Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 11
Enamel demineralization around metal and ceramic brackets: an in vitro study
Authors Almosa NA , Sibai BS, Rejjal OA, Alqahtani N
Received 15 October 2018
Accepted for publication 14 January 2019
Published 28 February 2019 Volume 2019:11 Pages 37—43
DOI https://doi.org/10.2147/CCIDE.S190893
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Christopher E. Okunseri
Naif A Almosa,1 Beshayer S Sibai,2 Olla A Rejjal,3 Nasser Alqahtani1
1Department of Pediatric Dentistry and Orthodontics, King Saud University, Riyadh, Saudi Arabia; 2National Guard Hospital, Dammam, Saudi Arabia; 3State University of New York, Buffalo, NY, USA
Aim: To evaluate the degree of enamel demineralization of teeth bonded with ceramic and metal brackets.
Materials and methods: In this cross-sectional experimental in vitro study, 60 extracted human premolar teeth were selected according to the experimental criteria. They were divided into three groups; 20 premolar teeth in each group. Teeth in group 1 were bonded with “ceramic brackets”, and teeth in group 2 were bonded with “metal brackets”, while teeth in group 3 served as the “control group” without any brackets. Teeth in all groups were then immersed in demineralization media, de-bonded, sectioned into three parts (proximal 1, middle, and proximal 2), and evaluated to determine the level of enamel demineralization under a Scanning Electron Microscope.
Results: On tooth level, the results show that the control group has significantly less enamel demineralization compared to the other two experimental groups, with mean values of 145.3 µm and 192.7 µm, respectively (P=0.000). The mean value of enamel demineralization in the metal group is 55.93 µm, compared to 72.55 µm in the ceramic group, which is significantly less (P≤0.05), while there is no difference between the control and metal group with regard to enamel demineralization. On section level, the control group has significantly less enamel demineralization in all three sections compared to the ceramic group, while a significant difference is found in one of the proximal sections when compared with the metal group. Moreover, the ceramic group has significantly higher enamel demineralization in the middle section compared to the metal group (73.54 µm, 46.5 µm, respectively) (P=0.000), while there is no statistical significant difference between the two experimental groups in proximal sections.
Conclusion: In vitro, non-bonded teeth show least demineralization compared to the bonded teeth. Teeth bonded with ceramic brackets show significantly higher enamel demineralization compared to teeth bonded with metal brackets.
Keywords: ceramic, demineralization, metal, orthodontic brackets, decalcification, white spot lesions, orthodontic material, adhesive
Introduction
Plaque accumulation and demineralization around orthodontic brackets has been the scope of many studies. Recently, dental caries and periodontal diseases are known to be one of the most common diseases around the world. The tooth demineralization process, which is the removal of hard minerals from the tooth surface, leading to dental caries, can be affected by many factors, primarily plaque.1 The composition of the bacteria mainly constitutes of Streptococcus Mutans, involved in caries process initiation, and Lactobacilli, which flourish in the carious environment and aid in caries progression.2 Other etiological factors displayed in a model of overlapping circles presented in the 1960s include a susceptible tooth surface, dietary intake, time, fluoride, salivary flow, and social and demographic factors.2
Many studies have shown that proliferation of facultative bacteria occurs around orthodontic brackets as the appliance alters the microbial environment around it. Plaque accumulation is increased around the brackets, which can lead to enamel demineralization.3 Post-orthodontic appliance removal, clinically detectable areas of enamel white spots can be recognized on teeth surfaces. In most teeth, enamel demineralization can be initiated after 4 weeks of brackets bonding, ranging from slight loss of translucency to distinct white spots.4 These changes are likely to be observed at the junction between the bonding resin and the enamel, gingival to the bracket base.5
The differences between metal and ceramic brackets have been studied in multiple aspects comparing different characteristics between the two types.6 Regarding plaque accumulation, some studies have proved no significance between both brackets,7 while Brusca et al8 reported the metal brackets have less bacterial accumulation compared to ceramic brackets.
