Back to Journals » Clinical Epidemiology » Volume 14
Elucidating Pathways Mediating the Relationship Between Male Sex and COVID-19 Severity
Authors Stalter RM, Atluri V, Xia F, Thomas KK, Lan KF, Greninger AL, Patel RC
Received 31 August 2021
Accepted for publication 9 December 2021
Published 25 January 2022 Volume 2022:14 Pages 115—125
DOI https://doi.org/10.2147/CLEP.S335494
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eyal Cohen
Randy M Stalter,1 Vidya Atluri,2 Fan Xia,1 Katherine K Thomas,3 Kristine F Lan,2 Alexander L Greninger,4 Rena C Patel2
1Department of Epidemiology, University of Washington, Seattle, WA, USA; 2Department of Medicine, University of Washington, Seattle, WA, USA; 3Department of Global Health, University of Washington, Seattle, WA, USA; 4Department of Laboratory Medicine and Pathology, University of Washington, Seattle, WA, USA
Correspondence: Randy M Stalter, Department of Epidemiology, University of Washington, UW Box 359927 325 Ninth Avenue, Seattle, WA, 98104, USA, Tel +1-412-760-8134, Email [email protected]
Purpose: To examine associations between male sex and SARS-CoV-2 test positivity, severe COVID-19 disease, and death in a single-site cohort, and assess whether male sex impacts risk for severe COVID-19 disease through socioeconomic status (SES), comorbidities, or inflammation.
Materials and Methods: We conducted a retrospective cohort study with data collected from University of Washington Medicine EMR from March 1 to September 29, 2020. All persons, regardless of age, were included if they had a conclusive diagnostic COVID-19 PCR test result. Our exposure was sex assigned at birth. We used Poisson regression to assess associations between sex and COVID-19 test positivity, disease severity and COVID-19 related death, and linear regression to compare viral cycle threshold at the first positive test. We conducted mediation analyses to assess interventional indirect effects of male sex on severe COVID-19 risk through socioeconomic status (SES, based on area deprivation and insurance type), comorbidities, and inflammation status. Models controlled for age and race/ethnicity.
Results: Of 32,919 males and 34,733 females included, 1469 (4.5%) and 1372 (4.0%) tested positive for SARS-CoV-2, respectively. Males were 14% more likely to test positive (RR = 1.14; 95% CI: 1.06– 1.23), had 80% higher risk for severe COVID-19 disease (RR = 1.80; 95% CI: 1.39– 2.33) and had 58% higher risk for death (RR = 1.58; 95% CI: 1.10– 2.26) compared to females after adjusting for age and race/ethnicity. Mediation analyses indicated non-significant interventional indirect effects of male sex on severe COVID-19 disease through elevated inflammatory markers, SES and comorbidities, but the greatest effect was through the inflammation pathway.
Conclusion: Males appear to be at higher risk at all steps of the continuum of COVID-19 illness. The strongest mediating signal, albeit non-significant, is with inflammatory pathways. Further elucidation of causal pathways linking sex and COVID-19 severity is needed in larger cohorts.
Keywords: SARS-CoV-2, disease severity, mediation, sex-based differences
Introduction
More than a year into the global COVID-19 pandemic, we have learned much more about the disease, including that males appear more vulnerable to acquiring the infection, developing severe disease, and death compared to females.1 From the early reports from China2–6 to the latest global sex-disaggregated data tracking,7 case fatality appears higher in males than females nearly universally. Yet, exactly why that is the case continues to evade us.
Several hypotheses exist for why males may face greater vulnerability to COVID-19 as compared to females that largely fall into two major pathways. The first is due to socio-behavioral factors, such as healthcare-seeking behaviors, gendered behaviors (eg, smoking), or social networks and socialization behaviors.8–10 The second is due to biologic factors, such as sex hormone-regulated expression of angiotensin converting enzyme-2 (ACE-2) receptors, which facilitate viral entry, or immune responses.9,11,12 Furthermore, evaluations of interactions between sex and factors such as race/ethnicity, socioeconomic status (SES), and existing comorbidities and sex are often lacking, clouding our understanding of any associations observed with sex and COVID-19 outcomes. Limited work has been done to explore causal pathways by which male sex affects risk of severe disease. Identifying these pathways could yield crucial insights into future clinical management and vaccination plans.
