual patients were not followed from start to end of the workflow for data collection, overall time impact of the SPS for each patient type was assessed using summary statistics of the average elapsed time per device and workflow activity associated with each pre- and post-cohort. Figure 2 illustrates the composition of clinical activities and measurement devices involved in the workflow of each patient type—post-refractive (Figure 2a), astigmatic (Figure 2b), and conventional (Figure 2c).
Results
Performance Time and Transcription Points for Each Activity and Device, by Setting
Average total performance time, sample size, and number of TPs for each device and task identified in the cataract surgery care workflow are summarized in Table 3.
 |
Table 3 Average Time per Device: Data Entry for Integrated Devices in Pre-, Intra-, and Postoperative Workflow Phases |
For data entry into each device used during preoperative diagnostic workup, there was a statistically significant difference in the average time spent using devices that integrate with the SPS compared to devices used in the SoC (p<0.0001) (Table 3). With SoC methods, time spent on preoperative diagnostic devices ranged from 21.8 to 48.2 seconds per patient. With the SPS, time spent on preoperative diagnostic devices was 5.5 seconds per device per patient regardless of device type. SoC devices each have 7 TPs, whereas the SPS required only one TP per device used in the preoperative workup.
For surgery planning activities, the SPS achieved statistically significant time savings for post-refractive (p<0.0001), astigmatic (p=0.0005), and conventional (p=0.0004) patient types (Table 4). The average per-eye time to develop a surgery plan and have it reviewed and approved for post-refractive, astigmatic, and conventional patient eyes took 223.3, 211.5, and 183.0 seconds, respectively, using SoC methods compared to 113.1, 90.6, and 87.9 seconds, respectively, with the SPS. Per-eye TPs involved in post-refractive, astigmatic, and conventional surgery planning activities with SoC methods were found to be 26, 23, and 14 TPs, respectively; per-eye TPs with the SPS across the same three patient types involved 9, 9, and 7 TPs, respectively.
 |
Table 4 Average Time per Activity: Surgery Planning (in Clinic), by Patient Type [per Eye] |
In the intraoperative phase, integration capabilities of the SPS offered a statistically significant difference in time saved to perform data entry compared to standard methods (p<0.0001) (Table 3). Time to enter data into the intraoperative aberrometer requires 81.1 seconds per eye with the SoC compared to 6.5 seconds per eye with the SPS. In the SoC, 29 TPs were measured per eye in the intraoperative aberrometer for a per-patient (ie two eyes) total of 58 TPs. With the SPS, the intraoperative aberrometer required only one TP per eye for a total of 2 TPs per patient.
For data entry into the intraoperative aberrometer cloud-based database, the SPS offered statistically significant time savings compared to SoC methods for preoperative and postoperative data entry (p<0.0001) (Table 3). By obviating the need for manual data input into the intraoperative aberrometer cloud-based database, the SPS took no time and requires zero TPs; by contrast, manual data input with SoC methods takes an average 147.2 seconds. Data entry into the intraoperative aberrometer cloud-based database with SoC methods required 100.0 seconds for the preoperative data input and 47.2 seconds in the postoperative data input. Per eye in the SoC, 20 TPs were required to use the intraoperative aberrometer cloud-based database preoperatively and 12 TPs are required to use the database postoperatively; at the per patient level, 40 and 24 TPs were required to use the database pre- and postoperatively, respectively.
Average Total Performance Time for the Full Cataract Care Workflow, by Patient Cohort
Post-Refractive Patients
The average time to perform all tasks and procedures in the post-refractive cataract surgery workflow was 1058.6 seconds (17.6 minutes) per patient with SoC methods and 266.7 seconds (4.4 minutes) per patient with the SPS. The greatest SPS time savings was realized by obviating pre- and postoperative data input into the intraoperative aberrometer cloud-based database (294.5 seconds, 4.9 minutes) followed by preoperative diagnostic device integration (127.8 seconds, 2.1 minutes) and streamlining surgery plan development by obviating manual IOL power calculation (199.6 seconds, 3.3 minutes). Overall, the SPS reduced post-refractive cataract surgery workflow time by 791.9 seconds (13.2 minutes) per patient for a relative time efficiency of 75% per patient. Time efficiency results of the SPS compared to SoC methods in the post-refractive patient care workflow can be seen in Figure 3a.