De-bonding is the procedure of removing the brackets, along with adhesive resin from the tooth surface.9 De-bonding of orthodontic brackets can be done in several methods, and each method has its own effect on enamel resistance to demineralization after the de-bonding.10 Enamel loss can occur during bracket removal, which depends on bracket material and the de-bonding method. Metal brackets have shown less risk in enamel fracture than ceramic brackets. In addition, the de-bonding method has a great effect on enamel as well. It has been reported that high-speed tungsten carbide bur and ultrasonic scaler have shown the most detrimental effect on the tooth surface.11
Demineralization detection and quantification should ideally be non-invasive, reproducible, accurate, and simple to use.12 One of the trustful tools to detect enamel demineralization is the Scanning Electron Microscope (SEM), which is characterized by higher energy beam electrons that are scattered to display information reflecting subsurface changes in the composition of the material meant to be measured. To our knowledge, there is no study investigating the difference between metal and ceramic bracket materials in the aspect of enamel demineralization under SEM. Thus, the scope of this study is to investigate enamel demineralization for teeth bonded with ceramic and metal brackets using SEM. The null hypothesis is that there is no difference between teeth bonded with enamel and ceramic brackets with regard to enamel demineralization.13
Materials and methods
This in-vitro cross-sectional study was approved by the Ethics Committee at King Saud University, College of Dentistry Research Center, Riyadh, Saudi Arabia (Reg. No. IR 0220). It was carried out at King Khaled University Dental Hospital, and comprised of 60 human premolar teeth extracted for orthodontic purposes. The inclusion criteria included teeth with intact buccal surfaces and free from caries, restorations, cracks, and fluorosis. Teeth with white spot lesions, hypoplastic enamel, anatomical anomalies, and endodontically treated teeth were excluded. A written informed consent form was provided to all patients whose teeth were used in this research.
The 60 premolar teeth were divided into three groups, 20 in each group. In the first group (G1); 20 premolar teeth were bonded with APC Plus Adhesive Ceramic brackets (APC™ PLUS Adhesive Coated Appliance System, 3M Unitek, Monrovia, CA, USA), in the second group (G2); 20 premolar teeth were bonded with APC Plus Adhesive Metal brackets (APC™ PLUS Adhesive Coated Appliance System, 3M Unitek), while in the third group (G3); 20 premolars were not bonded with any brackets, comprising the “control group”. Immediately after extraction, all teeth were stored in double deionized water solution supersaturated with 1% thymol and maintained at room temperature before bonding procedures.14
All included teeth were polished with a rubber cup for 20 seconds before the beginning of the experiment. The brackets positions on the buccal surfaces of teeth in G1 and G2 were etched with 35% phosphoric acid (Unitek Etching Gel, 3M Unitek) for 15 seconds, then rinsed with water for 10 seconds and dried. After that, each buccal surface of teeth in G1 and G2 was coated with a thin uniform layer of bonding agent (Transbond XT, 3M Unitek) with a disposable micro-brush, then, gently air dried and light cured according to manufacturer’s instructions.14 Brackets were then positioned properly centered mesio-distally and occluso-gingivally, parallel to the long axis of the tooth. The bracket was pressed lightly in place, and excess cement was removed with a plastic instrument (3M™ ESPE™ Composite Placement Instruments, 3M Unitek). Light cure (Elipar™ DeepCure-S LED Curing Light, 3M Unitek) was used after bracket placement for 20 seconds.1 Then, all teeth in all three groups were immersed individually in 40 mL of standard 500 mL demineralization solution at pH 4.5 for 7 days at 37°C. The demineralization solution was prepared by weighting 0.166 g CaCl2.2H2O and adding it to 200 mL deionized H2O and dissolving in a 500 mL Erlenmeyer flask. Then, 0.154 g CH3COONH4, 0.204 g Na2HPO4 and 2H2O, 25 mL glacial acetic acid were added to the solution. The mixing procedure was performed under the chemical fume hood.15
After 7 days of placing all teeth in demineralization solution, they were removed and the brackets were de-bonded for teeth in G1 and G2 using ETM 346 plier (ETM Narrow/Wide Direct Bond Removing Orthodontic Plier, Ormco, Orange, CA, USA), and the excess resin cement was removed by low speed hand piece with round carbide latch-type bur. After that, all teeth were rinsed under running water and stored in distilled water until the time of sectioning. One and the same operator for standardization purposes performed bonding and de-bonding procedures.
