Back to Journals » Neuropsychiatric Disease and Treatment » Volume 10
Emerging therapeutic options for acute migraine: focus on the potential of lasmiditan
Authors Rizzoli P
Received 17 January 2014
Accepted for publication 31 January 2014
Published 31 March 2014 Volume 2014:10 Pages 547—552
DOI https://doi.org/10.2147/NDT.S25531
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Paul B Rizzoli
Department of Neurology, Brigham and Women's Faulkner Hospital, John R. Graham Headache Center, Boston, MA, USA
Abstract: The serotonin receptor agonist triptan drugs (5-HT1B/1D receptor agonists) have been in use for over 20 years in the abortive management of migraine. Although clearly effective, their ability to produce vasoconstriction in cerebral and coronary arteries, thought to be mediated by their high affinity for the 5-HT1B receptor, has been a limitation to their use in certain patient populations. Variable potency triptan binding at the 5-HT1F receptor occurs in addition to binding at the 5-HT1B and 5-HT1D receptors. A more selective serotonin agonist without 5-HT1B-mediated vasoconstriction might prove efficacious yet safer. The 5-HT1F receptor has been targeted as a site of action for such a drug. In experimental models, 5-HT1F receptor agonists have been shown to block neurogenic inflammation and c-Fos expression in neural tissue and, as well, show no evidence of vasoconstriction in vascular tissue models in vitro. In clinical trials, efficacy in the abortive management of migraine has been established. Lasmiditan (LY573144), a selective 5-HT1F receptor agonist (K1=2.21 µM), showed efficacy in its primary endpoint, with a 2-hour placebo-subtracted headache response of 28.8%, though with frequent reports of dizziness, paresthesias, and vertigo. Study results support an emerging central neuronal mechanism of migraine pathophysiology. This review traces the history and use of 5-HT1F receptor agonists, now referred to as neurally acting anti-migraine agents in migraine management.
Keywords: 5-HT1F receptor, migraine, serotonin, lasmiditan, treatment, vasoconstriction
Introduction
Migraine and related headaches are highly prevalent worldwide.1 Migraine itself accounts for 30% of the global burden of disease and produces half of the world’s neurological disease-related disability.2 New and more potent therapies for this common and often disabling condition would certainly be welcome. A recent review discussed both promising new therapies and new uses for existing therapies.3 Topiramate, currently used as a preventive agent, may also be helpful for migraine vertigo. Occipital nerve stimulation may be useful in some patients with intractable chronic migraine, and other nerve stimulation procedures also look promising in migraine. Neurotransmitter modulators that include a neuronal nitric oxide synthase inhibitor, several glutamate receptor antagonists, and a kainite receptor antagonist are under active study. In addition to the currently used abortive agents, triptans, and nonsteroidal anti-inflammatory medications, other promising new treatments include calcitonin gene-related peptide (CGRP) receptor antagonists, transcranial magnetic stimulation, and, reviewed below, the serotonin receptor 1F (5-HT1F) agonists.
Most of the current migraine abortive agents were developed at a time when headache was thought to result from abnormal vasodilatation of intracranial vessels. The search for new migraine treatments was thus focused on agents that produced cerebral vasoconstriction. Sumatriptan was the first 5-HT agonist developed based on this search and was effective in aborting migraine. Other so-called “triptan” drugs soon followed and were also effective. Triptans affect a variety of 5-HT receptor subgroups that are found both peripherally and centrally in the nervous system. Whether the principal site of action of triptans is central or peripheral is uncertain, as is the specific mechanism of action. Triptan vasoconstrictive effects are generally thought to result from activation of the post-synaptic 5-HT1B receptors found on vascular smooth muscle. If not the major site of action of the beneficial effects of triptans, 5-HT1B receptors do appear to be the site of action for the most significant limitation of triptans; their ability to produce clinically relevant peripheral vasoconstrictive side effects at times leads to myocardial ischemia or infarction.
