Back to Journals » Infection and Drug Resistance » Volume 16
Emergence of Tigecycline and Carbapenem-Resistant Citrobacter freundii Co-Carrying tmexCD1-toprJ1, blaKPC-2, and blaNDM-1 from a Sepsis Patient
Authors Huang J, Zhao J, Yi M, Yuan Y, Xia P, Yang B, Liao J, Dang Z, Xia Y
Received 10 July 2023
Accepted for publication 22 August 2023
Published 5 September 2023 Volume 2023:16 Pages 5855—5868
DOI https://doi.org/10.2147/IDR.S426148
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Jinzhu Huang,* Jinxin Zhao,* Miao Yi, Yaling Yuan, Peiwen Xia, Bingxue Yang, Jiajia Liao, Zijun Dang, Yun Xia*
Department of Laboratory Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yun Xia, Department of Laboratory Medicine, The First Affiliated Hospital of Chongqing Medical University, No. 1 Youyi Road, Yuzhong District, Chongqing, 400016, People’s Republic of China, Tel +86-13668043942, Fax +86-23-89012513, Email [email protected]
Purpose: This research aims to profile ten novel strains of carbapenem-resistant Enterobacteriaceae (CRE) co-carrying blaKPC and blaNDM.
Methods: Clinical CRE strains, along with corresponding medical records, were gathered. To ascertain the susceptibility of the strains to antibiotics, antimicrobial susceptibility tests were conducted. To validate the transferability and cost of fitness of plasmids, conjugation experiments and growth curves were employed. For determining the similarity between different strains, ERIC-PCR was utilised. Meanwhile, whole genome sequencing (WGS) was performed to characterise the features of plasmids and their evolutionary characteristics.
Results: During the course of this research, ten clinical CRE strains co-carrying blaKPC and blaNDM were gathered. It was discovered that five out of these ten strains exhibited resistance to tigecycline. A closer examination of the mechanisms underlying tigecycline resistance revealed that tmexCD 1-toprJ 1, blaKPC-2, and blaNDM-1 existed concurrently within a single Citrobacter freundii strain (CF10). This strain, with a minimum inhibitory concentration (MIC) of 32 mg/L to tigecycline, was obtained from a sepsis patient. Furthermore, an investigation of genome evolution implied that CF10 belonged to a novel ST type 696, which lacked analogous strains. Aligning plasmids exposed that similar plasmids all had less than 70% coverage when compared to pCF10-tmexCD1, pCF10-KPC, and pCF10-NDM. It was also found that tmexCD 1-toprJ 1, blaKPC-2, and blaNDM-1 were transferred by Tn 5393, IS 5, and Tn 6296, respectively.
Conclusion: This research presents the first report of coexistence of tmexCD 1-toprJ 1, blaKPC-2, and blaNDM-1 in a carbapenem and tigecycline-resistant C. freundii strain, CF10.
Importance: Tigecycline is considered a “last resort” antibiotic for treating CRE infections. The ongoing evolution of resistance mechanisms to both carbapenem and tigecycline presents an alarming situation. Moreover, the repeated reporting of both these resistance mechanisms within a single strain poses a significant risk to public health. The research revealed that the genes tmexCD 1-toprJ 1, blaKPC-2, and blaNDM-1, which cause carbapenem and tigecycline-resistance in the same strain, were located on mobile elements, suggesting a potential for horizontal transmission to other Gram-negative bacteria. The emergence of such a multi-resistant strain within hospitals should raise significant concern due to the scarcity of effective antimicrobial treatments for these “superbugs”.
Keywords: blaKPC, blaNDM, carbapenem-resistantEnterobacteriaceae, tigecycline-resistance, tmexCD 1-toprJ 1
Corrigendum for this paper has been published.
