Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
End-of-life care in individuals with respiratory diseases: a population study comparing the dying experience between those with chronic obstructive pulmonary disease and lung cancer
Authors Kendzerska T , Nickerson JW , Hsu AT , Gershon AS , Talarico R, Mulpuru S , Pakhale S , Tanuseputro P
Received 5 April 2019
Accepted for publication 10 July 2019
Published 31 July 2019 Volume 2019:14 Pages 1691—1701
DOI https://doi.org/10.2147/COPD.S210916
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Tetyana Kendzerska,1,2 Jason W Nickerson,3,4 Amy T Hsu,1–3 Andrea S Gershon,5 Robert Talarico,1,2 Sunita Mulpuru,1 Smita Pakhale,1 Peter Tanuseputro1–3
1Department of Medicine, University of Ottawa, The Ottawa Hospital Research Institute, Ottawa, Ontario, Canada; 2ICES, Ottawa, Ontario, Canada; 3Bruyère Research Institute, Ottawa, Ontario, Canada; 4Centre for Health Law, Policy and Ethics, Faculty of Law, University of Ottawa, Ottawa, Ontario, Canada; 5Department of Medicine, the Sunnybrook Health Science Center/ICES, Toronto, Ontario, Canada
Purpose: Among individuals with COPD and/or lung cancer, to describe end-of-life health service utilization, costs, and place of death; to identify predictors of home palliative care use, and to assess benefits associated with palliative care use.
Patients and methods: We conducted a retrospective population-based study using provincial linked health administrative data (Ontario, Canada) between 2010 and 2015. We examined health care use in the last 90 days of life in adults 35 years and older with physician-diagnosed COPD and/or lung cancer identified using a validated algorithm and the Ontario Cancer Registry, respectively. Four mutually exclusive groups were considered: (i) COPD only, (ii) lung cancer only, (iii) COPD and lung cancer, and (iv) neither COPD nor lung cancer. Multivariable generalized linear models were employed.
Results: Of 445,488 eligible deaths, 34% had COPD only, 4% had lung cancer only, 5% had both and 57% had neither. Individuals with COPD only received less palliative care (20% vs 57%) than those with lung cancer only. After adjustment, people with lung cancer only were far more likely to receive palliative care (OR=4.22, 4.08–4.37) compared to those with neither diagnosis, while individuals with COPD only were less likely to receive palliative care (OR=0.82, 0.81–0.84). Home palliative care use was associated with reduced death and fewer days in acute care, and less cost, regardless of the diagnosis.
Conclusion: Although individuals with lung cancer were much more likely to receive palliative care than those with COPD, both populations were underserviced. Results suggest greater involvement of palliative care may improve the dying experience of these populations and reduce costs.
Keywords: pulmonary disease, chronic obstructive, lung neoplasms, palliative care, health services
Plain language summary
We conducted this population-based study to: (i) describe the health service use and costs in the last 90 days of life, as well as place of death among individuals with chronic lung diseases such as COPD and/or lung cancer; (ii) identify predictors of receiving home palliative care; and (iii) assess benefits associated with palliative care in these populations.
Our findings from a large population-based study suggest that home palliative care services were underutilized in both COPD and lung cancer populations, and that increased use of home palliative care services in the last three months of life has the potential to reduce costs and influence the place of death.
To our knowledge, this study is the first large population-based study to report the predictors of palliative care use among decedents with COPD and/or lung cancer and assess the benefits associated with palliative care at the population level for both diseases across health sectors.
Introduction
Chronic obstructive pulmonary disease (COPD) and lung cancer are respiratory diseases that are associated with morbidity, progressive deterioration, and high health care utilization.1–8 Individuals with COPD and lung cancer would benefit from palliative care during the last months of life as the symptom burden is high, leading to reduced quality of life.2–10 Without provision of adequate palliative care at home, individuals with COPD and lung cancer have no recourse but to seek end of life care through hospital admissions.