An effective anti-migraine agent that did not produce vasoconstriction would be a welcome advance. CGRP receptor antagonists and anti-CGRP antibodies, currently in development, appear to be effective without notable vasoconstrictive effects. CGRP receptor antagonists compete for receptor binding sites with endogenous CGRP, reducing its vasoconstrictive properties, while the anti-CGRP antibodies bind to CGRP and neutralize its effects; both seem able to treat established migraine attacks without associated vasoconstriction. CGRP receptor antagonists, however, did not appear to be more effective than triptans and problems with liver toxicity have limited clinical development.4
Opinions are mixed about whether vasoconstriction is the major reason that triptans are effective in treating migraine.5 Other central or neuronal mechanisms of action of the triptans have been suggested, including direct modulation of neurotransmitter release or other direct modulation of neurons within the trigeminal system. Triptans appear to have effects at 5-HT1F receptors, which are found on trigeminal neurons. In animal models, triptan drug activation at 5-HT1F receptors does not produce the vasoconstrictive effects seen with 5-HT1B activation. 5-HT1F receptors are activated to varying degrees by most or all of the triptan drugs, but these data suggest that a pure 5-HT1F agonist might act through direct neural mechanisms. It seems plausible that a selective 5-HT1F agonist might provide efficacy without vasoconstrictive side effects. This new class has been termed “neurally acting anti-migraine agents”.6 One of them, lasmiditan, has entered clinical trial testing.
Receptor physiology
5-HT1F receptors5 have been identified in various locations in the trigeminovascular system, perhaps most notably peripherally in the trigeminal ganglion and centrally in the trigeminal nucleus caudalis. They are members of the G-family of protein receptors acting through inhibition of adenylate cyclase. They are located on peripheral and central ends of sensory trigeminal neurons,6 and appear to function by hyperpolarizing nerve terminals and thereby inhibiting trigeminal impulses.7
Further, studies5 show that human trigeminal ganglion neurons that are positive for 5-HT1B/1D/1F receptors are also positive for glutamate receptors. If 5-HT1F receptors are shown to inhibit glutamate release, this would provide another potential mechanism of migraine relief. Glutamate is an excitatory amino acid that appears to lower the threshold for neuronal depolarization and thereby raise the risk for subsequent attacks of migraine in susceptible individuals, possibly through trigeminovascular activation and production of central sensitization.8
5-HT1F receptors are also present in the cortex and cerebellum. Drug effects at either or both locations may be responsible for some of the prominent central nervous system (CNS) side effects that are seen with lasmiditan. These side effects are consistent with drug entry into the CNS and of a central as well as peripheral site of action for lasmiditan.
LY334370
In 2001, an early prototype selective 5-HT1F receptor agonist was tested in migraineurs in the acute abortive management of a single attack with the endpoint of 2-hour headache reduction.9 This compound showed a high binding affinity (K1=1.6 nM) for the 5-HT1F receptor and showed no vasoactive properties. In Phase II clinical trials, statistically significant headache improvement was noted for the 60 mg (P=0.012) and 200 mg (P=0.001) doses: placebo 8%, 60 mg 37%, and 200 mg 52% headache reduction at 2 hours.
The appearance of liver toxicity in dogs halted its development. Similar toxicity has not occurred in other animals tested with this or any other 5-HTF1 receptor agonist. Further, triptans that bind the 5-HT1F receptor have not been reported to produce this toxicity. Thus, the authors concluded that the toxicity was both species and drug specific and not related to the mechanism of action of the compound.
Based on indirect evidence, a central site of action was suspected, probably in the trigeminal nucleus caudalis, though peripheral sites of action could not be ruled out. The adverse event profile was different from that reported by triptan users and consisted of frequent fatigue, dizziness, somnolence, and paresthesia. A similar adverse event profile is noted in other 5-HTF1 agonists. Although development of this agent did not advance, interest continued in the development of other selective 5-HT1F agonists.
Lasmiditan (COL-144, LY573144)
Preclinical studies
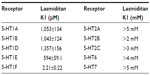
Nelson et al6 reported the results of preclinical testing of another 5-HT1F selective blocker, structurally unique compared with the triptans, that showed marked affinity and specificity for the 5-HT1F receptor (Table 1). As depicted in Table 1, the lower the dissociation constant (K1) the more tightly bound the drug. Results of binding studies with other 5-HT subtypes are shown for comparison and confirm both the binding affinity and specificity of this agent. Notable are the much lower binding affinities (higher K1s) for lasmiditan at the 5-HT1B and 5-HT1D receptors, values much lower than many of the triptan drugs.10 Lasmiditan also demonstrated negligible binding affinity across a host of other CNS receptors, ion channels, and other binding sites, confirming its specificity.