Introduction
Carbapenem-resistant Enterobacteriaceae (CRE) infections are highly prevalent in China, with Klebsiella pneumoniae, Escherichia coli, Enterobacter cloacae, and Citrobacter freundii being the primary culprits.1 The fatality rate for patients suffering from carbapenem-resistant K. pneumoniae (CRKP) infections is reported to be 24.3%, and this figure rises to 40% for those patients with lower respiratory tract infections.2 Strains co-carrying KPC (Klebsiella pneumoniae carbapenemase) and NDM (NewDelhimetallo‑β‑lactamase) have been reported globally in recent years, including instances in India,3 Turkey, Brazil,4 and China.5 Previous reports have described C. freundii strains, specifically WCHCF65 from hospital sewage and HN380 from clinical urine, co-carrying blaKPC and blaNDM.6,7 The occurrence of both blaKPC and blaNDM in a single strain is a matter of serious concern due to the associated higher-level carbapenem resistance and the acceleration of carbapenemase gene transmission.8
Tigecycline is generally regarded as the antimicrobial of “last resort” for CRE infections.9 Nonetheless, resistance to tigecycline has emerged since its approval, with frequent reports within Enterobacteriaceae.10 Previous research has indicated that chromosomal mutations and overexpression of efflux pumps were closely tied to tigecycline resistance.11 In addition, a plasmid-mediated resistance-nodulation-division (RND) family multidrug efflux pump gene cluster, tmexCD1-toprJ1, has recently been identified and characterized.12 Currently, tmexCD1-toprJ1-like-positive K. pneumoniae strains have predominantly been found in China and Vietnam.13,14 While they are commonly located in animals, food, and the environment, these strains are rarely isolated from patients.15,16 An increased tmexCD1-toprJ1 presence has been noted to elevate the minimum inhibitory concentration (MIC) of K. pneumoniae, E. coli, and Salmonella against tetracyclines (including tigecycline and eravacycline), quinolones, cephalosporins, and aminoglycosides between four to thirty-two fold.12
Apart from the efflux pumps’ contribution to tigecycline resistance, the proliferation of mutant tet(A) genes is of concern, given their potential for increasing tigecycline resistance in K. pneumoniae upon transmission.17 The tet(A) gene, associated with the major facilitator superfamily (MFS) family efflux pump, could escalate the accumulation of tigecycline, thus enhancing resistance. Notable in various sources such as food, clinical samples, environmental components and human microflora from diverse countries,18–20 any source could harbour the mutated tet(A) gene.21,22 Consequently, the acquisition of a tmexCD1-toprJ1-positive plasmid by Enterobacteriaceae species, particularly CRE, could generate pan-drug-resistant strains, potentially leading to untreatable infections. Hence, curbing the emergence and spread of tigecycline-resistance CRE is an epidemiological urgency.
The molecular epidemiological characteristics of ten CRE strains co-carrying blaKPC and blaNDM have been described herein. One strain was discovered to contain the mobile tigecycline resistance gene, tmexCD1-toprJ1.
Materials and Methods
Samples and Clinical Information Collection
Ongoing surveillance of CRE in China enabled the collection of clinic CRE isolate samples from the First Affiliated Hospital of Chongqing Medical University from January to December 2021. The Vitek 2 Compact (BioMérieux) was employed to confirm these isolates. The CRE collection criteria required an MIC ≥4 mg/L for imipenem or meropenem. PCR and Sanger sequencing confirmed all isolates co-carrying blaKPC and blaNDM. All PCR primers are provided in Table S1. Standard and predetermined case report forms collected patient clinical information, including demographics, sample source and type, underlying diseases, clinical presentations, antimicrobial therapy, and outcomes.
Antimicrobial Susceptibility Testing (AST) and String Test
The broth microdilution method, as per Clinical and Laboratory Standards Institute (CLSI) guidelines,23 assessed antimicrobial agent susceptibility using aztreonam, ceftazidime, imipenem, cefatriaxone, cefepime, cefoxitin, meropenem, amikacin, tigecycline, levofloxacin, and colistin. The tigecycline breakpoints with an MIC ≥4 mg/L for Enterobacteriaceae were interpreted based on the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines (http://www.eucast.org/Clinical-breakpoints/). E. coli ATCC25922 served as a control. The string test validated the hypermucoviscous phenotype of K. pneumoniae.24 In brief, the test is considered positive if a bacteriology inoculation loop or needle can create a viscous string longer than 5 mm by stretching bacterial colonies on an agar plate.
ERIC-PCR and Tigecycline-Resistant Mechanism
The Enterobacterial Repetitive Intergenic Consensus (ERIC) methodology serves as a molecular tool employed in epidemiological investigations and bacterial species genotyping.25 The ERIC-PCR assay was conducted with the PCR primers specified in Table S1, as described earlier. The unweighted pair-group method with an arithmetic mean evaluated genetic diversity and categorised isolates with ≥80% identity into a single cluster.26 PCR and Sanger sequencing detected and confirmed tigecycline-resistant genes, ramR, acrR, rpsJ, oqxR, tet(A), tmexCD1-toprJ1, and tet(X). Mutation analysis aligned sequences from each sample against references from wild-type strains, such as E. coli plasmid RP1 (X00006) for the tet(A) genes and K. pneumoniae MGH78578 (CP000647) for the remaining genes.
Conjugation Experiments and Transconjugant Characteristics
The plasmid-encoded blaKPC, blaNDM, and tmexCD1-toprJ1 genes’ transferability was ascertained via conjugation assays. In these assays, KPC-NDM-CRE (CRE co-harbouring blaKPC and blaNDM) strains acted as donors, and the rifampin-resistant E. coli 600 strain was the recipient. Mueller-Hinton agar (MHA, Oxoid) plates with rifampin (200 mg/L) and either meropenem (2 mg/L) or tigecycline (2 mg/L) were used to select transconjugants. Subsequent AST and PCR amplification confirmed successful plasmid transfer. Plasmid fitness cost was gauged using a growth curve assay,27 wherein bacteria were diluted overnight with 1:100 Luria-Bertani broth and cultured at 37 °C and 200 rpm. The OD600 value was tested hourly, with the process repeated thrice.