Integration capabilities of the SPS obviated a total of 184 TPs from the traditional post-refractive patient care workflow. Total TPs measured in SoC and SPS study arms can be seen in Table 5.
 |
Table 5 Number of Transcription Points in Post-Refractive, Astigmatic, and Conventional Patient Workflows (SoC vs SPS) [per Patient] |
Astigmatic Patients
The average time to perform all tasks and procedures in the astigmatic cataract surgery workflow was 964.9 seconds (16.1 minutes) per patient with SoC methods and 211.2 seconds (3.5 minutes) per patient with the SPS. The greatest SPS time savings was realized by obviating pre- and postoperative data input into the intraoperative aberrometer cloud-based database (294.5 seconds, 4.9 minutes) followed by streamlining surgery plan development by obviating manual IOL power calculation (235.8 seconds, 3.9 minutes) and integration of the intraoperative aberrometer (149.1 seconds, 2.5 minutes). Overall, the SPS reduced astigmatic cataract surgery workflow time by 753.7 seconds (12.6 minutes) for a relative time efficiency of 78% per patient. Time efficiency results of the SPS compared to SoC methods in the astigmatic patient care workflow can be seen in Figure 3b.
Integration capabilities of the SPS obviated a total of 166 TPs from the traditional astigmatic patient care workflow. Total TPs measured in SoC and SPS study arms can be seen in Table 5.
Conventional Patients
The average time to perform all tasks and procedures in the conventional cataract surgery workflow was 451.1 seconds (7.5 minutes) per patient with SoC methods and 192.8 seconds (3.2 minutes) per patient with the SPS. The greatest SPS time savings was realized by streamlining surgery plan development by obviating manual IOL power calculation (184.0 seconds, 3.1 minutes) followed by preoperative diagnostic device integration (69.0 seconds, 1.1 minutes) and streamlining surgery plan review and approval (5.4 seconds, 0.1 minutes). Overall, the SPS reduced conventional cataract surgery workflow time by 259.2 seconds (4.3 minutes) per patient for a relative efficiency of 57% per patient. Time efficiency results of the SPS compared to SoC methods in the conventional patient care workflow can be seen in Figure 3c.
Integration capabilities of the SPS obviated a total of 25 TPs from the traditional conventional patient care workflow. Total TPs measured in SoC and SPS study arms can be seen in Table 5.
Discussion
This study describes the time savings offered by the SPS, a digital platform aimed to facilitate clinic-to-operating room (OR) device integration and cloud-based surgery planning. By leveraging time-and-motion methods and workflow mapping, we were able to visualize and quantify the time associated with the various steps in the cataract surgery process. Our results find that the SPS offers statistically significant time savings for all integrated technologies (p<0.0001) in addition to significant time savings for surgery planning for post-refractive (p<0.0001), astigmatic (p=0.0005), and conventional (p=0.0004) cataract patients. When assessing the overall workflow for each patient type, the SPS demonstrates most time savings for the post-refractive care workflow and the greatest incremental time efficiency benefit in the astigmatic care workflow compared to SoC out of all patient types identified. With device integration capabilities and simplified surgery planning, the SPS reduces the patient workflow time for post-refractive, astigmatic, and conventional cataract patients by averages of 13.2, 12.6, and 4.3 minutes per patient, respectively. The end-to-end incremental time efficiency impact of the SPS relative to SoC time across post-refractive, astigmatic, and conventional workflows demonstrated reductions of 75%, 78% and 57%, respectively. Across all patient types, minimizing or obviating manual transcription contributes the most to overall time saved. The SPS’ ability to facilitate clinic-to-OR integration obviates up to 184, 166, and 25 TPs per patient in post-refractive, astigmatic, and conventional cohorts, respectively. By obviating the need for manual data transcription (ie IOL calculation and lens ordering), practices that use the SPS can avoid one of the major upstream causes of potential data entry errors. Assuming a 5.17% rate of errors in data transcription as noted in previous literature,9 the SPS may minimize errors in up to 9, 8, and 1 TPs for post-refractive, astigmatic, and conventional cataract workflows, respectively.
Although few studies have investigated automated integration solutions in cataract surgery, a study by Gujral and Hovanesian assessed the time impact of a web-based software (Veractiy Surgical, Zeiss, Oberkochen, Germany) on cataract surgery planning.11 Results of this study are consistent with our finding that average time savings was greatest for post-refractive patients among all other patient types; however, limitations of the Gujral study are noteworthy. The Gujral study assumed a single surgeon responsible for all activities associated with surgery planning such that involvement of other healthcare professionals in surgery plan development, such as discrete manual data transcription activities that may be completed by a technician, was not evaluated. Additionally, the Gujral study measured the elapsed time for surgery planning which involved the continuous observation of the surgeon’s work and did not identify or distinguish discrete methodological variations. Finally, the Gujral study did not include time measurements pertaining to intraoperative aberrometry, which is used by many practices in refractive cataract surgery, especially in the US.
