Back to Journals » Infection and Drug Resistance » Volume 17
EMR Combined with CRB-65 Superior to CURB-65 in Predicting Mortality in Patients with Community-Acquired Pneumonia
Authors Sun Y , Wang H, Gu M , Zhang X , Han X , Liu X
Received 24 October 2023
Accepted for publication 29 January 2024
Published 8 February 2024 Volume 2024:17 Pages 463—473
DOI https://doi.org/10.2147/IDR.S443045
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Yi Sun,1,2 Hong Wang,3 Minghao Gu,4 Xingyu Zhang,5 Xiudi Han,1, Xuedong Liu,1
1Department of Respiratory and Critical Care Medicine, Qingdao Municipal Hospital Group, Qingdao, Shandong Province, 266000, People’s Republic of China; 2School of Clinical Medicine, Shandong Second Medical University, Weifang, Shandong Province, 261000, People’s Republic of China; 3Hospital-Acquired Infection Control Department, Qingdao Municipal Hospital Group, Qingdao, Shandong Province, 266000, People’s Republic of China; 4School of Medicine, Qingdao University, Qingdao, Shandong Province, 266000, People’s Republic of China; 5Human Resources Department, Qingdao Municipal Hospital Group, Qingdao, Shandong Province, 266000, People’s Republic of China
Correspondence: Xuedong Liu; Xiudi Han, Email [email protected]; [email protected]
Background: Data about eosinophil-to-lymphocyte ratio (ELR) and eosinophil-to-monocyte ratio (EMR) in patients with community-acquired pneumonia (CAP) are rare. We aimed to evaluate the role of EMR and ELR in predicting disease severity and mortality in patients with CAP.
Methods: A total of 454 patients (76 with severe CAP (SCAP), 378 with non-SCAP) were enrolled from November 18, 2020, and November 21, 2021. Laboratory examination on day 1 after admission was measured. The ELR and EMR values were calculated for patients. Propensity score matching (PSM) was performed to balance potential confounding factors. Binary logistic regression model was fitted to identify the potential risk factors for disease severity and Cox proportional hazards regression model analysis for mortality in CAP. Receiver operating characteristic (ROC) analysis was performed to distinguish disease severity and mortality.
Results: EMR and ELR at admission were significantly lower in SCAP patients than in non-SCAP patients (P< 0.001). EMR < 0.018 ([OR] = 12.104, 95% CI: 4.970– 29.479), neutrophil (NEU) ([OR]=1.098, 95% CI:1.005– 1.199), and age ([OR]=1.091, 95% CI:1.054– 1.130) were independent risk factors for disease severity of CAP. EMR < 0.032 ([HR] = 5.816, 95% CI: 1.704– 9.848) was an independent predictor of in-hospital mortality. Combining EMR or ELR with CRB-65 improved the overall accuracy of disease severity prediction (AUC from 0.894 to 0.937), the same as CURB-65. The area under the curve of EMR (AUC=0.704; 95% CI: 0.582– 0.827) to predict in-hospital mortality was higher than that of CURB-65 (AUC=0.619; 95% CI: 0.484– 0.754). Otherwise, EMR combined with CRB-65 (AUC=0.721; 95% CI: 0.592– 0.851) had significantly higher diagnostic accuracy for in-hospital mortality than that of CURB-65 alone.
Conclusion: EMR combined with CRB-65 was superior to CURB-65 in predicting mortality in patients with CAP. This new combination was simpler and easier to obtain for physicians in clinics or admission, and it was more convenient for early recognition of patients with poor prognoses.
Keywords: community-acquired pneumonia, EMR, ELR, CURB-65, CRB-65, severity of disease, mortality
A Letter to the Editor has been published for this article.
A Response to Letter by Mr Rusdi has been published for this article.
Introduction
Community-acquired pneumonia (CAP) is the leading cause of death among all infectious diseases and an important health problem, with a significant impact on healthcare systems worldwide.1–3 In the United States of America (USA), CAP causes around 102,000 deaths per year, with mortality rates of 13%, 23.4%, and 30.6% at 1, 6, and 12 months, respectively.4 Accurate assessment of CAP severity and prognosis is critical for diagnosis and treatment. The CURB-65 score—Confusion, Urea>7 mmol/L, Respiratory Rate≥30 breaths/min, Blood Pressure<90 mmHg (systolic) or ≤60 mmHg (diastolic), and Age≥65 years and CRB-65 score (a simplified version of CURB-65 without the blood urea) are widely used clinical scoring systems to predict the disease severity of CAP. CURB-65 and CRB-65 are applied owing to the advantages of being concise and easy-to-use.5,6 However, neither CURB-65 nor CRB-65 could effectively evaluate the outcome of older patients with CAP because of the improper weighting of age variables.7 At the same time, the vast majority of decisions concerning treatment location are made outpatient service without ready access to the results of blood urea nitrogen (BUN) (the “U” in CURB). Therefore, a better prediction index is the need of the hour for rapid, early clinical assessment.