Analyzing the Prevalence of Reported Strains Co-Carrying blaKPC and blaNDM
The prevalence of reported strains co-carrying blaKPC and blaNDM was analysed by collecting relevant information from PubMed and National Center for Biotechnology Information (NCBI) databases. These included species, temporal data, geographical location, source of specimens, molecular typing, plasmid typing, and other characteristics.
Whole Genome Sequencing (WGS) and Plasmid Analysis
The CF10 strain genome was sequenced using the third-generation Nanopore platform (Oxford, UK) and the second-generation Illumina X Ten sequencing platform (Illumina, San Diego, CA). The other nine CRE strains were sequenced using the second-generation MGI2000 platform (Genomics, China). The software fastp version 0.23.0 (https://github.com/OpenGene/fastp) was employed for data cleaning, with reads of low sequencing quality, higher N proportions, and smaller lengths post-quality pruning removed. SPAdes v.3.5.0 (http://cab.spbu.ru/software/spades/) assembled the trimmed second-generation sequencing data. Prokka v.1.10 (https://github.com/tseemann/prokka) was utilised for gene prediction, while ResFinder v.4.1 (http://genepi.food.dtu.dk/resfinder) predicted antibiotic resistance genes. Virulence genes were predicted using the virulence factor database (VFDB; http://www.mgc.ac.cn/cgi-bin/VFs/v5/main.cgi). The MLST v.2.0 (https://pubmlst.org/) and PlasmidFinder v.2.1 (https://cge.food.dtu.dk/services/PlasmidFinder/) were used to analyse multilocus sequence typing (MLST) and plasmid incompatibility types. ICEberg v.2.0 (http://db-mml.sjtu.edu.cn/ICEberg/) predicted mobile elements like insertion sequences and transposons. A complete K. pneumoniae K-locus reference database can be found at https://github.com/katholt/Kaptive. The CGView server (http://cgview.ca/) visualised the plasmid’s circular representation,28 while BLAST v.2.0 performed linear alignment and comparison of sequences, the results of which were visualised by Easyfig v.2.2.2.29
Evolution Analysis
To conduct evolution analysis, a search for homologous sequences in the NT and PLSDB databases identified 20 plasmids resembling pCF10-tmexCD (with a bidirectional threshold coverage of ≥45%), 28 plasmids resembling pCF10-NDM (with a coverage of ≥48%), and 12 plasmids resembling pCF10-KPC (with a coverage of ≥60%). This was followed by the generation of phylogenetic trees of plasmids using KSNP3, which identified single nucleotide polymorphisms (SNPs) directly based on k-mer and built maximum likelihood trees without a reference sequence. Hierarchical clustering was carried out using the Cluster package from the software package R. Minimum spanning tree analysis was executed using PHYLOVIZ. The evolutionary pathway was constructed using the phylogenetic trees’ genetic distance and sequence coverage. The R ggtree package generated the phylogenetic tree, and the pathway was visualised using a scatter plot (R language).
Ethical Approval Statements
This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Evaluation Committee and the Biomedical Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (2022-0310). Given the study’s retrospective and anonymous nature, the Ethics Committee deemed written informed consent from participants unnecessary.
Results
Clinical Characteristics of KPC-NDM-CRE Strains
This study confirmed ten strains co-harbouring blaKPC and blaNDM. These comprised three K. pneumoniae, five E. coli, one E. cloacae, and one C. freundii strains. Of these, four strains were isolated from the intensive care unit (ICU), and two out of ten patients had sepsis. Additionally, two of the three patients infected with CRKP succumbed to the infection. Table 1 presents these clinical characteristics.
 |
Table 1 Clinical Characteristics of Patients with KPC-NDM-CRE |
Antimicrobial Susceptibility Profiles and String Test
Table S2 reveals that all strains exhibited resistance to cephalosporins, penicillin, and β-lactam compounds. However, all strains were susceptible to aztreonam/avibactam and colistin. The EL82 strain was the only one susceptible to carbapenem, while five strains resisted tigecycline. The CF10 strain demonstrated resistance to tigecycline, with a MIC value of 32 mg/L. All K. pneumoniae strains tested negative in the string test.
Microbiological Characteristics
Molecular typing (Figure 1a) classified K. pneumonia into two MLSTs and two capsule antigen serotypes, and E. coli into three MLSTs and three serotypes. K. pneumonia and E. coli commonly exhibited ST11 and ST410 types. K. pneumonia commonly presented with the capsule antigen serotype KL64. E. cloacae and C. freundii were members of ST50 and ST969, respectively, with ST696 emerging as a new MLST type for C. freundii. ERIC-PCR analysis of genetic relationships disclosed identities ranging from 18% to 85% between the ten strains. Intriguingly, KP21 and KP36 displayed the highest similarity at 85%.
While the NDM plasmids were successfully transferred by two strains (EC39 and EC42), the KPC plasmids could not be transferred by conjugation. The EC42T-NDM transconjugant was susceptible to meropenem and imipenem, contrasting with the resistance exhibited by EC42 (Table S3). The fitness cost of acquiring the blaNDM plasmids was also evaluated. Interestingly, no significant difference in growth rates was observed between the recipient strain E. coli 600 and the transconjugants bearing NDM plasmids (Figure 1c).