Despite similar mortality rates and symptom burden among individuals with lung cancer and COPD, previous research suggests that patients with COPD underutilize palliative care services, and may have worse dying experiences compared to patients with lung cancer.11–18 However, there is a poor understanding of patient-preference and system-level factors associated with the use of palliative care in these populations.
Palliative care has been shown to improve quality of life and mood,19–21 survival,20 and reduce health care costs22,23 in individuals with lung cancer. However, the impact of palliative care on health care utilization and care-costs is lacking in the COPD population. To improve the systematic delivery and use of palliative care among patients with COPD, a better understanding of the health service requirements needs to be studied at a population level. Currently, the number of population-based studies examining end-of-life care in COPD and lung cancer are limited,14,15,23–25 and tend to focus on separate aspects of health care utilization for COPD versus lung cancer patients.
Thus, we conducted this population-based study to: (i) understand the health service utilization and costs in the last 90 days of life, as well as place of death among individuals with COPD and/or lung cancer; (ii) identify predictors of receiving home palliative care; and (iii) assess benefits associated with palliative care in these populations.
Methods
Study design
We conducted a retrospective population-based observational cohort study of all decedents in Ontario, Canada, eligible for provincial health insurance, and described their receipt of health care services in the last 90 days of life captured through linked provincial health administrative databases between April 1, 2010, and March 31, 2015.
The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board.
Data sources
ICES (formerly Institute for Clinical Evaluative Sciences) is an independent, non-profit research institute which holds a comprehensive high-quality collection of administrative claims and billing data in the province of Ontario whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement. The datasets used for this study are held securely in a deidentified form at ICES and were linked using unique encoded identifiers and analyzed at ICES. Please see details in the data supplement and at https://datadictionary.ices.on.ca.
Study population
We used the Registered Persons Database to capture all deaths in the province over a 5-year period between April 1, 2010 and March 31, 2015. We identified individuals with prevalent physician-diagnosed COPD from the Ontario COPD Database, using an algorithm previously validated in Ontario – based on one or more COPD hospital discharges and/or one or more COPD ambulatory care visits after 35 years of age – with a sensitivity of 85% and specificity of 78%.26 Lung cancer was identified from the Ontario Cancer Registry27–30 using US Surveillance, Epidemiology and End Results Site Recode definitions as International Classification of Diseases for Oncology -3 code C34.0–C34.9 (http://seer.cancer.gov/siterecode/icdo3_dwhoheme/index.html).
For the analysis, we stratified our study cohort into individuals with (i) COPD without lung cancer (“COPD only”), (ii) lung cancer without COPD (“lung cancer only”), and (iii) with both concurrent COPD and lung cancer as mutually exclusive groups. Remaining decedents who were not identified as having COPD or lung cancer by our algorithms comprised the final comparison group of individuals with “neither COPD nor lung cancer”.
Variable definition
Age was determined at death and stratified by decades. Sex, rural status,31 and neighborhood income quintiles32 were determined at 1 year prior to death. We also examined the prevalence of 17 chronic conditions separately using previously described definitions33–37 as well as the total number of chronic comorbidities (0–10 and 10+). Among patients with COPD, having concurrent lung cancer, older age, female gender, and comorbidities have been shown to be associated with an increased likelihood of receiving palliative care, regardless of COPD severity.15,38,39 In individuals with lung cancer, older age, female gender, and having fewer comorbidities have been shown to be associated with an increased likelihood of receiving palliative care.23
Outcomes
We considered the following outcomes: location of death, receipt of health services, and health care cost in the last 90 days of life.
Location of death was categorized as death in acute care settings (both emergency room visits and hospital admissions), subacute care (complex continuing care facilities), long-term care (nursing homes) and community. We categorized places of care through receipt of health services in different settings of care, which captured the number of days spent in acute care, subacute care, long-term care, and at home with receipt of home care. Palliative home care and physician home visits were defined using Ontario Health Insurance Plan and Home Care databases.40,41 All physician home visits in the last 90 days of life, regardless if billed specifically as a “palliative” service, were considered palliative care in nature. Please see details on definitions in the data supplement (Table S1).