Eosinophils represent 1–4% of circulating leukocytes and have a pivotal role in the propagation of allergic conditions and immune and inflammatory networks.8 Serum eosinophils were found to be a predictor of mortality in a prospective study.9 However, a single indicator of inflammation is not a good predictor. In recent studies, the eosinophil-to-lymphocyte ratio (ELR) has been proposed as a novel marker of inflammation that can be easily calculated.10 ELR has been evaluated in patients with nasal polyposis and allergic rhinitis and has become another potential index of inflammation.11 Guo et al showed that ELR was significantly increased in patients with Kawasaki disease (KD).12 In addition, a retrospective study found that peripheral blood eosinophil counts and the eosinophil-to-monocyte ratio (EMR) at admission in pediatric patients with COVID-19 were significantly lower than those in healthy children.13 Recent studies have evaluated the prognostic value of EMR in patients with ST elevated myocardial infarction,14 acute ischemic stroke,15 and pulmonary embolism.16 However, for patients hospitalized with CAP, the prognostic capabilities of EMR and ELR remain unknown.
The aim of this study was therefore to explore the role of EMR and ELR in CAP and validate their utility as markers of disease severity and risk of death. Meanwhile, to evaluate the value of EMR or ELR combined with CRB-65 in assessing the severity and in-hospital mortality in patients with CAP.
Methods
Study Design
This was a nested case-control, single‐center study about hospitalized patients with CAP. All patients diagnosed with CAP were recruited from the intensive care unit (ICU), emergency department, or Department of Pulmonary and Critical Care Medicine of Qingdao Municipal Hospital between November 18, 2020, and November 21, 2021. Ethics approval was obtained from the Ethics Committee of Qingdao Municipal Hospital (No. 2020CXJJ001-052). Patients were informed in detail about the study design and were requested to provide written consent before participation.
Inclusion/Exclusion Criteria
CAP was defined as detailed in “Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America”.17 Severe CAP was defined as detailed in “Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults”.18 At the same time, patients were divided into a survival group and a non-survival group by tracking the survival of patients in the hospital. The exclusion criteria were as follows: hospital-acquired pneumonia (HAP), tuberculosis, pulmonary tumor, noninfectious interstitial lung disease, pulmonary edema, atelectasis, pulmonary embolism, or pulmonary vasculitis. Diseases affecting the expression level of eosinophils, like acute asthma, allergy, parasitic infection, pulmonary eosinophilia, rheumatism, and hematological disease. Pregnant women and immunocompromised19 patients were also excluded.
Data Collection
We developed a case report form to collect data including age, sex, and other demographic information and clinical outcomes. The following laboratory parameters were measured within 24 h of admission: complete blood cell count (CBC); biochemical indices, such as urea nitrogen, etc.; C-reactive protein (CRP). The CURB-65 score, CRB-65 score, and PSI were calculated by two clinicians. Patients were classified as a low-risk group with CURB-65 of 0–2 and a high-risk group with CURB-65 of 3–5, and PSI low-risk with I–III and PSI high-risk with IV–V.