Identification of Antimicrobial Resistance Genes and Virulence Genes
WGS data were employed to predict the antimicrobial resistance genes and virulence genes of the ten KPC-NDM-CRE isolates, which were depicted in Figure 1a and b. Apart from the high frequency and diversity of resistance genes, the three K. pneumoniae and five E. coli strains also exhibited a substantial number of virulence genes. The distribution of resistance genes is outlined in Table S4. Notably, the iutA gene recorded the highest occurrence rate of virulence genes, followed by iucABCD, fimA, and fimH. Table S5 summarised the results of the mutation sites of the five tigecycline-resistant CRE isolates. The tet(A) variants with type 1 mutations (I5R, V55M, I75V, T84A, S201A, F202S, and V203F) were found in all tigecycline-resistant isolates.21 A93V, classified as a type 2 mutation, was detected in KP36 and EC85. Interestingly, CF10 had two copies of tet(A) genes, and the mutations in one of the copies included the type 1 mutations along with 20 other mutation sites. Two isolates revealed nucleotide changes in ramR (A19V) compared to that of the reference strain MGH78578 (CP000647). The tet(X) was absent in five strains, but tmexCD1-topJ1 was detected in CF10.
Reported Prevalence of Co-Carrying blaKPC and blaNDM Isolates
A total of 51 strains co-harbouring blaKPC and blaNDM were collected (Table S6) from Pubmed and NCBI. Among these, ten strains of ST15 CRKP have been prevalently found in Turkey,30 and three strains of ST11 CRKP were transmitted in Oman31 as clones. K. pneumoniae accounted for the highest proportion at 45% among the 51 strains, followed by E. cloacae at 11.8%. KPC-NDM-CRE have been reported globally, with China having the highest number of strains at 47% (Figure 2). The primary source of these bacteria was blood, potentially responsible for 16% of deaths, with most fatalities having been infected with K. pneumoniae. The most prevailing types of K. pneumoniae strains were ST11 and ST15. The strain IR98, discovered in India in 2010, carried both blaKPC and blaNDM, and was also tigecycline-resistant.3 Additionally, 25.5% of strains were non-susceptible to tigecycline, and potentially more as 21.5% of the strains did not have a tigecycline susceptibility result. Notably, KPC-NDM-CRE strains have been identified in hospital sewage, river sediment, vegetables, and retail food.32 Further details are provided in Table S6.
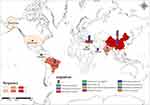 |
Figure 2 Prevalence and distribution of co-carrying blaKPC and blaNDM isolates. Different colours are used to represent different organisms and frequencies. |
Genetic Characteristics of CF10
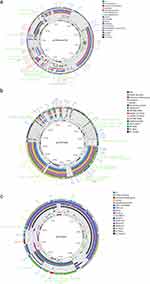
WGS data revealed that the CF10 strain had a chromosome of 5,010,143 bp and eight plasmids. The pCF10-tmexCD1 plasmid had a length of 212,154 bp, with a total of 186 open reading frames (ORFs) and a GC content ratio of 51.54% (Table S7). It belonged to the IncHIA+IncHIB group as it contained a replication gene repHIA and an additional repHIB gene (Figure 3a). BLASTn search analysis indicated that the pCF10-tmexCD1 plasmid had a 99.95% identity and 57% query coverage (the ratio of the query sequence length that aligned with the database sequence) with the following plasmids: pMH13-051M_1 (AP018572.2), pEC-13-33-NDM-1 (MZ836798.1), p7_SCLZS62 (CP082175.1), and pCHS4.3-1 (OL964513.1). The pCF10-tmexCD1 plasmid, interestingly, comprised a 52-kb multidrug-resistant region (MDR) flanked by multiple transposase genes, with IS26 being the most common (Figure 4a). These resistance genes in MDR included the tmexCD1-torJ1, blaTEM-1B, aph(6’)-Id, aph(3’)-Ib, aadA2, mph(A), msr(E), qnrS1, sul2, sul1, tet(A)1, tet(A)2, and dfrA12 gene clusters.
Furthermore, a 32-kb sequence containing the tmexCD1-toprJ1 gene bore a strong resemblance to pHNAH8I-1 (MK347425.1) from K. pneumoniae, found in a chicken farm in Anhui province in 2017, with 99.96% identity and 67% query coverage. Comparative analysis of the genetic context (Figure 4b) revealed a common genetic structure, Tn5393-int1-like-int2-like-hp1-hp2-tnfxB1-tmexCD1-toprJ1, which was most likely derived from Tn5393. In addition to Tn5393, IS26 also participated in the transfer of tmexCD1-toprJ1, qnrS, and tet(A) genes, which were located upstream of tet(A) and downstream of qnrS. In contrast to the strA-strB of Tn5393-3’ retained in pHNAH8I-1, which formed the tnpR-strA-strB structure, a Tn6361 remnant was found downstream of tnpR in pCF10-tmexCD1, forming the tnpR-qnrS1-IS26 structure. The ΔTn6361 was likely acquired in pCF10-tmexCD1 through massive recombination events occurring between the two copies of tnpR.