To better elucidate underlying associations between sex and COVID-19, and potential causal pathways, we conducted a retrospective cohort analysis to assess interaction and mediation by sociodemographic, clinical, and biologic factors. Our hypothesis was that sex differences would not exist in COVID-19 test positivity, but males would be at greater risk for more severe COVID-19 disease, including death, than females. Furthermore, we hypothesized that certain factors, namely SES, comorbidities, and inflammation, mediate the relationship between male sex and severe COVID-19 disease.
Materials and Methods
Study Setting, Site, and Population
We conducted a retrospective cohort study of electronic medical record (EMR) data collected from March 1 through September 29, 2020, from three hospitals within the University of Washington Medicine (UWM) system. These facilities included an academic medical center, a county hospital and a community hospital affiliated with UWM. The UW Human Subjects Division approved this study (STUDY00005397). This study met the regulatory criteria for waiver of the consent process requirement, including evaluation of a public benefit or service program and being of minimal risk to participants. We conducted this study in accordance with the Declaration of Helsinki.
All persons, regardless of age, were included if they had a conclusive diagnostic COVID-19 polymerase chain reaction (PCR) test result and had their sex recorded in the EMR.
Variable Definitions
Our exposure was sex assigned at birth as recorded in individuals’ EMR file. We defined COVID-19 test positivity as a positive SARS-CoV-2 PCR test result; persons with a positive antibody or antigen test result only were not considered COVID-19 positive. We excluded persons with inconclusive PCR results from analysis. We defined COVID-19 severity, our primary outcome, using the 11-point World Health Organization clinical progression scale, which categorizes severity from uninfected with no viral DNA detected (score of 0) to death (score of 10).13 Because we were unable to collect certain indicators of clinical progression as delineated in the scale, such as COVID-19 symptoms and PaO2/FiO2 ratio and SpO2/FiO2 ratio when mechanical ventilation was required, we could not accurately classify individuals on certain elements of the ordinal scale. Therefore, outcomes were dichotomized. Scores >5, which requires hospitalization with non-invasive ventilation or higher order of ventilatory or life support, were considered severe COVID-19 infections as delineated by the scale. We defined death as death by any cause occurring any time after an individual’s COVID-19 diagnosis. We defined hospitalization as having a record of inpatient admission following a COVID-19 diagnosis. We did not consider an emergency room visit only as a hospitalization. Individuals who were hospitalized may not have necessarily met the criteria for severe COVID-19 disease. We recorded individuals’ SARS-CoV-2 cycle threshold measured at the time of the first positive PCR test result by one of the following platforms: Hologic Panther Fusion, Roche Cobas, and a laboratory-developed test based on primer sets from the Centers for Disease Control and Prevention. All three assays have been shown to have good sensitivity for detection of SARS-CoV-2.11 Where multilocus testing was performed, we used the lowest cycle threshold value.
Using the EMR, we collected information on covariates/mediators at the time of the first positive SARS-CoV-2 PCR result for individuals who received any positive result and the last negative result for individuals who never tested positive. These variables included age, race/ethnicity group, binary SES based on whether individuals had income-based insurance and/or lived in an area with area deprivation greater than the national median,14,15 Charlson Comorbidity Index (CCI) score (possible range: 0–33),16 and inflammation variable indicating the number of the following inflammatory markers (possible range: 0–8) above the upper limit of the normal reference range:17 interleukin (IL)-6, erythrocyte sedimentation rate (ESR), ferritin, high sensitivity C reactive protein (HSCRP), lactate dehydrogenase (LDH), lymphocytes, neutrophils, and white blood cells. Additional information on covariate construction can be found in the Supplemental Methods.