Statistical Analyses
Continuous data were presented using descriptive statistics, eg, median and 25th and 75th percentiles (interquartile range [IQR]). Categorical data were presented using frequency counts and percentages. Normality was assessed using the Shapiro–Wilk W-test and graphical methods. Standard tests were used to check univariate associations between categorical and categorical (Fisher’s exact tests or chi-squared tests) or categorical and continuous variables (Mann–Whitney U-test). We used propensity-score matching to balance confounding factors in baseline characteristics between the non-CAP group and SCAP group and between survivors and non-survivors. We matched participants with SCAP versus non-SCAP based on one-to-two matching and matched participants with survivors versus non-survivors based on one-to-three matching without replacement within a predefined propensity score radius (caliper width: 0.1). The variables used for the estimation of propensity score were sex, chronic cardiovascular disease, and diabetes mellitus between the non-CAP group and SCAP group. This matching process yielded 134 patients from the 378 patients in the non-SCAP group and 67 patients in the SCAP group. The variables used for the estimation of propensity score were chronic airway disease and chronic cardiovascular disease between survivors and non-survivors. This matching process yielded 63 patients from the 433 patients in the survivors and 21 patients in the non-survivors. The differences in patient characteristics between the groups of patients with and without adverse in-hospital events were calculated with the help of the Wilcoxon-Whitney U-test for continuous variables and Fisher’s exact or chi-squared test for categorical variables, as appropriate. Univariate and multivariate logistic regression models were analyzed to investigate associations between EMR and ELR and disease severity. The multivariate regression models were adjusted for age, WBC, NEU, CRP, EMR, ELR, and NLR. The results were presented as odds ratios (ORs) and 95% confidence intervals (CI). Univariate and multivariate Cox proportional hazards regression models were analyzed to investigate associations between EMR and ELR and in-hospital mortality. The multivariate Cox proportional hazards regression models were adjusted for EMR, ELR, and NLR. The results were presented as hazard ratios (HRs) and 95% CI. Stepwise regression analysis was used to screen the indicators and obtain the probability prediction model of the joint indicators; The prediction accuracy was evaluated using the area under the receiver operating characteristic (ROC) curves. We considered the optimal cutoff point by calculating Youden’s index max point giving the highest sum of sensitivity and specificity and maximum diagnostic efficiency. Comparisons between ROC curves were made using DeLong tests. All statistical analyses were carried out using SPSS software (v 26.0, IBM Corporation, Armonk, NY, USA), Prism v9.3.1 (GraphPad Software, San Diego, CA, USA), MedCalc® Statistical Software version 20.0 (MedCalc Software Ltd, Ostend, Belgium).
Results
Patient Characteristics
In all, 454 patients with CAP were enrolled in the study and divided into the non-SCAP (n=378) and SCAP (n=76) groups. The demographic and clinical characteristics of the participants are presented in Table 1. Before propensity score matching analysis, the proportion of male patients with SCAP was higher than that of non-SCAP (P<0.05), and SCAP had a higher proportion of patients with chronic cardiovascular disease and diabetes (P<0.001). ELR and EMR in the SCAP group were lower than those in the non-SCAP group (Figure 1a and b). EMR and ELR were significantly higher in survivors than non-survivors (0.210±0.013 vs 0.028±0.013, 0.073±0.004 vs 0.011±0.004, respectively, P<0.001; Figure 1c and d).
 |
Table 1 Demographic and Clinical Characteristics of the CAP Patients Before and After Propensity Score Matching Analysis |
The results showed that there was still a significant difference in EMR and ELR between the two groups after propensity score matching analysis (P<0.001; Table 1). Serum EMR and ELR were significantly lower in CAP patients in the high-risk group than those in the low-risk group (P<0.001; Figure 2a and b), consistent with results from the classification of PSI (P<0.001; Figure 2c and d).
Predictive Value of EMR and ELR for Patients with SCAP After Propensity Score Matching Analysis
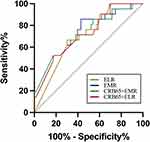
The AUC for EMR was 0.818(P<0.001), with the optimal threshold EMR concentration of 0.018 (95% CI: 0.756–0.880, 89.60% sensitivity and 64.20% specificity). The AUC for ELR was 0.787 (P<0.001), with the optimal threshold ELR concentration of 0.010 (95% CI: 0.781–0.857, 89.60% sensitivity and 59.70% specificity) for predicting disease severity. Thus, CURB-65 (AUC=0.937, 95% CI: 0.904–0.970) and CRB-65(AUC=0.894, 95% CI: 0.894–0.939) had greater predictive power than EMR and ELR levels for SCAP. However, combining EMR or ELR with CRB-65 improved the overall accuracy of prediction from 0.894 to 0.937 (Table 2 and Figure 3), the same as CURB-65 (AUC=0.937, 95% CI: 0.904–0.970). There is no significant difference in the three curves after DeLong test analysis (P>0.05).
 |
Table 2 Area Under the Curve and Thresholds for Predicting Patients with SCAP After Propensity Score Matching Analysis |
The optimum cut-off values for EMR and ELR were identified by drawing ROC curves. The cut-off values are shown in Table 2. As shown in Table 3, the univariate logistic regression analysis showed that age, WBC, Neu, CRP, ELR<0.001, EMR<0.018 and NLR were significantly associated with the SCAP (all P <0.05). After adjusting for these potential confounding factors, EMR < 0.018 ([OR] = 12.104, 95% CI: 4.970–29.479), neutrophil (NEU) ([OR]=1.098, 95% CI:1.005–1.199) and age ([OR]=1.091, 95% CI:1.054–1.130) were significantly associated with an increased risk of SCAP.