Health care cost
For costing, we took the perspective of the payer, which is the Ontario Ministry of Health and Long-Term Care, to estimate person-level health care expenditures in the last 90 days of life. Methodologies for deriving the unit and total costs for each care episode across all health sectors are described elsewhere.42 All costs were expressed in 2015 Canadian dollars; we inflated past costs using health care-specific yearly consumer index reported by Statistics Canada. Health sector cost for the population was the sum of all costs among decedents captured within each respective sector.
Analysis
We used frequencies, proportions, means (standard deviations [SD]) and medians (interquartile ranges [IQR]) as applicable, to describe the study populations separately by group of interest. All statistical tests were two-tailed and p<0.05 was used to determine statistical significance. To address our second objective, we used multivariable logistic regressions to determine the association between decedent characteristics and the receipt of palliative home care and/or physician home visit in the last 90 days for the entire cohort. To address our third objective, for each group of interest, controlling for age, sex, neighborhood income quintile, neighborhood rurality, and number of comorbidities, we estimated (i) multivariable logistic regressions to assess the relationship between receipt of palliative care and odds of death in acute care; (ii) negative binomial regressions to assess the relationship between the receipt of palliative care and total number of days spent in a hospital; and (iii) log-gamma regressions to assess the relationship between the receipt of palliative care and health care cost.43 For modeling purposes, home care was considered a three-level mutually exclusive categorical variable: palliative home care, usual home care without the palliative element (reference group) and no home care of any type. All statistical analyses were performed in the secure environment of ICES following Ontario privacy standards using SAS 9.3 (SAS Institute Inc., Cary, NC).
Results
Study population characteristics
There were 445,488 eligible deaths during our 5-year study period: 150,999 (33.9%) individuals had COPD only, 15,638 (3.5%) had lung cancer only, 24,082 (5.4%) had both COPD and lung cancer, and 254,769 (57.2%) had neither. Individuals with COPD, with or without lung cancer were older, had lower income status and more comorbidities, and were more likely to live in rural area compared to individuals with lung cancer only (Table 1).
 |
Table 1 Population characteristics by the condition of interest, N (%) |
Outcome description
Description of all outcomes stratified by the group of interest is presented in Table 2.
 |
Table 2 Location of death, receipt of health care services and cost in the last 90 days of life by the condition of interest |
Regardless of the group of interest, approximately 50% of the individuals died in acute care. Individuals with COPD only were less likely to die in community as compared to those with COPD and lung cancer or lung cancer only (20.8%, 28.2%, and 32.4%, respectively). Decedents with lung cancer with or without COPD spent more days in acute and sub-acute care than individuals with COPD only, which also was reflected by the increased cost of these groups.
Compared to individuals with lung cancer only, individuals with COPD only were less likely to receive palliative care (20% vs 57%).
Predictors of the receipt of palliative care
After adjustment for confounding variables listed above, lung cancer with or without COPD was significantly associated with receiving home palliative care (OR of 4.22, 95% CI: 4.08–4.37 for lung cancer only), while individuals with COPD only were significantly less likely to receive palliative care (OR of 0.82, 95%CI: 0.81–0.84) compared to those with no lung cancer or COPD (Table 3). Other predictors of receiving palliative care included female gender, living in urban areas, higher income, and multiple comorbidities. The relationship between age and receiving palliative care was complex. Being older was significantly associated with increase in physician home visits with the highest likelihood between age of 50 and 79 years old. Being between 50 and 69 years old was associated with a higher likelihood of receiving palliative home care, while being 80 years and older was associated with a decreased likelihood (OR: 0.38, 95%CI: 0.36–0.40), compared to decedents who were 35–49 years old (Table 3).