Early studies tested whether these binding affinities would correlate with physiologic effects in animal models. In a rabbit saphenous vein model, lasmiditan showed no evidence of vasoconstriction while comparable doses of sumatriptan produced 50% maximal vessel contraction. Separate in vivo animal models of migraine, one measuring dural plasma protein extravasation and one of trigeminal c-Fos expression, demonstrated that lasmiditan both effectively blocked dural plasma protein extravasation and inhibited stimulation-induced c-Fos expression in the trigeminal nucleus caudalis.
One persistent question about the triptans has been the degree to which they cross the blood–brain barrier and whether central activity is required to produce therapeutic effects. Most information now suggests that as a group triptans are poorly CNS penetrant, which supports the view that relevant clinical benefits are predominantly due to peripheral, rather than central, effects. This implies that the ability of triptans to bind the central 5-HT1F receptor in vitro11 may be misleading in regard to their clinical effects, which may instead be mostly peripheral. If activation of these central receptors is an important therapeutic mechanism, then the ease of passage of study agents across the blood–brain barrier is especially relevant. A wide range of preclinical and clinical evidence supports the view that lasmiditan has good central penetrance.7
Clinical studies
The results of two industry-sponsored randomized, double-blind, placebo-controlled trials of lasmiditan in human subjects have been reported. Ferrari et al12 examined the optimal intravenous dosage of lasmiditan for the treatment of a single migraine attack. This adaptive treatment design began with a starting dose of 2.5 mg with adjustments up or down for subsequent cohorts based on predefined safety and efficacy rules for outcomes in the prior cohort. The primary endpoint, headache response, was defined as the 2-hour improvement from moderate or severe headache to mild or no headache.
Subjects with migraine were asked to report to the hospital within 4 hours of onset of a typical moderate-to-severe event for intravenous infusion of the study drug. The subject population was mainly female and Caucasian with a mean age of 38.4 years. Data on 130 subjects were used in the analysis. Lasmiditan doses between 2.5 mg and 45 mg were studied in an adaptive trial study method. Efficacy was based on attainment of the primary endpoint of headache response in conjunction with prearranged stopping rules. This resulted in an effective dose determination of 20 mg, at which point the trial was terminated. Based on the primary endpoint, the 20 mg dose produced a headache response at 2 hours of 64.3% compared to a placebo response of 45.2% (P=0.0126 for the linear association between response rates and dose levels). Efficacy was noted as early as 20–40 minutes after the start of treatment. Outcomes for multiple secondary endpoints correlated with the primary endpoint. No serious adverse events were noted. Mild and transient paresthesias were reported in about one-third of treated subjects and 25% of subjects reported dizziness compared with 14% in the placebo group. No significant clinical changes were noted. Electrocardiography parameters were unchanged. Since this study demonstrated the clinical efficacy of the non-vasoconstrictive compound lasmiditan for the treatment of migraine, it was widely viewed as confirming the potential clinical usefulness of non-vasoconstrictive compounds in migraine treatment. Dosing information was used to help select optimal oral dosing for future trials.
A second study13 examined oral lasmiditan in a double-blind, placebo-controlled, parallel-group fashion. Subjects with episodic migraine not on preventive agents were assigned to one of the following treatment groups: 50 mg, 100 mg, 200 mg, 400 mg lasmiditan, or placebo. They were to abortively treat one moderate-to-severe migraine event.
The same primary endpoint was used: headache response at 2 hours. Demographic characteristics of subjects were similar to the prior study.12 Associated symptoms and side effects were recorded. Secondary endpoints included headache recurrence at 24 hours, use of a rescue drug, disability, and the subject’s global impression of benefit.
Although 512 subjects were screened and randomly assigned into the study, 121 subjects did not end up using the study drug to treat a headache. Of the remaining 391 subjects who entered, roughly 75 subjects were randomized into each treatment group. The typical subject was female with episodic migraine with a frequency of one event per month usually lasting about 2 hours. Sixty percent of the treated headache events were rated as moderate in severity and 40% were rated as severe before treatment. Treatment was reported as having been initiated in most subjects within 15 minutes of headache onset. Fifty percent of subjects reported a return of headache within 24 hours after treatment; this was similar to the 24-hour return rate reported in the placebo group of 57.1%.