Missing Data
We used multiple imputations by chained equations to account for missingness of key analysis variables, including age, race/ethnicity and SES. Imputation models included variables from our primary models as predictors (sex, severe COVID-19 status, age, race/ethnicity, SES, comorbidities) and other sociodemographic and clinical characteristics available within our dataset, including facility, testing calendar month, marital status, and language preference. Twenty imputed datasets were generated. For inflammatory status, we imputed a zero for individuals who had no laboratory measurements available within 14 days of their first positive SARS-CoV-2 test with the assumption that individuals who were missing measurements likely were not ill enough to elicit blood testing and results of these tests would have indicated no elevated markers.
Statistical Analysis
We used Poisson regression with robust standard errors to assess the relative risk (RR) between sexes for COVID-19 test positivity, severe disease, hospitalization and death, and linear regression to compare viral cycle threshold at the first positive test. Results were stratified by sociodemographic, clinical, and biologic characteristics and separate interaction models were fit to assess for effect modification by these factors. We conducted mediation analyses to quantify the effect of male sex on severe COVID-19 risk through different causal pathways. The mediators we considered, which we hypothesized to lie on the causal pathway between male sex (exposure) and COVID-19 disease severity (outcome), were low SES, CCI score, and inflammation status as defined above; our proposed causal diagram is shown in Figure 1. We first quantified the total effect of male sex on COVID-19 severity. Then, we decomposed the total effect into interventional indirect and direct effects, which act through and independent of the mediating pathways, respectively. The interventional indirect effect through each mediator indicates the reduction in severe COVID-19 risk that would be seen if the distribution of the mediator was shifted from what it would be under male sex to female sex. For inference, nonparametric, bootstrapped confidence intervals were computed for each effect. To evaluate whether imputation of missing values for the inflammation variable biased our estimates, we re-ran the mediation models among the subset of individuals who had laboratory measurements recorded. All models controlled for age and race/ethnicity. All data analyses were conducted using R version 3.6.1 (R Core Team, Vienna, Austria).
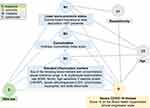 |
Figure 1 Basic causal diagram of the relationship between male sex and COVID-19 severity. |
Results
Individuals’ Characteristics
Of 74,426 individuals who had a clinical encounter involving SARS-CoV-2 testing within the three UWM hospitals during our study period, 67,693 had a conclusive PCR test result (Figure 2). Forty-one of these individuals did not have their sex recorded in the EMR and were excluded. In total, we included 67,652 individuals in analyses to assess differences in risk of test positivity. A positive test was recorded for 2841 individuals (4.2% test positivity).
 |
Figure 2 Flow diagram of participant inclusion. |
Among individuals who underwent testing, 49% of males and 44% of females were ≥50 years of age (Table 1). Approximately one-third of males and females were married or in a domestic partnership. Most commonly, individuals identified as non-white (41%) while <10% identified as non-Latinx Black, Latinx, Asian/Asian American or Native Alaskan/American/Hawaiian. Males and females had comparable SES indicators (insurance type, area deprivation). Hypertension and other cardiovascular disease, asthma and other respiratory disease and autoimmune diseases were the most prevalent comorbidities. Among individuals who received a positive SARS-CoV-2 test result, there were notable differences in certain characteristics. Nearly a quarter of individuals identified as Latinx and over 10% identified as non-Latinx Black. Additionally, over a quarter of individuals preferred a language other than English.
 |
Table 1 Individual Characteristics at Time of SARS-CoV-2 PCR Test |
Association of Male Sex with Test Positivity
Among 32,919 males who had a recorded SARS-CoV-2 test, 1469 (4.5%) received a positive result compared to 1372 of 34,733 (4.0%) females. In multivariable regression adjusting for age and race/ethnicity, males had an estimated 14% higher risk of testing positive compared to females (RR = 1.14; 95% confidence interval [CI]: 1.06–1.23; p<0.001) (Supplemental Table 1). Within race/ethnicity groups, positivity was highest among individuals who identified as Latinx (12.5% of males, 10.6% of females), Native Alaskan/American/Hawaiian (6.6% of males, 7.2% of females) and non-Latinx Black (5.9% of males and 6.9% of females), and lowest among non-Latinx white (2.6% of males and 2.1% of females). Among individuals who identified as non-Latinx Black, there was no significant difference in positivity between males and females; this comparison significantly differed from the RR of positivity between white males and females in interaction models (p=0.02).