The effect of the receipt of palliative care services on location of death, days spent in a hospital and health care cost
Regardless of disease, crude percentages suggested that individuals who did not receive palliative care in the last 90 days of life died more often in acute care compared to those who received palliative care (Figure 1 and Table S2). The largest differences were observed in decedents with lung cancer with or without COPD. Individuals with lung cancer, regardless of COPD status, who did not receive palliative care, spent more days in acute care compared to those who received palliative care (Table S2). Mean health care costs in the last 90 days of life were less for individuals who received palliative care compared to those who did not, with the largest differences observed among lung cancer patients (Table S2).
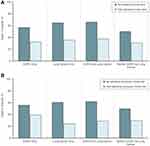 |
Figure 1 Proportion of decedents who died in hospital, by receipt of palliative care in the last 90 days of life: (A) by receipt of palliative home care, and (B) by receipt of physician home visits. |
After adjustment for confounding variables, the receipt of palliative home care and physician home visits in the last 90 days of life were significantly associated with reduced death in acute care among all groups (Figure 2A, Table S3). Patients with lung cancer only who received a physician home visit and patients with COPD only who received palliative home care had one-third the odds of death in an acute care setting compared to decedents who only received usual home care.
In terms of acute care days, receipt of physician home visits and palliative home care were significantly associated with fewer days spent in hospital (Figure 2B, Table S4), when compared to decedents who received usual home care in our regression models. The largest effect was observed among individuals with cancer only who received a physician home visit (IRR: 0.68, 95% CI: 0.71–0.65). These trends are also reflected in the average health care cost associated with palliative care services, whereby costs among decedents who received a physician home visits were 15–18% lower and cost among those who received palliative home care were 11–15% lower than individuals who only had usual home care visits (Figure 2C, Table S5).
Discussion
This population-based study of more than 400,000 individuals who died between 2010 and 2015 demonstrates that palliative care was underutilized among individuals with lung cancer and/or COPD in the last 3 months of life. Despite the similarity of health care needs and the available health care system that provides comprehensive care and social supports, palliative care utilization differed between individuals with the two diseases; the proportion of individuals with lung cancer who accessed palliative care was twice the proportion of individuals with COPD. While it may not be surprising, given the challenges in predicting the trajectory of the disease, that decedents with COPD only were less likely to receive palliative care than those with lung cancer, it was interesting that they were also less likely to receive palliative care than people with neither disease. Furthermore, we identified variables that suggest inequitable access to palliative home care in the population. For example, men who were 80 years of age and older with COPD only, living in urban areas, and in the lowest income status were the least likely to receive palliative home care. To our knowledge, this study is the first large population-based study to report the predictors of palliative care use among decedents with COPD and/or lung cancer and assess the benefits associated with palliative care at the population level for both diseases across health sectors.
Our findings are congruent with other studies demonstrating that individuals with COPD received less end of life palliative care, and were more likely to be institutionalized in a long-term care setting, compared with cancer patients.11,14,15,17,18,25,39,44 The reasons for underutilization of palliative care among patients with COPD are multifactorial.45–47 For example, COPD itself has a longer course of illness with the perception of a less defined prognosis. Further, our study and others have found a high prevalence of COPD among older populations, particularly those in long-term care, where COPD may be a secondary or complicating diagnosis, but not the primary reason for admission, which may result in poorer long-term planning, including for palliative care.48 Moreover, there is a lack of predictive models and guidelines to assist health professionals with the timing of referral to palliative care for patients with noncancer illnesses, and possible confusion as to which provider should initiate the referral.49 Lack of knowledge among providers of the available palliative care resources and the benefits of home palliative care may also limit referrals.50
Our results also confirm findings that patients with COPD are less likely to die in the community and more likely to die in acute or sub-acute settings, compared to those with only lung cancer24 despite both groups’ preference for treatment focused on comfort (rather than on prolonging life).12
We consistently found evidence that lung cancer co-diagnosis significantly increased the chances of receiving palliative care among individuals with COPD.15 This is consistent with other studies and suggests that concomitant lung cancer diagnosis, not COPD, may be the dominant driver of receiving palliative care for patients with COPD.