The pCF10-NDM was a 138,196-bp plasmid with an IncFII(Yp)-type replicon that contained 155 predicted ORFs and a GC content ratio of 51.49% (Table S7). Additionally, a whole plasmid BLASTn search found pCF10-NDM to have 99.96% identity and 47% query coverages with p205880-NDM in K. pneumoniae (MH909345.1), pKOX_NDM1 in K. michiganensis (JQ314407.1), and pNDM1_015096 in K. pneumoniae (CP043589.1). The aligned region between the pCF10-NDM and references contained both conjugation-related regions and blaNDM-bearing MDR (Figure 3b). Almost identical to pNDM-SCNJ07 (MK933278.1) from an Enterobacter hormaechei isolated in Chengdu, the genetic context of blaNDM-1 between them had a 99.95% identity (Figure 4c). In both plasmids, the transposition units had been organised in an IS5-ISEhe3-ISEhe-ISCR21-groEL-groES-cutA-dsbC-trpF-bleMBL-blaNDM-1-ISKpn26-ISKpn26 structure. More often than not, the blaNDM-1 gene was surrounded by downstream genes (bleMBL-trpF-dsbC-cutA1-groL) flanked by IS5, which implied IS5’s involvement in the mobilisation of the blaNDM-1 gene.
The 49-kb pCF10-KPC was identified as an IncN3-type plasmid, comprising 85 predicted ORFs and having a GC content ratio of 51.52% (Table S7). Two resistance genes (blaKPC-2 and blaMOX-3) and five mobile elements were present in the pCF10-KPC plasmid (Figure 3c). The backbone of pCF10-KPC, when searched through BLASTn, showed 99.9% identity and 64% query coverage with pZZ40-KPC (MN891679.1), pBKPC18-1 (CP022275.1), pCRE1.4 (CP034398.1), and pD18-1 (CP022277.1). The sequences aligned between these plasmids lacked any multi-resistant regions. A high degree of homology was found in the genetic context of blaKPC-2 to the plasmid pKP048 (FJ628167) from K. pneumoniae (Figure 4d). This plasmid was a 12-kb fragment bordered by Tn3 transposase with a genetic structure tnpA-tnpR-ISKpn27-blaKPC-2-ISKpn6-klcA-tnpA. This unit was recognised as a transposon from the Tn6296 group,33 a transposon unit of the Tn21 subfamily of the Tn3 family, which has been largely viewed as a significant vector for blaKPC-2, first found in plasmid pKP048.34
Evolutionary Pathway of CF10
In the plasmids phylogenetic tree analysis, no similar plasmids were identified with a bidirectional coverage of ≥90%. Bidirectional coverage thresholds were subsequently adjusted to 45%, 48%, and 60% for tmexCD1, NDM, and KPC plasmids, respectively (Figure 5a–c). The MLST minimum spanning tree also demonstrated CF10’s emergence as a new ST type 696, a derivative of ST95 (Figure 5d). A comparison with the WGS of CF10 from the GenBank database revealed no similar genome sequences with a query coverage ≥90%. The phylogenetic tree indicated that CF10 occupied a distinct branch (Figure 6a). Additionally, GCF_905330765_2 was identified as the genome sequence most similar to CF10 in the phylogenetic tree, with a difference of 104,098 SNPs between them. The phylogenetic distance evolutionary pathway showed that the tmexCD1, NDM, and KPC plasmids were closely associated with the CF10 genome (Figure 6b).
Discussion
The proliferation of CRE infections in hospitals has curtailed treatment alternatives, and the emergence of carbapenem resistance has posed significant clinical challenges.9 With tigecycline dubbed the “last resort” of defence against CRE, the combined resistance of tigecycline and carbapenem in CRE has emerged as a pressing global health issue.35 In this study, the molecular epidemiological characteristics of ten CRE co-carrying blaKPC and blaNDM were examined. Notably, resistance to tigecycline was found in five of the ten KPC-NAM-CRE. Critically, the coexistence of blaKPC-2, blaNDM-1, and tmexCD1-toprJ1 genes in a C. freundii strain CF10 was reported for the first time. To date, such a presence has been reported solely in K. pneumoniae in China. Our findings undeniably raise an epidemiological alarm, offering deeper insights into the mechanisms of tigecycline resistance and underscoring the urgent need for enhanced infection control within hospital settings.
Based on patients data, the two individuals infected with tigecycline-resistant ST11-KL64 CRKP succumbed while in the hospital, illustrating the heightened mortality rate associated with CRKP, exacerbated by tigecycline resistance. Long-standing selective pressure from tigecycline has resulted in a build-up of gene mutations and increased efflux pump gene expression, leading to decreased tigecycline susceptibility.36 Previously, mutations in the tet(A) genes were identified as the chief contributors to tigecycline resistance among CRKP isolates.2 In our investigation, all tigecycline-resistant CRE presented with type 1 mutations in the tet(A) variants. It has been noted that ST11-KL64 CRKP is gradually overtaking ST11-KL47 as the dominant hypervirulent CRKP clone in China, leading to heightened patient mortality.37 The enhanced toxicity and tigecycline resistance of these ST11-KL64 strains amplify the death risk. The emergence and rising prevalence of ST11-K64 CRKP strains underscore the urgency for immediate containment strategies.