At the physical laboratory, each tooth in all three groups was mounted in putty material using Genie VPS Impression Putty Rapid Set (Sultan Healthcare, York, PA, USA), and sectioned with a low speed double-sided diamond disk (0.6 mm) with continuous water irrigation (ISOMET 2000 Precisions Saw, Buehler, Lake Bluff, Il, USA), as shown in Figure 1. Each tooth was cut bucco-lingually into three parts; proximal 1 (P1), middle (M), and proximal 2 (P2) (Figure 2). Then, a total of 180 sections were mounted on stubs and prepared for gold plating. Gold coating is performed before the reading under SEM to enhance the conductive layer of metal on the sample, which inhibits charging, reduces thermal damage, and improves the secondary electron signal required for topographic examination in the SEM. After gold plating, each section was studied under the SEM (JEOL JSM-6360LV, Tokyo, Japan), which was operating at 15 KV, and a magnification of ×100 (Figure 3) to measure the depth of demineralization from the buccal enamel surface to the deepest point in each section in microns using image analysis software (SMile ViewTM, JEOL Ltd, Tokyo, Japan).
  | Figure 1 Low speed, double-sided diamond disk (ISOMET 2000 Precisions SAW). |
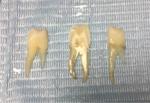  | Figure 2 Sectioned tooth (proximal 1, middle, proximal 2). |
  | Figure 3 Sample of a sectioned tooth bonded with (A) ceramic, (B) metal, and (C) control under SEM. Abbreviation: SEM, Scanning Electron Microscopy. |
Statistical analyses
Data were entered into MS Excel and analyzed using SPSS 21.0 version statistical software. Descriptive statistics (mean, SD, and range) were used to describe the enamel demineralization values. One-way analysis of variance was used to compare the mean values in relation to the three groups, followed by Tukey’s test for multiple comparisons of mean values to compare the enamel demineralization among different segments. A P-value of ≤0.05 was used to indicate the statistical significance of results. Sample size was calculated: Alpha=0.05, assumed SD=40, power=0.9; thus, the sample size for each group should be at least 20.
Ethical statement
The study protocol was approved by the Research and Ethics Committee at the College of Dentistry, King Saud University, Riyadh, Saudi Arabia (registration number IR 0220).
Results
Tooth level comparison
To compare between the control group (G3) and the experimental groups (G1 and G2) on tooth level, all sections were merged together for G3 vs G1+G2. The results reveal that the experimental groups have significantly higher enamel demineralization compared to the control group on tooth level (P≤0.001) (Table 1). Moreover, the metal group (G2) has significantly less enamel demineralization compared to the ceramic group (G1) on tooth level (P≤0.001) (Table 2).
  | Table 1 Comparing enamel demineralization between experimental groups and the control group on tooth level |
  | Table 2 Comparing enamel demineralization between metal and ceramic groups on tooth level |
Segment level comparison
Figure 4 reveals the difference in enamel demineralization between the control group and the ceramic group on each section (P1, M, and P2). The control group has significantly less enamel demineralization compared to the ceramic group on all segments (P≤0.05) (P1, M, and P2). In addition, Figure 5 shows the difference in enamel demineralization between the control group and the metal group on each section (P1, M, and P2). There is a statistically significant difference between the two groups in one of the proximal sides only.