We found that the receipt of palliative home care and physician home visits in the last 90 days were associated with less deaths in the acute care setting, fewer days spent in acute care settings, and lower costs with the largest impact in preventing deaths in the acute care setting. This suggests that patients are more comfortable with death in the home setting when they are supported by a palliative care team and as such, do not seek end-of-life care through hospital admissions. The potentially lower cost associated with palliative care was also shown in the population-based study on the National Inpatient Sample database for patient with stage IV lung cancer.23 In general, but not for our conditions of interest, a home-based palliative care program for end-of-life – which typically is associated with additional hours of support and more specialized palliative care – has been shown to be associated with less acute care use, lower costs of care, and higher likelihood of dying at home as compared to usual care in a smaller single center study.22
The strengths of our study include its real-world and population-level relevance, and power to examine the effects of palliative care use. Our study has several limitations. It is challenging to identify individuals with end-stage COPD; therefore, as a proxy, we performed our analysis on deceased individuals in the last 90 days of their life. Thus, it is possible that some individuals included in our analyses did not have end-stage COPD as they may have died from other unrelated causes. Similar to other population-based studies, our results are prone to unmeasured confounding. The death certificate, diagnostic and fee codes used to identify population and outcomes of interest may cause misclassification bias and does not provide information on important aspects of the end-of-life process such as preferences of treatment or decision-making levels. Thus, whether the palliative or acute care administered was in concordance with patients’ wishes could not be explored. Further, we may underestimate the percentage of the receipt of palliative care in individuals with COPD only as some of them may receive palliative care as a part of the comprehensive care plan in a long-term care facility or in a hospital. Those individuals may be less likely to receive palliative services than people living in the community and it may therefore not be a true reflection of their level of access.
Conclusion
The findings from our large population-based study suggest that palliative care services were underutilized in both COPD and lung cancer populations, and that increased use of palliative care services in the last three months of life has the potential to reduce costs and influence the place of death. Our findings identify an important care-gap among patients with lung cancer and COPD in the last months of life. Future work should focus on better understanding of disease trajectories in COPD and when is the “right time” to consider palliative care. There is a greater need for understanding the patient and provider perspectives of preferences for care at the end-of-life, identifying systemic inequities in availability of palliative services among patient sub-groups and on designing comprehensive care models to meet patient needs at the end of life.
Research ethics and patient consent
The ICES (formerly Institute for Clinical Evaluative Sciences) is a prescribed entity under section 45 of Ontario’s Personal Health Information Protection Act (PHIPA). Section 45 is the provision that enables analysis and compilation of statistical information related to the management, evaluation and monitoring of, allocation of resources to, and planning for the health system. Section 45 authorizes health information custodians to disclose personal health information to a prescribed entity, like ICES, without consent for such purposes.
Projects conducted wholly under section 45, by definition, do not require review by a Research Ethics Board. A confirming letter from the REB of Sunnybrook Health Sciences Centre, ICES’ Research Ethics Board of Record, is available upon request.
As a prescribed entity, ICES must submit to tri-annual review and approval of its privacy and security policies, procedures, and practices by Ontario’s Information and Privacy Commissioner. These include policies, practices, and procedures that require internal review and approval of every project by ICES’ Privacy and Compliance Office. ICES was approved by the Commissioner for the fifth time in 2017.
Data management and sharing
While data sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS. The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Acknowledgments
Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI) and Cancer Care Ontario (CCO), however, the analyses, conclusions, opinions, and statements expressed herein are those of the author, and not necessarily those of CIHI and CCO. No endorsement by CIHI and CCO is intended or should be inferred. This work was supported by a research grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC) to the Health System Performance Research Network (Grant #06034), the Bruyère Research Institute (BRI) through the Big Data Research Program, and ICES, which is funded by an annual grant from the MOHLTC. The opinions, results, and conclusions reported in this paper are those of the authors and are independent of the funding sources. No endorsement by ICES, BRI, or the Ontario MOHLTC is intended or should be inferred.