In our research, the genes tmexCD1-toprJ1, blaKPC-2, and blaNDM-1 were identified within the singular CF10 strain but were found on separate plasmids: pCF10-tmexCD1, pCF10-NDM, and pCF10-KPC. It is plausible that such plasmids have become tailored to C. freundii over an extended time. Conjugation tests only successfully introduced the blaNDM-bearing plasmids from EC39 and EC42. The MIC values for EC39T-NDM towards imipenem and meropenem decreased substantially, yet EC39T-NDM remained resistant. This suggests other potential resistance mechanisms, like efflux pumps and porin loss,11 might be at play in EC39. In contrast, EC40T-NDM displayed susceptibility to both antibiotics after a 16-fold decrease in MIC values, possibly because of a truncated blaNDM gene in EC40T-NDM. Moreover, the high transferability combined with the low fitness cost of blaNDM-bearing plasmids emphasises the need to halt the proliferation of CRE strains containing both blaKPC and blaNDM.
Interestingly, a BLASTn analysis using the megablast function revealed that no plasmids demonstrated more than 90% identity and over 70% coverage with pCF10-tmexCD1, pCF10-NDM, and pCF10-KPC in the NCBI’s non-redundant Nucleotide collection (nr/nt) database. Both the MLST’s minimum spanning tree and the phylogenetic tree indicated that CF10 represented a novel branch. WGS data suggested that the CF10 strain, including its plasmids, did not share evolutionary origins with known strains and plasmids due to limited query coverages (≤70%). IS26 was found surrounding various resistance genes such as sul2, qnrS, and tet(A), highlighting its role in the lateral transfer of these genes, as observed in Gram-negative bacteria.38 Furthermore, it was reported that the plasmid-harboured tmexCD1-toprJ1 gene was present in 2.5% and 52.4% tigecycline-nonsusceptible K. pneumoniae strains isolated from humans and animals, respectively.13 The current prevalence of tmexCD-toprJ across various clinical pathogens necessitates ongoing monitoring.39 The rise of tmexCD1-toprJ1 within CRE strains has hastened the dissemination of tigecycline resistance, underscoring the need for worldwide oversight to thwart multi-resistance transmission. Crucially, discovering strains with coexistent blaKPC and blaNDM in both river sediment and food products highlights a transition from the natural environment to clinical strains, expediting the lateral transfer of resistance genes and amplifying public health safety concerns.40
This research has its limitations. The genomic and evolutionary traits of the nine sequenced CRE strains from the second generation were not studied. A more comprehensive WGS data deserves further analysis of the evolution characteristics. The impacts of tigecycline resistance gene mutations on susceptibility were not experimentally verified.
Conclusion
To conclude, this study has unveiled ten clinical CRE strains co-carrying blaKPC and blaNDM. We have presented the first instance of a tigecycline-resistant C. freundii strain, CF10, containing tmexCD1-toprJ1, blaKPC-2, and blaNDM-1 plasmids. This represents a formidable “multidrug-resistant superbug” responsible for sepsis within a hospital setting.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (X1919XYTSC). We appreciate the technical support of visionmedicals and hugobiotech for evolutionary analysis.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang Y, Wang Q, Yin Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae Infections: report from the China CRE network. Antimicrob Agents Chemother. 2018;62(2). doi:10.1128/AAC.01882-17
2. Tumbarello M, Viale P, Bassetti M, De Rosa FG, Spanu T, Viscoli C. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study--authors’ response. J Antimicrob Chemother. 2015;70(10):2922. doi:10.1093/jac/dkv200
3. Kumarasamy K, Kalyanasundaram A. Emergence of Klebsiella pneumoniae isolate co-producing NDM-1 with KPC-2 from India. J Antimicrob Chemother. 2012;67(1):243–244. doi:10.1093/jac/dkr431
4. Pereira PS, Borghi M, Albano RM, et al. Coproduction of NDM-1 and KPC-2 in Enterobacter hormaechei from Brazil. Microb Drug Resist. 2015;21(2):234–236. doi:10.1089/mdr.2014.0171