 |
Table 3 Binary Logistic Regression Analysis for SCAP Incidence After Propensity Score Matching Analysis |
Predictive Value of EMR and ELR for Patients with in-Hospital Mortality After Propensity Score Matching Analysis
The results showed that there was still a significant difference in EMR and ELR between the two groups after eliminating the bias of chronic airway disease and chronic cardiovascular disease (P<0.05; Table 4). ROC curve analysis was performed to investigate the relation between EMR and ELR with in-hospital mortality (Figure 4 and Table 5). The AUC for ELR was 0.701 (P<0.05), with the optimal threshold ELR concentration of 0.006 (69.80% sensitivity and 66.70% specificity) for predicting death. The AUC for EMR was 0.704 (95% CI: 0.582–0.827, P<0.05). The AUCs of ELR and EMR were higher than those of CURB-65 (AUC=0.619, 95% CI: 0.484–0.754) (Table 5). EMR combined with CRB-65 (AUC=0.721, 95% CI: 0.592–0.851) and ELR combined with CRB-65 (AUC=0.719, 95% CI: 0.596–0.842) had significantly higher diagnostic accuracy for in-hospital mortality than CURB-65 alone (AUC=0.619, 95% CI: 0.484–0.754) (all P<0.05).
 |
Table 4 Demographic and Clinical Characteristics of the CAP Patients After Propensity Score Matching Analysis |
 |
Table 5 Area Under the Curve and Thresholds for Predicting in-Hospital Mortality in CAP Patients After Propensity Score Matching Analysis |
The optimum cut-off values for EMR and ELR were identified by drawing ROC curves. The cut-off values are shown in Table 5. As shown in Table 6, the univariate Cox proportional hazards regression analysis showed that age, EMR< 0.032, ELR<0.006 and NLR were significantly associated with in-hospital mortality in CAP (all P <0.05). After adjusting for these potential confounding factors, EMR < 0.032 ([HR] = 5.816, 95% CI: 1.704–9.848, P = 0.005) was significantly associated with an increased risk of in-hospital mortality.
 |
Table 6 Univariate and Multivariate Cox Proportional Hazards Regression Analysis for in-Hospital Mortality in CAP Patients After Propensity Score Matching Analysis |
Discussion
In this study, it has been demonstrated that EMR < 0.018 was an independent predictor of disease severity, and EMR < 0.032 was an independent predictor of in-hospital mortality for CAP patients. To our knowledge, this is the first study to investigate the utility of EMR and ELR as biomarkers of CAP in adults. The major findings were: (1) Serum EMR and ELR were significantly lower in SCAP patients than those in non-SCAP patients. (2) The ROC curves showed that EMR(AUC=0.704, 95% CI: 0.582–0.827, P<0.05) and ELR(AUC=0.701, 95% CI: 0.582–0.820, P<0.05) have a slightly better predictive capability for in-hospital mortality than CURB-65 (AUC=0.619, 95% CI: 0.484–0.754, P=0.104). Furthermore, EMR combined with CRB-65 (AUC=0.721, 95% CI: 0.592–0.851) and ELR combined with CRB-65 (AUC=0.719, 95% CI: 0.596–0.842) were superior to CURB-65 in predicting mortality in patients with CAP.
New indexes, calculated by subtypes of white blood cells, are better than single inflammatory cells in reflecting systemic inflammation. EMR and ELR are new parameters but the association between EMR or ELR and disease severity and in-hospital mortality in CAP patients has not been fully clarified. Our study shows that lower EMR levels are associated with a poor prognosis and mortality in patients with CAP. We found that the reduction in EMR levels was due to a decrease in eosinophilic counts, while monocyte counts were not statistically different between the two groups. In contrast to our current findings, other researchers found that monocyte counts were statistically significant in 2019-nCoV pneumonia patients. Severe 2019-nCoV pneumonia patients have higher monocyte count and lower lymphocyte count than non-severe 2019-nCoV pneumonia patients.20 The reason for this discrepancy may be that the pathogens that cause pneumonia are different among us, and their sample size is small.