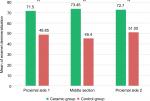  | Figure 4 Comparing enamel demineralization between ceramic and control groups in three different sections. Note: **Statistically significant difference. |
  | Figure 5 Comparing enamel demineralization between the control and metal groups in three different sections. Note: *Statistically significant difference. |
The results show that the ceramic group (G1) has significantly higher enamel demineralization (P≤0.001) in the middle (M) section compared to the metal group, while there is no statistical significant difference between the two experimental groups in proximal sections, as shown in Figure 6.
  | Figure 6 Comparing enamel demineralization between the ceramic and metal groups in three different sections. Note: **Statistically significant difference. |
Discussion
Caries control and limiting tooth demineralization has been the main concern of most dentists around the world. Preventing the spread of disease is more effective than treatment in most cases. Enamel demineralization has a high prevalence among orthodontic patients with fixed orthodontic appliances,16 and it has been proved that increased plaque accumulation occurs around orthodontic brackets, which in return can increase the susceptibility for caries.3 The focus of most recent studies is how to decrease the negative effect of orthodontic treatment on the tooth surface. In order to prevent or decrease the enamel demineralization around fixed orthodontic brackets, several improvements and innovations have been developed over the past few years. Maintaining good oral hygiene, including proper brushing technique and fluoride containing dentifrices, is effective in reducing plaque accumulation around the brackets. Moreover, regular fluoride application can also help in limiting surface demineralization during orthodontic treatment.6 Comparing different de-bonding techniques, bracket removal with slow speed tungsten, carbide bur, and de-bonding pliers have been proved to be the least effective on tooth enamel.7
Regarding the difference between metal and ceramic brackets, in the scope of this study, it has been proved that ceramic brackets have a more porous and less smooth structure, which exhibits higher adherence of microorganisms. This result is in agreement with the report of Ahn et al,17 who stated that metal brackets have less plaque accumulation compared to ceramic ones.
In contrast, in another study conducted by Lindel et al,18 teeth bonded with metal and ceramic brackets were compared in the mean of differences in long-term biofilm formation. Stainless steel brackets showed a significantly higher amount of biofilm around them compared to ceramic brackets in different teeth locations (incisor, canine, and second premolar). The biofilm concentration was highest in mesial and distal sides of the stainless steel brackets, while it was occlusal and gingival around ceramic brackets. This has been explained by the fact that bracket surface alters its characteristics over time due to wear from food and drink, oral hygiene, or corrosion. As a result, roughness can be increased by promoting the attachment of long-term biofilm.18
From a restorative point of view, microleakage can occur at the tooth–restoration interface due to the escape and leakage of fluids and bacteria at the junction, which in return increases the likelihood of recurrent caries and postoperative sensitivity. In orthodontics, microleakage will most probably cause the formation of white-spot lesions on the enamel at the adhesive–enamel interface. Arhun et al19 found that microleakage is more likely to occur around the ceramic brackets compared to metal brackets. Lindel et al has supported the present study, as he found that the result in enamel demineralization occurring in the ceramic brackets is higher than that in the metal ones. The study indicates that metal brackets have lower potential for bacterial accumulation (Streptococcus mutans) compared to plastic and ceramic brackets.18
O’Reilly and Featherstone2 state that enamel demineralization is likely to occur around orthodontic brackets after 1 month of bracket placement, even with the use of fluoride dentifrices. This finding supports the result that indicates that bonded teeth have a higher tendency for enamel demineralization in comparison with control non-bonded teeth.
In this study, the depth of enamel demineralization around ceramic brackets has shown the highest numbers compared to metal brackets and control teeth. On the other hand, control teeth have shown the least scores. These results explain how the presence or absence of orthodontic brackets can affect the enamel surface significantly. The absence of the brackets in the control group has reduced the adherence of the demineralization media, which in return decreased the detrimental effect on the tooth surface.
Teeth bonded with metal brackets have shown less demineralization compared to teeth bonded with ceramic brackets. The nature of the esthetic brackets shows a rougher surface compared to the metal smooth surface.19 As a result, demineralization media concentration around the ceramic brackets will be increased, which will lead to a higher demineralization depth. It has been also discussed that ceramic porosity is higher, which could also be another reason when comparing it to metal.18
Conclusion
Non-bonded teeth have the least enamel demineralization compared to bonded teeth. Teeth bonded with ceramic brackets have shown significantly higher enamel demineralization compared to teeth bonded with metal brackets.
