Back to Journals » International Journal of General Medicine » Volume 14
Encephalopathy Induced by Preventive Administration of Acyclovir in a Man with Symptomatic Multiple Myeloma and Renal Dysfunction
Authors Sugimoto K, Kenzaka T , Sugimoto R, Kitao A, Akita H
Received 10 November 2020
Accepted for publication 26 January 2021
Published 11 February 2021 Volume 2021:14 Pages 413—417
DOI https://doi.org/10.2147/IJGM.S291320
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Kazuma Sugimoto,1 Tsuneaki Kenzaka,1,2 Ryu Sugimoto,1 Akihito Kitao,3 Hozuka Akita1
1Department of Internal Medicine, Hyogo Prefectural Tamba Medical Center, Tamba, Hyogo, Japan; 2Division of Community Medicine and Career Development, Kobe University Graduate School of Medicine, Kobe, Hyogo, Japan; 3Division of Medical Oncology/Hematology, Kobe University Graduate School of Medicine, Kobe, Hyogo, Japan
Correspondence: Tsuneaki Kenzaka
Division of Community Medicine and Career Development, Kobe University Graduate School of Medicine, 2-1-5, Arata-cho, Hyogo-ku, Kobe, Hyogo, 652-0032, Japan
Tel +81 78 382 6732
Fax +81 78 382 6283
Email [email protected]
Background: Acyclovir (ACV) neurotoxicity is a neuropsychiatric condition induced by the anti-herpetic drugs ACV and valacyclovir (VACV). It is presumed that elevated blood levels of ACV and its metabolite 9-carboxymethoxymethylguanine are involved in the development of ACV-induced encephalopathy; age and renal dysfunction are risk factors. Here, we report a case of encephalopathy caused by the administration of VACV for herpes zoster prophylaxis in a patient with renal dysfunction owing to multiple myeloma.
Case Presentation: Renal dysfunction was diagnosed in a 70-year-old man visiting our hospital for a medical checkup. His creatinine clearance rate was 8 mL/min. He was diagnosed with symptomatic multiple myeloma, and bortezomib/dexamethasone (BD) therapy for multiple myeloma and VACV for herpes zoster prophylaxis were initiated. We administered 500 mg/day of VACV three times a week, a lower dosage than recommended, after adjusting for his renal impairment. His renal function was monitored twice per week during therapy. During the second course of BD therapy, 6 weeks after starting treatment, he was hospitalized owing to impaired consciousness (Glasgow Coma Scale score: E2, V4, M4), and his BD and VACV therapy were suspended. Brain magnetic resonance imaging and cerebrospinal fluid analysis showed no abnormalities. Three days after discontinuing BD and VACV therapy, his consciousness recovered completely, and impaired consciousness did not recur after resuming BD therapy. His clinical diagnosis was thus ACV-induced encephalopathy.
Conclusion: VACV is often prescribed to patients with multiple myeloma receiving BD therapy to prevent herpes zoster. ACV-induced encephalopathy is commonly observed in patients with renal dysfunction; especially among patients with multiple myeloma with Bence–Jones proteinuria, renal tubules are easily damaged and plasma ACV concentrations are likely to increase and induce ACV-induced encephalopathy. Careful monitoring of the level of consciousness is necessary during preventive ACV therapy in patients with renal dysfunction.
Keywords: acyclovir neurotoxicity, valacyclovir, herpes zoster, Bence–Jones proteinuria
Introduction
Acyclovir (ACV) neurotoxicity is a neuropsychiatric condition induced by the administration of the anti-herpetic drugs ACV and valacyclovir (VACV).1 VACV is the prodrug of ACV. Usually, various neuropsychiatric symptoms, such as disturbance of consciousness, tremor, and myoclonus, occur within 2 days after initiating the therapy.1–3 Hallucinations are also common.1–3 It is presumed that elevated blood levels of ACV and its metabolite, 9-carboxymethoxymethylguanine (CMMG), are involved in the development of ACV-induced encephalopathy4 and that age and renal dysfunction are risk factors.5
Bortezomib/dexamethasone (BD) therapy is one of the standard regimens for patients with symptomatic multiple myeloma who have severe renal impairment.6 In bortezomib-containing regimens, low-dose oral ACV is recommended for herpes zoster prophylaxis.7,8
We present a case of encephalopathy caused by the administration of VACV for herpes zoster prophylaxis in a patient with renal dysfunction due to multiple myeloma.