Currently, the role of EMR has been evaluated in inflammation, acute ischemic stroke,21 and pulmonary embolism. A retrospective study included 208 children with a confirmed diagnosis of COVID-19 and a control group comprising 117 healthy children found EMR on admission, was significantly lower in patients than that in the control group.13 Our findings showed that EMR in the SCAP group was significantly lower than that in the non-SCAP group. Külahçıoğlu et al revealed that a lower EMR could independently predict long-term mortality in patients with intermediate-high- and high-risk pulmonary embolism,16 while no study was performed in patients with CAP. Our research found that EMR<0.032 was an independent risk factor for in-hospital mortality in patients with CAP. Moreover, EMR has a good ability to predict in-hospital mortality in patients with CAP. The role of ELR was also paid attention in various infections9,10 and cancer.12,22 The ELR value was lower in pneumonic COVID‐19 patients than that with non‐pneumonic COVID‐19 infection, contrary to the NLR value23 In the present study, ELR was significantly decreased in non-survivors than that in survivors, suggesting that ELR is a useful biomarker for distinguish the in-hospital mortality in CAP.
CURB-65 is a classic tool for evaluating CAP severity.24 CRB-65 is a simplified version. The diagnostic efficiency of CRB-65 is inferior to that of CURB-65. However, EMR or ELR combined with CRB-65 can significantly increase the ROC value to 0.937 for evaluating disease severity. Encouragingly, this new combination has the same predictive ability for evaluating disease severity as CURB-65. This reminds us that in the process of evaluation of hospital admission, we can quickly obtain the results of routine blood tests to classify patients and assess whether further hospitalization treatment is needed. Thus, this study recommends the measurement of EMR or ELR to serve as a simpler and more accurate predictor. EMR was more effective in predicting in-hospital mortality in patients with CAP. ELR and EMR were superior to other indices such as NLR. The combination of EMR and CRB-65 scores improved the predictive accuracy of CRB-65 score for in-hospital mortality, and EMR < 0.032 was an independent predictor of in-hospital mortality for CAP patients. This finding indicated that EMR combined with CRB-65 was a good predictive index that could reflect the in-hospital mortality of CAP. This could further guide clinicians in assessing disease in patients with CAP.
Since a single inflammatory cell count has disadvantages in summarizing the overall systemic inflammation, it is a current research hotspot to propose novel indicators by combining different white blo, like NLR. Above all, EMR was a potential prognostic marker with its convenience of calculating as eosinophils and monocytes were collected in the blood routine test.
A limitation of our study is the relatively small size of the single-center cohort and there was no healthy control group to corroborate our findings. Therefore, the representation of severe CAP cases is limited. Another limitation is the large proportion of non-SCAP in our samples, and in the course of analyzing the results, we found that the eosinophils in SCAP can reach very low values and are often difficult to measure. Finally, due to the small number of samples, we lack research on the etiology of this part of patients. In the future, we will expand the number of samples.
Conclusions
The combination of EMR and CRB-65 score is a more accurate predictor of CAP-related in-hospital mortality than CURB-65 alone. This new combination was simpler and easier to obtain for physician in clinic or admission, and more convenient for early recognition of patients with poor prognosis.
Ethics Approval
Ethics approval was obtained from the Ethics Committee of Qingdao Municipal Hospital (No. 2020CXJJ001-052). This study was in compliance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests for this work.
References
1. Ferreira-Coimbra J, Sarda C, Rello J. Burden of Community-Acquired Pneumonia and Unmet Clinical Needs. Adv Ther. 2020;37(4):1302–1318. doi:10.1007/s12325-020-01248-7
2. Cilloniz C, Dominedo C, Pericas JM, Rodriguez-Hurtado D, Torres A. Community-acquired pneumonia in critically ill very old patients: a growing problem. Eur Respir Rev. 2020;29(155):155. doi:10.1183/16000617.0126-2019
3. Prina E, Ranzani OT, Torres A. Community-acquired pneumonia. Lancet. 2015;386(9998):1097–1108. doi:10.1016/S0140-6736(15)60733-4
4. Ramirez JA, Wiemken TL, Peyrani P, et al. Adults Hospitalized With Pneumonia in the United States: incidence, Epidemiology, and Mortality. Clin Infect Dis. 2017;65(11):1806–1812. doi:10.1093/cid/cix647
5. Bahlis LF, Diogo LP, Kuchenbecker RS, Fuchs SC. Clinical, epidemiological, and etiological profile of inpatients with community-acquired pneumonia in a public hospital in the interior of Brazil. J Bras Pneumol. 2018;44(4):261–266. doi:10.1590/s1806-37562017000000434
6. Barrera L. Editorial Commentary: community-acquired pneumonia, comparison of three mortality prediction scores in the emergency department. Colomb Med (Cali). 2022;53(3):e1015377. doi:10.25100/cm.v53i3.5377
7. Kaya AE, Ozkan S, Usul E, Arslan ED. Comparison of pneumonia severity scores for patients diagnosed with pneumonia in emergency department. Indian J Med Res. 2020;152(4):368–377. doi:10.4103/ijmr.IJMR_595_18
8. Agnello L, Giglio RV, Bivona G, et al. The Value of a Complete Blood Count (CBC) for Sepsis Diagnosis and Prognosis. Diagnostics. 2021;11:10.