Acknowledgment
The authors would like to thank the College of Dentistry Research Center and Deanship of Scientific Research at King Saud University, Saudi Arabia for supporting this research project.
Disclosure
The authors report no conflicts of interest in this work.
References
Al Maaitah EF, Adeyemi AA, Higham SM, Pender N, Harrison JE. Factors affecting demineralization during orthodontic treatment: a post-hoc analysis of RCT recruits. Am J Orthod Dentofacial Orthop. 2011;139(2):181–191. | ||
O’Reilly MM, Featherstone JD. Demineralization and remineralization around orthodontic appliances: an in vivo study. Am J Orthod Dentofacial Orthop. 1987;92(1):33–40. | ||
Ogaard B, Rølla G, Arends J. Orthodontic appliances and enamel demineralization. Part 1. Lesion development. Am J Orthod Dentofacial Orthop. 1988;94(1):68–73. | ||
Gwinnett AJ, Ceen RF. Plaque distribution on bonded brackets: a scanning microscope study. Am J Orthod. 1979;75(6):667–677. | ||
Ajlouni R, Bishara SE, Oonsombat C, Soliman M, Laffoon J. The effect of porcelain surface conditioning on bonding orthodontic brackets. Angle Orthod. 2005;75(5):858–864. | ||
Brandão GA, Pereira AC, Brandão AM, de Almeida HA, Motta RR. Does the bracket composition material influence initial biofilm formation? Indian J Dent Res. 2015;26(2):148–151. | ||
Brusca MI, Chara O, Sterin-Borda L, Rosa AC. Influence of different orthodontic brackets on adherence of microorganisms in vitro. Angle Orthod. 2007;77(2):331–336. | ||
Zachrisson BU, Årthun J. Enamel surface appearance after various debonding techniques. Am J Orthod. 1979;75(2):121–137. | ||
Cehreli ZC, Lakshmipathy M, Yazici R. Effect of different splint removal techniques on the surface roughness of human enamel: a three-dimensional optical profilometry analysis. Dent Traumatol. 2008;24(2):177–182. | ||
Hosein I, Sherriff M, Ireland AJ. Enamel loss during bonding, debonding, and cleanup with use of a self-etching primer. Am J Orthod Dentofacial Orthop. 2004;126(6):717–724. | ||
Yap J, Walsh LJ, Naser-Ud Din S, Ngo H, Manton DJ. Evaluation of a novel approach in the prevention of white spot lesions around orthodontic brackets. Aust Dent J. 2014;59(1):70–80. | ||
Nelson DG. Backscattered electron imaging of partially-demineralized enamel. Scanning Microsc. 1990;4(1):31–41; discussion 42. | ||
Ten Cate JM, Duijsters PP. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res. 1982;16(3):201–210. | ||
Kumar VL, Itthagarun A, King NM. The effect of casein phosphopeptide-amorphous calcium phosphate on remineralization of artificial caries-like lesions: an in vitro study. Aust Dent J. 2008;53(1):34–40. | ||
Moura JS, Rodrigues LK, Del Bel Cury AA, Lima EM, Garcia RM. Influence of storage solution on enamel demineralization submitted to pH cycling. J Appl Oral Sci. 2004;12(3):205–208. | ||
Benkaddour A, Bahije L, Bahoum A, Zaoui F. Orthodontics and enamel demineralization: clinical study of risk factors. Int Orthod. 2014;12(4):458–466. | ||
Ahn SJ, Lee SJ, Lim BS, Nahm DS. Quantitative determination of adhesion patterns of cariogenic streptococci to various orthodontic brackets. Am J Orthod Dentofacial Orthop. 2007;132(6):815–821. | ||
Lindel ID, Elter C, Heuer W, et al. Comparative analysis of long-term biofilm formation on metal and ceramic brackets. Angle Orthod. 2011;81(5):907–914. | ||
Arhun N, Arman A, Çehreli SB, Arıkan S, Karabulut E, Gülşahi K. Microleakage beneath ceramic and metal brackets bonded with a conventional and an antibacterial adhesive system. Angle Orthod. 2006;76(6):1028–1034. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