Author contributions
All co-authors were involved in the following: study conception and design, interpretation of data, revising the manuscript critically for the accuracy and important intellectual content, final approval of the version to be published and agreee to be accountable for all aspoects of the work. Tetyana Kendzerska was additionally involved in the literature search and drafting of the manuscript. Robert Talarico was additionally involved in data analyses. Tetyana Kendzerska and Peter Tanuseputro had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure
The authors declare no relevant competing interests exist in this work.
References
1. Chapman KR, Bourbeau J, Rance L. The burden of COPD in Canada: results from the confronting COPD survey. Respir Med. 2003;97(Suppl C):S23–S31. doi:10.1016/S0954-6111(03)80022-7
2. Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: global burden of disease study. Lancet. 1997;349(9064):1498–1504. doi:10.1016/S0140-6736(96)07492-2
3. Doucet M, Rochette L, Hamel D. Incidence, prevalence, and mortality trends in chronic obstructive pulmonary disease over 2001 to 2011: a public health point of view of the burden. Can Respir J. 2016;2016:7518287. doi:10.1155/2016/7518287
4. Gershon AS, Wang C, Wilton AS, Raut R, To T. Trends in chronic obstructive pulmonary disease prevalence, incidence, and mortality in Ontario, Canada, 1996 to 2007: a population-based study. Arch Intern Med. 2010;170(6):560–565. doi:10.1001/archinternmed.2010.17
5. Jinjuvadia C, Jinjuvadia R, Mandapakala C, Durairajan N, Liangpunsakul S, Soubani AO. Trends in outcomes, financial burden, and mortality for acute exacerbation of Chronic Obstructive Pulmonary Disease (COPD) in the United States from 2002 to 2010. COPD. 2017;14(1):72–79. doi:10.1080/15412555.2016.1199669
6. Lopez-Campos JL, Ruiz-Ramos M, Soriano JB. Mortality trends in chronic obstructive pulmonary disease in Europe, 1994–2010: a joinpoint regression analysis. Lancet Respir Med. 2014;2(1):54–62. doi:10.1016/S2213-2600(13)70232-7
7. Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294(10):1255–1259. doi:10.1001/jama.294.10.1255
8. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
9. Spathis A, Booth S. End of life care in chronic obstructive pulmonary disease: in search of a good death. Int J Chron Obstruct Pulmon Dis. 2008;3(1):11–29.
10. Pantilat SZ, O’Riordan DL, Dibble SL, Landefeld CS. Longitudinal assessment of symptom severity among hospitalized elders diagnosed with cancer, heart failure, and chronic obstructive pulmonary disease. J Hosp Med. 2012;7(7):567–572. doi:10.1002/jhm.1925
11. Au DH, Udris EM, Fihn SD, McDonell MB, Curtis JR. Differences in health care utilization at the end of life among patients with chronic obstructive pulmonary disease and patients with lung cancer. Arch Intern Med. 2006;166(3):326–331. doi:10.1001/archinte.166.3.326
12. Claessens MT, Lynn J, Zhong Z, et al. Dying with lung cancer or chronic obstructive pulmonary disease: insights from SUPPORT. Study to understand prognoses and preferences for outcomes and risks of treatments. J Am Geriatr Soc. 2000;48(5 Suppl):S146–S153. doi:10.1111/j.1532-5415.2000.tb03124.x
13. Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55(12):1000–1006. doi:10.1136/thorax.55.12.1000
14. Goodridge D, Lawson J, Duggleby W, Marciniuk D, Rennie D, Stang M. Health care utilization of patients with chronic obstructive pulmonary disease and lung cancer in the last 12 months of life. Respir Med. 2008;102(6):885–891. doi:10.1016/j.rmed.2008.01.007