Case Presentation
Renal dysfunction was diagnosed in a 70-year-old man who visited our hospital for a medical checkup. His serum creatinine level and creatinine clearance rate were 8.78 mg/dL (normal range: 0.53–1.02 mg/dL) and 8 mL/min (normal range: 80–180 mL/min), respectively. He was diagnosed with Bence–Jones protein λ-type multiple myeloma based on the presence of 40% plasma cells in his bone marrow (10% or more of plasma cells is considered definitive of the disease) and Bence–Jones proteinuria (M proteinuria of 4.8 g/day). Additionally, the diagnosis of symptomatic multiple myeloma (International Staging System stage 3) was based on the presence of renal dysfunction. Renal biopsy revealed cast nephropathy known as myeloma kidney, in which large amounts of Bence–Jones proteins formed casts that blocked the tubules (Figure 1). BD therapy was initiated with concurrent VACV for herpes zoster prophylaxis. We administered a reduced dose VACV of 500 mg three times a week because of the patient’s renal impairment, based on the drug information on VACV provided in the UpToDate database.9 His renal function was monitored twice per week during therapy. Six weeks later, during his second course of BD therapy, the patient was hospitalized because of impaired consciousness. He displayed no other symptoms during hospitalization.
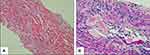 |
Figure 1 Histology of kidney tissue showing myeloma cast nephropathy. (A) Hematoxylin and eosin stain (magnification ×200). (B) Periodic acid-Schiff stain (magnification ×400). |
On admission, his vital signs were as follows: Glasgow Coma Scale score, E2, V4, M4; body temperature, 36.5°C; blood pressure, 145/79 mmHg; pulse rate, 73 beats/min; respiratory rate, 15 breaths/min; and SpO2, 96%. His vital signs were normal, and there were no remarkable neurological abnormalities except for disturbance of consciousness. Table 1 summarizes the results of patient’s blood test on admission. The results, including renal function, were unchanged. Brain magnetic resonance imaging and cerebrospinal fluid analysis—cell counts 1/µL, protein 40 mg/dL, glucose 98 mg/dL, reference blood glucose level 125 mg/dL—revealed no abnormalities. There was no new electrolyte, endocrine hormone abnormality, or suggestion of epilepsy. Therefore, we suspected drug-induced disturbance of consciousness and suspended the BD and VACV therapy. Three days after discontinuing the drugs, his level of consciousness returned to normal, and the BD therapy was restarted after 20 days of drug interruption. The Naranjo score10 for estimating the probability of adverse drug reactions was 7 points. In this scoring system, ≥ 9 points indicate “high probability for adverse reactions” and 5–8 points indicate “probability for adverse reactions”.10 In all Japan, the laboratories do not have facilities to measure ACV/CMMG levels. Though his blood level of ACV could not be measured, the clinical diagnosis was ACV neurotoxicity based on his response to the suspension of the therapy, the high Naranjo score, and the lack of other contributing factors. We theorized that ACV blood levels gradually increased over the long-term administration of oral VACV owing to renal dysfunction. Figure 2 illustrates his clinical course.
 |
Table 1 Results of the Patient’s Admission Blood Tests |
The patient underwent 9 cycles of BD therapy and achieved complete remission. We administered 250 mg of famciclovir for herpes zoster prophylaxis, three times a week, between cycles 4 to 9. One year after the end of treatment, he remained in remission. His creatinine level recovered and remained stable at 4–5 mg/dL in response to the treatment. He did not exhibit any sequelae of ACV encephalopathy.
Discussion
We presented a case of ACV-induced encephalopathy caused by the administration of VACV for herpes zoster during the treatment of multiple myeloma in a man with renal dysfunction. To the best of our knowledge, this is the first report of ACV neurotoxicity in a patient taking low-dose VACV for herpes zoster prophylaxis. This case illustrates that ACV or VACV should be used with caution in patients with myeloma-associated renal dysfunction, even if used in low doses for herpes zoster prophylaxis.
In all Japan, the laboratories do not have facilities to measure ACV/CMMG levels. However, we diagnosed ACV-induced encephalopathy based on his clinical course, the high Naranjo score, the lack of other contributing factors. ACV or VACV can cause renal tubular obstruction secondary to crystal-induced nephropathy, and direct action of the ACV aldehyde can cause acute kidney injury; these can lead to increased blood concentrations of ACV and CMMG and cause encephalopathy.2,11 In this case, our patient exhibited Bence–Jones proteinuria. Increased excretion of Bence–Jones proteins may have damaged the tubular epithelium or formed casts that blocked the renal tubules, leading to myeloma cast nephropathy. It is the most common cause of myeloma-associated renal injury and may cause renal dysfunction.12,13 Though the renal dysfunction in our patient was stable at a low level, we theorized that long-term preventive oral VACV therapy gradually led to increased plasma concentrations of ACV and CMMG, resulting in encephalopathy.