A significant linear association was noted between both headache response rates and headache-free rates at 2 hours compared with placebo. All studied doses of lasmiditan were considered efficacious compared with placebo (200 mg P=0.032; 400 mg P=0.0007). Efficacy was noted as early as 30 minutes post-dose in the 400 mg cohort. Migraine-associated symptoms improved as well in all treatment groups.
The 200 mg dose produced efficacy felt by the authors comparable to historical triptan controls, though the study did not directly compare the two drugs. The placebo-subtracted improvement to pain-free rate in this study was 11.6% compared with roughly 6%–31% in a combination of efficacy studies of the triptans.14,15
Based on this study, lasmiditan demonstrated a benefit roughly comparable to that of the triptans, but with a higher 24-hour recurrence rate.
Treatment emergent adverse events, including dizziness, paresthesia, fatigue, and vertigo, were noted in the active treatment group and increased with increasing doses of lasmiditan. CNS and vestibular symptoms were reported most commonly. Of these side effects, most were mild or moderate except for dizziness which was classified as severe in 15%–17% of subjects and was associated with use of higher doses. Since this was a single-dose study, a dropout rate is not meaningful. However, the global impression of benefit for the highest administered dose (that also included the most severe adverse events) was 34% compared with 16% for placebo.
Dose-dependent efficacy in the abortive management of a single migraine attack was seen as confirmation of the utility of activation of the 5-HT1F receptor in migraine management. Given receptor locations in the trigeminovascular system, proposed mechanisms of action might include inhibition of protein leakage, blockage of secondary trigeminal neuron activation, and/or inhibition of neuropeptide release, including possibly glutamate. Activation of cerebellar 5-HT1F receptors could explain some of the reported adverse events. Due to the single treatment structure of both of these studies, long-term efficacy could not be assessed.
This study’s authors concluded that lasmiditan was efficacious in the abortive management of a moderate-to-severe migraine attack at a recommended dosage of 100 mg. The study was unable to determine to what degree the treatment emergent adverse events might limit use clinically. Similarly, the seemingly high 24-hour recurrence rate was not discussed and was of unclear significance.
Discussion
It seems clear from the current research that the selective 5-HT1F receptor agonist lasmiditan is efficacious in the acute management of migraine.16,17 Further research in which subjects treat multiple attacks is necessary to determine true clinical utility. Future head-to-head comparisons will help clarify relative efficacy. Whether these drugs will be subject to medication overuse headache is another important clinical question.
Since peripheral effects have not been ruled out, confirmation is needed that the primary site of action for lasmiditan is central inhibition in the trigeminal nucleus caudalis. It is possible that the fairly high incidence of CNS adverse effects of lasmiditan may limit its clinical utility.
The potential of this new neurally acting anti-migraine agent class of migraine abortive agents has one expert17 questioning if their discovery signals the end of the vascular hypothesis of migraine, at least as the primary pathophysiology in migraine. It is difficult to refute vascular effects entirely. Vasodilator agents are known to trigger migraine attacks in humans. Further, vasodilatation has been detected during a migraine attack and, in combination with peripheral neuronal sensitization, could contribute to some of the resulting migraine symptoms.17 However, the ability of lasmiditan to abort a migraine attack through activation of a centrally located neuronal receptor without discernible vascular effects certainly seems to bolster a primarily neural hypothesis of migraine pathophysiology. Whether or not this drug or this group becomes commercially successful, a shift in research focus away from vascular mediators and toward centrally acting agents could be expected on the basis of this information. Previously, CGRP receptor antagonists had also been noted to be effective in migraine without evidence of vasoconstrictor activity; however, because CGRP is a potent vasodilator, an indirect vascular effect could still be inferred as playing some role in the mechanism of action. Other migraine abortive agents such as nonsteroidal anti-inflammatory drugs, though also without a clear vascular mechanism of action, could be acting through less specific analgesic actions and thus are not informative as to pathophysiology.