15. Bloom CI, Slaich B, Morales DR, Smeeth L, Stone P, Quint JK. Low uptake of palliative care for COPD patients within primary care in the UK. Eur Respir J. 2018;51:2. doi:10.1183/13993003.01879-2017
16. Hyasat K, Sriram KB. Evaluation of the patterns of care provided to patients with COPD compared to patients with lung cancer who died in hospital. Am J Hosp Palliat Care. 2016;33(8):717–722. doi:10.1177/1049909115586395
17. Faes K, De Frene V, Cohen J, Annemans L. Resource use and health care costs of COPD patients at the end of life: a systematic review. J Pain Symptom Manage. 2016;52(4):588–599. doi:10.1016/j.jpainsymman.2016.04.007
18. Lau KS, Tse DM, Tsan Chen TW, Lam PT, Lam WM, Chan KS. Comparing noncancer and cancer deaths in Hong Kong: a retrospective review. J Pain Symptom Manage. 2010;40(5):704–714. doi:10.1016/j.jpainsymman.2010.02.023
19. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741–749. doi:10.1001/jama.2009.1198
20. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. doi:10.1056/NEJMoa1000678
21. Casarett D, Pickard A, Bailey FA, et al. Do palliative consultations improve patient outcomes? J Am Geriatr Soc. 2008;56(4):593–599. doi:10.1111/j.1532-5415.2007.01610.x
22. Brumley RD, Enguidanos S, Cherin DA. Effectiveness of a home-based palliative care program for end-of-life. J Palliat Med. 2003;6(5):715–724. doi:10.1089/109662103322515220
23. Mrad C, Abougergi MS, One Step DB. Forward, two steps back: trends in aggressive inpatient care at the end of life for patients with stage IV lung cancer. J Oncol Pract. 2018. JOP1800515. doi:10.1200/JOP.18.00515
24. Cohen J, Beernaert K, Van Den Block L, et al. Differences in place of death between lung cancer and COPD patients: a 14-country study using death certificate data. NPJ Prim Care Respir Med. 2017;27(1):14. doi:10.1038/s41533-017-0017-y
25. Husted MG, Kriegbaum M, Kirkegaard N, Lange P. The use of healthcare resources in the last 3 years of life in patients with COPD and lung cancer in Denmark. A retrospective nationwide study. BMJ Support Palliat Care. 2014;4(2):146–151. doi:10.1136/bmjspcare-2013-000601
26. Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying individuals with physcian diagnosed COPD in health administrative databases. COPD. 2009;6(5):388–394.
27. Robles SC, Marrett LD, Clarke EA, Risch HA. An application of capture-recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol. 1988;41(5):495–501.
28. McLaughlin JR, Kreiger N, Marrett LD, Holowaty EJ. Cancer incidence registration and trends in Ontario. Eur J Cancer. 1991;27(11):1520–1524.
29. Hall S, Schulze K, Groome P, Mackillop W, Holowaty E. Using cancer registry data for survival studies: the example of the Ontario cancer registry. J Clin Epidemiol. 2006;59(1):67–76. doi:10.1016/j.jclinepi.2005.05.001
30. Brenner DR, Tammemagi MC, Bull SB, Pinnaduwaje D, Andrulis IL. Using cancer registry data: agreement in cause-of-death data between the Ontario cancer registry and a longitudinal study of breast cancer patients. Chronic Dis Can. 2009;30(1):16–19.
31. Kralj B. Measuring ‘rurality’ for purposes of health-care planning: an empirical measure for Ontario. Ont Med Rev. 2000;67:33-52.
32. Denny K, Davidson MJ. Area-based socio-economic measures as tools for health disparities research, policy and planning. Can J Public Health. 2012;103(8 Suppl 2):S4–S6.