Our study is the first to assess the overall workflow impact of a cloud-based digital solution in cataract surgery. The main strength of this study is the observational real-world study design in combination with time-and-motion techniques. Quantitative data collection methods and workflow mapping, in which an observer records the time and movements required to complete a set of discrete actions, identified process efficiencies and improvements when the SPS is adopted. Furthermore, our study aimed to determine the system’s ability to interface with individual devices and processes and offers an opportunity for the data to be generalizable to practices with different devices adopted in-house. A full list of integrated IOL power calculation formulae can be found in Supplementary Table 1. Our study was not without limitations. The study results expressed in this report are limited to data collected at one practice. Time to perform clinical activities such as surgery planning may vary by practice as each practice may employ different techniques to calculate IOL power. Time to perform clinical activities may also vary by healthcare professionals’ years of professional experience and familiarity with using different technologies. Although this study collected technicians’ years of professional experience in both ophthalmology and at the study site, we did not investigate the possible association of time to perform discrete clinical activities with healthcare professionals’ years of professional experience.
Conclusion
Results from this real-world study demonstrate substantial and statistically significant time savings in the cataract care workflow with the SPS, a digital cloud-based solution that offers simplified ophthalmic data processing for surgery planning and integration with various devices used in all settings (clinic and OR) and phases (preoperative, intraoperative, and postoperative) of the cataract surgery workflow. Enhanced workflow efficiencies may translate into higher patient volume, increased practice revenue, and improved patient experience in surgery clinics. Future studies should aim to evaluate and quantify the improved patient experience and cost-effectiveness of reducing the per-patient workflow time and increasing the patient intake.
Abbreviations
IOL, intraocular lens; OCT, optical coherence tomography; OLCR, optical low-coherence reflectometry; OR, operating room; ORA SYSTEM, Optiwave Refractive Analysis SYSTEM; SD, standard deviation; SoC, standard of care; SPS, surgical planning software; SS-OCT, swept source optical coherence tomography; TP, transcription point.
Acknowledgments
Special thanks to Thomas Goss, Robert Wenthold, Manasi Datar, and Salwa Masud from Veranex Solutions, Inc. (“Veranex”) for providing editorial assistance in the preparation of this manuscript and Michelle Saylor from Veranex for supporting a portion of the data collection.
Disclosure
This study was supported by Alcon Vision, LLC (“Alcon”) (Fort Worth, Texas). Alcon is the market authorization holder of SMARTCataract. Veranex (Boston, MA) received funding from Alcon to conduct this study as a third-party research consultancy. LP and KM are employees of Veranex, HC and DO are employees of Alcon, and ZZ is a consultant for Alcon.
References
1. Lee CM, Afshari NA. The global state of cataract blindness. Curr Opin Ophthalmol. 2017;28(1):98–103. doi:10.1097/ICU.0000000000000340
2. Rossi T, Romano MR, Iannetta D, et al. Cataract surgery practice patterns worldwide: a survey. BMJ Open Ophthalmol. 2021;6(1):e000464. doi:10.1136/bmjophth-2020-000464
3. National Eye Institute. Cataract Tables. Available from: https://www.nei.nih.gov/learn-about-eye-health/outreach-campaigns-and-resources/eye-health-data-and-statistics/cataract-data-and-statistics/cataract-tables.
4. Alcon. Alcon cataract refractive surgery diagnostics | MyAlcon professional. Available from: https://professional.myalcon.com/cataract-surgery/cataract-equipment/refractive-diagnostics/.
5. Ciulla TA, Tatikonda MV, ElMaraghi YA, et al. Lean six sigma techniques to improve ophthalmology clinic efficiency. Retina. 2018;38(9):1688–1698. doi:10.1097/IAE.0000000000001761
6. Lindholm JM, Laine I, Hippala H, Ylinen P, Tuuminen R. Improving eye care services with a lean approach. Acta Ophthalmol. 2018;96(7):724–728. doi:10.1111/aos.13703
7. Talley-Rostov A. Patient-centered care and refractive cataract surgery. Curr Opin Ophthalmol. 2008;19(1):5–9. doi:10.1097/ICU.0b013e3282f2d7a3
8. Lim S, Shahid H. Distribution and extent of electronic medical record utilisation in eye units across the United Kingdom: a cross-sectional study of the current landscape. BMJ Open. 2017;7(5):e012682. doi:10.1136/bmjopen-2016-012682
9. Keay L, Lindsley K, Tielsch J, Katz J, Schein O. Routine preoperative medical testing for cataract surgery. Cochrane Database Syst Rev. 2012. doi:10.1002/14651858.CD007293.pub3
10. Håkansson I, Lundström M, Stenevi U, Ehinger B. Data reliability and structure in the Swedish National Cataract Register. Acta Ophthalmol Scand. 2001;79(5):518–523. doi:10.1034/j.1600-0420.2001.790519.x
11. Gujral T, Hovanesian J. Cataract surgical planning using online software vs traditional methods: a time/motion and quality of care study. Clinical Ophthalmol. 2021;15:3197–3203. doi:10.2147/OPTH.S318935
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