9. Camon S, Quiros C, Saubi N, et al. Full blood count values as a predictor of poor outcome of pneumonia among HIV-infected patients. BMC Infect Dis. 2018;18(1):189. doi:10.1186/s12879-018-3090-0
10. Chen H, Chen S, Huang Z, et al. Relationship between blood parameters and Clonorchis sinensis infection: a retrospective single center study. Int Immunopharmacol. 2018;59:120–126. doi:10.1016/j.intimp.2018.04.003
11. Bruns J, Steiner D. [Rupture of the pes anserinus--a rare concomitant injury in anteromedial instability of the knee joint]. Aktuelle Traumatol. 1986;16(6):230–232.
12. Guo X, Liao J, Fan X, Xu M. Exploring the diagnostic value of eosinophil-to-lymphocyte ratio to differentiate Kawasaki disease from other febrile diseases based on clinical prediction model. Sci Rep. 2023;13(1):3399. doi:10.1038/s41598-023-30463-9
13. Ustundag Y, G. Kazanci E, Koloğlu RF. A retrospective study of age-defined hematologic inflammatory markers related to pediatric COVID-19 diagnosis. Int J Lab Hematol. 2022;44(4):722–728. doi:10.1111/ijlh.13838
14. Deng X, Wang X, Shen L, et al. Association of eosinophil-to-monocyte ratio with 1-month and long-term all-cause mortality in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. J Thorac Dis. 2018;10(9):5449–5458. doi:10.21037/jtd.2018.09.27
15. Chen Y, Ren J, Yang N, et al. Eosinophil-to-Monocyte Ratio is a Potential Predictor of Prognosis in Acute Ischemic Stroke Patients After Intravenous Thrombolysis. Clin Interv Aging. 2021;16:853–862. doi:10.2147/CIA.S309923
16. Kulahcioglu S, Tokgoz HC, Akbal OY, et al. Eosinophil-to-Monocyte Ratio as a Candidate for a Novel Prognostic Marker in Acute Pulmonary Embolism: is it a Consumptive Mechanism? Anatol J Cardiol. 2022;26(9):717–724. doi:10.5152/AnatolJCardiol.2022.1780
17. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi:10.1164/rccm.201908-1581ST
18. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2(Suppl 2):S27–72. doi:10.1086/511159
19. Gu M, Han X, Liu X, Sui F, Zhang Q, Pan S. Predictive Value of Annenxin A1 for Disease Severity and Prognosis in Patients with Community-Acquired Pneumonia. Diagnostics. 2023;13(3):548. doi:10.3390/diagnostics13030396
20. Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. doi:10.1016/j.intimp.2020.106504
21. Yu S, Luo Y, Zhang T, et al. Eosinophil-to-monocyte ratio is a potential biomarker in the prediction of functional outcome among patients with acute ischemic stroke. BMC Neurosci. 2021;22(1):8. doi:10.1186/s12868-021-00610-x
22. Brescia G, Barion U, Zanotti C, Parrino D, Marioni G. Pre- and postoperative blood neutrophil-to-lymphocyte and eosinophil-to-lymphocyte ratios in patients with sinonasal polyps: a preliminary investigation. Allergy Asthma Proc. 2017;38(5):64–69. doi:10.2500/aap.2017.38.4068
23. Damar Cakirca T, Torun A, Cakirca G, Portakal RD. Role of NLR, PLR, ELR and CLR in differentiating COVID-19 patients with and without pneumonia. Int J Clin Pract. 2021;75(11):e14781. doi:10.1111/ijcp.14781
24. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382. doi:10.1136/thorax.58.5.377
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.