Conclusion
The development of the new neurally acting anti-migraine agent class of migraine abortive drugs seems to provide additional proof of concept of a neural, and likely central, mechanism of migraine pathophysiology, further clarifying that a primarily vascular mechanism for migraine is unlikely. Lasmiditan, the most developed member of this group, is efficacious in the context of clinical trials for treatment of individual migraine attacks. Current studies provide “strong and direct evidence for a nonvascular mechanism for migraine-specific acute treatment”.18 Though further investigation is necessary, it seems possible that CNS adverse events may limit the clinical acceptability of lasmiditan. Whether this is a class effect or not is uncertain, although the original tested compound, LY334370, produced a similar pattern of side effects. It may be that other 5-HT1F agonists will share this limitation due primarily to their central site of action. Thus, adverse effects from these agents may become a significant barrier to commercial development. Nonetheless, development of future specific centrally acting receptor mediators could be expected, building on prior research observations on the one hand while further advancing our understanding of migraine pathophysiology on the other. Or, put another way, it is evident based on these advances that mechanism-based drug development in migraine is now within reach.17
Acknowledgments
With thanks to Dr Elizabeth Loder for her manuscript review and constructive comments.
Disclosure
The author reports no conflicts of interest in this work.
References
Smitherman TA, Burch R, Sheikh H, Loder E. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53(3):427–436. | |
Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. | |
Magis D, Schoenen J. Treatment of migraine: update on new therapies. Curr Opin Neurol. 2011;24(3):203–210. | |
Stovner LJ, Tronvik E, Hagen K. New drugs for migraine. J Headache Pain. 2009;10(6):395–406. | |
Ramadan NM, Skljarevski V, Phebus LA, Johnson KW. 5-HT1F receptor agonists in acute migraine treatment: a hypothesis. Cephalalgia. 2003;23(8):776–785. | |
Nelson DL, Phebus LA, Johnson KW, et al. Preclinical pharmacological profile of the selective 5-HT1F receptor agonist lasmiditan. Cephalalgia. 2010;30(10):1159–1169. | |
Olesen J. 5-Hydroxyptryptamine 1F (5-HT1F) receptor agonism. A possible new treatment principle for acute migraine attacks. Cephalalgia. 2010;30(10):1157–1158. | |
Ramadan NM. The link between glutamate and migraine. CNS Spectr. 2003;8(6):446–449. | |
Goldstein DJ, Roon KI, Offen WW, et al. Selective seratonin 1F (5-HT[1F]) receptor agonist LY334370 for acute migraine: a randomised controlled trial. Lancet. 2001;358(9289):1230–1234. | |
Geraud G, Keywood C, Senard JM. Migraine headache recurrence: relationship to clinical, pharmacological, and pharmacokinetic properties of triptans. Headache. 2003;43(4):376–388. | |
Maggioni F, Pini LA, Zanchin G. Preclinical pharmacological profile of the selective 5-HT1F receptor agonist lasmiditan. A comment. Cephalalgia. 2011;31(9):1061. | |
Ferrari MD, Farkkila M, Reuter U, et al. Acute treatment of migraine with the selective 5-HT1F receptor agonist lasmiditan – a randomised proof-of-concept trial. Cephalalgia. 2010;30(10):1170–1178. | |
Farkkila M, Diener HC, Geraud G, et al. Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a Phase II randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 2012;11(5):405–413. | |
Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Triptans (serotonin, 5-HT1B/1D agonists) in migraine: detailed results and methods of a meta-analysis of 53 trials. Cephalalgia. 2002;22(8):633–658. | |
Loder E, Goldstein R, Biondi D. Placebo effects in oral triptan trials: the scientific and ethical rationale for continued use of placebo controls. Cephalalgia. 2005;25(2):124–131. | |
Tfelt-Hansen PC, Olesen J. The 5-HT1F receptor agonist lasmiditan as a potential treatment of migraine attacks: a review of two placebo-controlled Phase II trials. J Headache Pain. 2012;13(4):271–275. | |
Olesen J, Ashina M. Emerging migraine treatments and drug targets. Trends Pharmacol Sci. 2011;32(6):352–359. | |
Charles A. Defining and refining 5-HT receptor targets for migraine. Lancet Neurol. 2012;11(5):383–384. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.