5. Wu W, Feng Y, Carattoli A, Zong Z. Characterization of an Enterobacter cloacae strain producing both KPC and NDM carbapenemases by whole-genome sequencing. Antimicrob Agents Chemother. 2015;59(10):6625–6628. doi:10.1128/AAC.01275-15
6. Wu W, Espedido B, Feng Y, Zong Z. Citrobacter freundii carrying blaKPC-2 and blaNDM-1: characterization by whole genome sequencing. Sci Rep. 2016;6(1):30670. doi:10.1038/srep30670
7. Feng J, Qiu Y, Yin Z, et al. Coexistence of a novel KPC-2-encoding MDR plasmid and an NDM-1-encoding pNDM-HN380-like plasmid in a clinical isolate of Citrobacter freundii. J Antimicrob Chemother. 2015;70(11):2987–2991. doi:10.1093/jac/dkv232
8. Li X, Zhu Y, Shen M, Du J, Zhang L, Wang D. Draft genome sequence of Enterobacter cloacae HBY, a ST128 clinical strain co-producing KPC-2 and NDM-1 carbapenemases. J Glob Antimicrob Resist. 2018;12:1–2. doi:10.1016/j.jgar.2017.10.022
9. Alhashem F, Tiren-Verbeet NL, Alp E, Doganay M. Treatment of sepsis: what is the antibiotic choice in bacteremia due to carbapenem resistant Enterobacteriaceae? World J Clin Cases. 2017;5(8):324–332. doi:10.12998/wjcc.v5.i8.324
10. Hoban DJ, Bouchillon SK, Johnson BM, Johnson JL, Dowzicky MJ. In vitro activity of tigecycline against 6792 Gram-negative and Gram-positive clinical isolates from the global Tigecycline Evaluation and Surveillance Trial (TEST Program, 2004). Diagn Microbiol Infect Dis. 2005;52(3):215–227. doi:10.1016/j.diagmicrobio.2005.06.001
11. Sheng ZK, Hu F, Wang W, et al. Mechanisms of tigecycline resistance among Klebsiella pneumoniae clinical isolates. Antimicrob Agents Chemother. 2014;58(11):6982–6985. doi:10.1128/AAC.03808-14
12. Lv L, Wan M, Wang C, et al. Emergence of a plasmid-encoded resistance-nodulation-division efflux pump conferring resistance to multiple drugs, including tigecycline, in Klebsiella pneumoniae. mBio. 2020;11(2). doi:10.1128/mBio.02930-19
13. Sun S, Gao H, Liu Y, et al. Co-existence of a novel plasmid-mediated efflux pump with colistin resistance gene mcr in one plasmid confers transferable multidrug resistance in Klebsiella pneumoniae. Emerg Microbes Infect. 2020;9(1):1102–1113. doi:10.1080/22221751.2020.1768805
14. Hirabayashi A, Ha VTT, Nguyen AV, Nguyen ST, Shibayama K, Suzuki M. Emergence of a plasmid-borne tigecycline resistance in Klebsiella pneumoniae in Vietnam. J Med Microbiol. 2021;70(3). doi:10.1099/jmm.0.001320
15. Peng K, Wang Q, Yin Y, et al. Plasmids shape the current prevalence of tmexCD1-toprJ1 among Klebsiella pneumoniae in food production chains. mSystems. 2021;6(5):e0070221. doi:10.1128/mSystems.00702-21
16. Li R, Peng K, Xiao X, Liu Y, Peng D, Wang Z. Emergence of a multidrug resistance efflux pump with carbapenem resistance gene blaVIM-2 in a Pseudomonas putida megaplasmid of migratory bird origin. J Antimicrob Chemother. 2021;76(6):1455–1458. doi:10.1093/jac/dkab044
17. Xu J, Zhu Z, Chen Y, Wang W, He F. The plasmid-borne tet(A) gene is an important factor causing tigecycline resistance in ST11 carbapenem-resistant Klebsiella pneumoniae under selective pressure. Front Microbiol. 2021;12:644949. doi:10.3389/fmicb.2021.644949
18. Shahada F, Sekizuka T, Kuroda M, et al. Characterization of Salmonella enterica serovar Typhimurium isolates harboring a chromosomally encoded CMY-2 beta-lactamase gene located on a multidrug resistance genomic island. Antimicrob Agents Chemother. 2011;55(9):4114–4121. doi:10.1128/AAC.00560-11
19. Sommer MOA, Dantas G, Church GM. Functional characterization of the antibiotic resistance reservoir in the human microflora. Science. 2009;325(5944):1128–1131. doi:10.1126/science.1176950
20. Akinbowale OL, Peng H, Barton MD. Diversity of tetracycline resistance genes in bacteria from aquaculture sources in Australia. J Appl Microbiol. 2007;103(5):2016–2025. doi:10.1111/j.1365-2672.2007.03445.x
21. Chiu S-K, Huang L-Y, Chen H, et al. Roles of ramR and tet(A) mutations in conferring ti resistance in carbapenem-resistant Klebsiella pneumoniae clinical isolates. Antimicrob Agents Chemother. 2017;61(8). doi:10.1128/AAC.00391-17
22. Akiyama T, Presedo J, Khan AA. The tetA gene decreases tigecycline sensitivity of Salmonella enterica isolates. Int J Antimicrob Agents. 2013;42(2):133–140. doi:10.1016/j.ijantimicag.2013.04.017