In this case, the VACV prophylaxis resulted in ACV-induced encephalopathy, even though we administered it at a dose lower than the recommended dose for patients with renal dysfunction. ACV-induced encephalopathy has been observed in patients administered with extremely high doses (10 mg/kg every hour) of the drug or in cases of renal failure without dose adjustment.4 It has often been reported in elderly people and patients with impaired renal function,5 but it has occurred in patients without renal dysfunction and young patients.14 In all cases, ACV-induced encephalopathy developed owing to the ACV or VACV treatment for herpes simplex or zoster virus. There were no reports that ACV-induced encephalopathy developed with prophylactic administration. Myeloma kidney with Bence–Jones proteinuria causes kidney renal tubular damage, which is disproportionate to the degree of renal impairment suggested by the creatinine level. Thus, it is presumed that it inhibits the excretion of drugs, including ACV, in renal tubules, resulting in an elevated blood concentration. It is difficult to measure ACV and CMMG blood levels. Therefore, even with the recommended level of ACV or VACV prophylaxis for renal impairment, it is not possible to predict ACV neurotoxicity, such as impaired consciousness and impaired renal function.
In conclusion, among patients with multiple myeloma with Bence–Jones proteinuria, the renal tubules are easily damaged, and the plasma concentration of ACV is likely to increase and induce ACV neurotoxicity. Careful monitoring of the level of consciousness is necessary during preventive ACV therapy in patients with renal dysfunction.
Abbreviations
ACV, acyclovir; BD, bortezomib/dexamethasone; CMMG, 9-carboxymethoxymethylguanine; VACV, valacyclovir.
Data Sharing Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Consent for Publication
Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
Author Contributions
All authors contributed to the conception, study design, execution, acquisition of data, analysis and interpretation, drafting and revising the article, and critically reviewing the article; provided final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Rashiq S, Briewa L, Mooney M, Giancarlo T, Khatib R, Wilson FM. Distinguishing acyclovir neurotoxicity from encephalomyelitis. J Intern Med. 1993;234:507–511. doi:10.1111/j.1365-2796.1993.tb00785.x
2. Asahi T, Tsutsui M, Wakasugi M, et al. Valacyclovir neurotoxicity: clinical experience and review of the literature. Eur J Neurol. 2009;16:457–460. doi:10.1111/j.1468-1331.2008.02527.x
3. Adair JC, Gold M, Bond RE. Acyclovir neurotoxicity: clinical experience and review of the literature. South Med J. 1994;87:1227–1231. doi:10.1097/00007611-199412000-00006
4. Chowdhury MA, Derar N, Hasan S, Hinch B, Ratnam S, Assaly R. Acyclovir-induced neurotoxicity: a case report and review of literature. Am J Ther. 2016;23:e941–e943. doi:10.1097/MJT.0000000000000093
5. Das V, Peraldi MN, Legendre C. Adverse neuropsychiatric effects of cytomegalovirus prophylaxis with valaciclovir in renal transplant recipients. Nephrol Dial Transplant. 2006;21:1395–1401. doi:10.1093/ndt/gfk031
6. Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 Phase III trial. J Clin Oncol. 2010;28:4621–4629. doi:10.1200/JCO.2009.27.9158
7. Chanan-Khan A, Sonneveld P, Schuster MW, et al. Analysis of herpes zoster events among bortezomib-treated patients in the phase III APEX study. J Clin Oncol. 2008;26:4784–4790. doi:10.1200/JCO.2007.14.9641
8. San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi:10.1056/NEJMoa0801479
9. UpToDate®. Valaciclovir: drug information. Available from: https://www.uptodate.com/contents/valacyclovir-drug-information?search=valacyclovir&topicRef=8337&source=see_link#F50991799.
10. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi:10.1038/clpt.1981.154
11. Sacchetti D, Alawadhi A, Albakour M, Rapose A. Herpes zoster encephalopathy or acyclovir neurotoxicity: a management dilemma. BMJ Case Rep. 2014;2014:bcr2013201941. doi:10.1136/bcr-2013-201941
12. Hutchison CA, Batuman V, Behrens J, et al. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Rev Nephrol. 2011;8:43–51. doi:10.1038/nrneph.2011.168
13. Leung N, Rajkumar SV. Renal manifestations of plasma cell disorders. Am J Kidney Dis. 2007;50:155–165. doi:10.1053/j.ajkd.2007.05.007
14. Izumo A, Sakai K, Tamura Y. Acyclovir-induced neurotoxicity in an elderly patient: report of a case. J Japan Soc Emerg Med. 2017;20:763–768.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