33. Pefoyo AJ, Bronskill SE, Gruneir A, et al. The increasing burden and complexity of multimorbidity. BMC Public Health. 2015;15:415. doi:10.1186/s12889-015-1733-2
34. Mondor L, Maxwell CJ, Bronskill SE, Gruneir A, Wodchis WP. The relative impact of chronic conditions and multimorbidity on health-related quality of life in Ontario long-stay home care clients. Qual Life Res. 2016;25(10):2619–2632. doi:10.1007/s11136-016-1281-y
35. Mondor L, Maxwell CJ, Hogan DB, et al. Multimorbidity and healthcare utilization among home care clients with dementia in Ontario, Canada: a retrospective analysis of a population-based cohort. PLoS Med. 2017;14(3):e1002249. doi:10.1371/journal.pmed.1002230
36. Thavorn K, Maxwell CJ, Gruneir A, et al. Effect of socio-demographic factors on the association between multimorbidity and healthcare costs: a population-based, retrospective cohort study. BMJ Open. 2017;7(10):e017264. doi:10.1136/bmjopen-2017-017264
37. Petrosyan Y, Bai YQ, Kone Pefoyo AJ, et al. The relationship between diabetes care quality and diabetes-related hospitalizations and the modifying role of comorbidity. Can J Diabetes. 2017;41(1):17–25. doi:10.1016/j.jcjd.2016.06.006
38. Meffert C, Hatami I, Xander C, Becker G. Palliative care needs in COPD patients with or without cancer: an epidemiological study. Eur Respir J. 2015;46(3):663–670. doi:10.1183/09031936.00208614
39. Gershon AS, Maclagan LC, Luo J, et al. End of life strategies among patients with advanced Chronic Obstructive Pulmonary Disease (COPD). Am J Respir Crit Care Med. 2018;198(11):1389-1396. doi:10.1164/rccm.201803-0592OC
40. Brown CR, Hsu AT, Kendall C, et al. How are physicians delivering palliative care? A population-based retrospective cohort study describing the mix of generalist and specialist palliative care models in the last year of life. Palliat Med. 2018. 269216318780223. doi:10.1177/0269216318780223
41. Isenberg S, Hsu A, Spruin S, Budhwani S, Gill A, Goldman R, Tanuseputro P. Evaluating the Impact and Costs of Home-Based Palliative Care at the System Level. The Journal of Pain and Symptom Management. 2019;57(2):410. doi:10.1016/j.jpainsymman.2018.12.122.
42. Wodchis WP, Bushmeneva K, Nikitovic M, McKillop I Guidelines on person-level costing using administrative databases in Ontario. Working Paper Series Toronto: Health System Performance Research Network. 2013;1.
43. Gregori D, Petrinco M, Bo S, Desideri A, Merletti F, Pagano E. Regression models for analyzing costs and their determinants in health care: an introductory review. Int J Qual Health Care. 2011;23(3):331–341. doi:10.1093/intqhc/mzr010
44. Halpin DMG. Palliative care for people with COPD: effective but underused. Eur Respir J. 2018;51:2. doi:10.1183/13993003.02645-2017
45. Brown CE, Jecker NS, Curtis JR. Inadequate palliative care in chronic lung disease. An issue of health care inequality. Ann Am Thorac Soc. 2016;13(3):311–316. doi:10.1513/AnnalsATS.201510-666PS
46. Rocker GM, Dodek PM, Heyland DK. Canadian researchers at the end of life N. Toward optimal end-of-life care for patients with advanced chronic obstructive pulmonary disease: insights from a multicentre study. Can Respir J. 2008;15(5):249–254. doi:10.1155/2008/369162
47. Boland J, Martin J, Wells AU, Ross JR. Palliative care for people with non-malignant lung disease: summary of current evidence and future direction. Palliat Med. 2013;27(9):811–816. doi:10.1177/0269216313493467
48. Nickerson JW. A needs assessment to determine the need for respiratory therapy in complex continuing care: a methods paper. Can J Respir Ther. 2015;51(3):55–59.
49. Wichmann AB, van Dam H, Thoonsen B, Boer TA, Engels Y, Groenewoud AS. Advance care planning conversations with palliative patients: looking through the GP’s eyes. BMC Fam Pract. 2018;19(1):184. doi:10.1186/s12875-018-0787-5
50. Rocker GM, Simpson AC, Horton R. Palliative care in advanced lung disease: the challenge of integrating palliation into everyday care. Chest. 2015;148(3):801–809. doi:10.1378/chest.14-2593
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