23. Humphries R, Bobenchik AM, Hindler JA, Schuetz AN, McAdam AJ. Overview of changes to the clinical and laboratory standards institute performance standards for antimicrobial susceptibility testing, M100, 31st Edition. J Clin Microbiol. 2021;59(12):e0021321. doi:10.1128/JCM.00213-21
24. Shon AS, Bajwa RPS, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4(2):107–118. doi:10.4161/viru.22718
25. Ye Y, Wu Q, Yao L, Dong X, Wu K, Zhang J. Analysis of a consensus fragment in ERIC-PCR fingerprinting of Enterobacter sakazakii. Int J Food Microbiol. 2009;132(2–3):172–175. doi:10.1016/j.ijfoodmicro.2009.03.018
26. Codjoe FS, Brown CA, Smith TJ, Miller K, Donkor ES, Duse AG. Genetic relatedness in carbapenem-resistant isolates from clinical specimens in Ghana using ERIC-PCR technique. PLoS One. 2019;14(9):e0222168. doi:10.1371/journal.pone.0222168
27. Hirabayashi A, Dao TD, Takemura T, et al. A Transferable IncC-IncX3 hybrid plasmid cocarrying blaNDM-4, tet(X), and tmexCD3-toprJ3 confers resistance to carbapenem and tigecycline. mSphere. 2021;6(4):e0059221. doi:10.1128/mSphere.00592-21
28. Stothard P, Grant JR, Van Domselaar G. Visualizing and comparing circular genomes using the CGView family of tools. Brief Bioinform. 2019;20(4):1576–1582. doi:10.1093/bib/bbx081
29. Sullivan MJ, Petty NK, Beatson SA. Easyfig: a genome comparison visualizer. Bioinformatics. 2011;27(7):1009–1010. doi:10.1093/bioinformatics/btr039
30. Tekeli A, Dolapci İ, Evren E, Oguzman E, Karahan ZC. Characterization of Klebsiella pneumoniae coproducing KPC and NDM-1 Carbapenemases from Turkey. Microb Drug Resist. 2020;26(2):118–125. doi:10.1089/mdr.2019.0086
31. Balushi MA, Kumar R, Al-Rashdi A, et al. Genomic analysis of the emerging carbapenem-resistant Klebsiella pneumoniae sequence type 11 harbouring Klebsiella pneumoniae carbapenemase (KPC) in Oman. J Infect Public Health. 2022;15(10):1089–1096. doi:10.1016/j.jiph.2022.08.014
32. Wang J, Yao X, Luo J, Lv L, Zeng Z, Liu J-H. Emergence of Escherichia coli co-producing NDM-1 and KPC-2 carbapenemases from a retail vegetable, China. J Antimicrob Chemother. 2018;73(1):252–254. doi:10.1093/jac/dkx335
33. Wang X, Xiao W, Li L, et al. Analysis of the molecular characteristics of a blaKPC-2-harbouring untypeable plasmid in Serratia marcescens. Int Microbiol. 2022;25(2):237–244. doi:10.1007/s10123-021-00172-2
34. Wang D, Zhu J, Zhou K, et al. Genetic characterization of novel class 1 Integrons In0, In1069 and In1287 to In1290, and the inference of In1069-associated integron evolution in Enterobacteriaceae. Antimicrob Resist Infect Control. 2017;6(1):84. doi:10.1186/s13756-017-0241-9
35. Chen J, Zeng Y, Zhang R, Cai J. In vivo emergence of colistin and tigecycline resistance in carbapenem-resistant hypervirulent Klebsiella pneumoniae during antibiotics treatment. Front Microbiol. 2021;12:702956. doi:10.3389/fmicb.2021.702956
36. Yan WJ, Jing N, Wang SM, et al. Molecular characterization of carbapenem-resistant Enterobacteriaceae and emergence of tigecycline non-susceptible strains in the Henan province in China: a multicentrer study. J Med Microbiol. 2021;70(3). doi:10.1099/jmm.0.001325
37. Zhou K, Xiao T, David S, et al. Novel subclone of carbapenem-resistant Klebsiella pneumoniae sequence type 11 with enhanced virulence and transmissibility, China. Emerg Infect Dis. 2020;26(2):289–297. doi:10.3201/eid2602.190594
38. Varani A, He S, Siguier P, Ross K, Chandler M. The IS6 family, a clinically important group of insertion sequences including IS26. Mob DNA. 2021;12(1):11. doi:10.1186/s13100-02100239-x
39. Dong N, Zeng Y, Wang Y, et al. Distribution and spread of the mobilised RND efflux pump gene cluster tmexCD-toprJ in clinical Gram-negative bacteria: a molecular epidemiological study. Lancet Microbe. 2022;3(11):e846–e856. doi:10.1016/S2666-5247(22)00221-X
40. Dang B, Zhang H, Li Z, Ma S, Xu Z. Coexistence of the bla(NDM-1)-carrying plasmid pWLK-NDM and the bla(KPC-2)-carrying plasmid pWLK-KPC in a Raoultella ornithinolytica isolate. Sci Rep. 2020;10(1):2360. doi:10.1038/s41598-020-59341-4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.