Association of Male Sex with COVID-19 Severity
Our primary outcome of interest was severe COVID-19 disease among individuals who received a positive test result. Of 1469 males who tested positive, 145 (9.9%) developed severe COVID-19, our primary outcome of interest, after their diagnosis in contrast to 77 of 1372 females (5.6%) (Table 2). Overall, males had 80% higher risk of severe COVID-19 compared to females in multivariable regression models (RR = 1.80, 95% CI: 1.39–2.33; p<0.001). Unlike the test positivity models, no significant interactions were observed between any of the tested subgroups. Higher male vs female, albeit insignificant, RRs were estimated for individuals who identified as non-Latinx Black (RR = 6.95, 95% CI: 0.96–50.13; p=0.05) or Native Alaskan/American/Hawaiian (RR=2.58, 95% CI: 0.59–11.18; p=0.21). Within comorbidity-based subgroups, the risk of severe COVID-19 was more than three-times higher among males compared to females among individuals without comorbidities (RR=3.14, 95% CI: 1.56–6.33; p=0.001).
 |
Table 2 Association of Male Sex with Severe COVID-19 Disease Among Individuals Who Received a Positive SARS-CoV-2 PCR Result |
Association of Male Sex with Hospitalization, Death and Cycle Threshold
Other secondary outcomes included COVID-19 related hospitalization, any-cause death and viral cycle threshold. Males were 30% more likely than females to be hospitalized (RR = 1.30, 95% CI: 1.11–1.51; p=0.001) and 58% more likely to die following a positive test (RR=1.58, 95% CI: 1.10–2.26; p=0.014) compared to females in adjusted models (Supplemental Tables 2 and 3). Interaction models showed no significant differences in RR estimates between analysis subgroups. Additionally, we observed no significant difference in mean cycle threshold between males (mean = 25.2, standard deviation [SD]=7.4) and females (mean = 26.1, SD = 7.7) at first COVID-19 positive test result (p = 0.69) after adjustment for age and race/ethnicity.
Mediators of the Relationship Between Male Sex and COVID-19 Severity
In mediation analyses, the total causal effect of male sex on COVID-19 disease severity indicated an 80% increase in severe disease risk (RR = 1.80; 95% CI: 0.84–2.77) (Table 3), an estimate similar to our main model but with a wider confidence interval due to use of the more conservative nonparametric bootstrapping method to estimate standard errors. Of 432 individuals with inflammatory markers measured, 283 (65.5%) had one or more elevated marker. Individuals with at least one elevated marker had a median of 3 of elevated markers observed (Interquartile range: 2–4). Estimated interventional indirect effects suggest that reductions in severe COVID-19 disease risk of −2% (RR = 0.98; 95% CI: 0.80–1.17), 1% (RR = 1.01; 95% CI: 0.85–1.18) and 5% (RR = 1.05; 95% CI: 0.78–1.31), could be achieved if the distributions of lower SES, comorbidities and elevated inflammatory markers were shifted from what it would be for males to females, respectively. A null estimate for the interventional indirect effects through dependence between mediators (RR = 1.00; 95% CI: 0.89–1.10) suggests minimal interaction between mediators in the model. The interventional direct effect, or the proportion of the male sex and severe COVID-19 relationship that does not act through the examined mediating pathways, accounted for most of the total causal effect (RR = 1.48; 95% CI: 0.61–2.35). Comparable interventional indirect estimates were estimated when models included an inflammatory markers variable based on observed (ie, non-imputed) laboratory values (Supplemental Table 4).
 |
Table 3 Evaluation of Factors Mediating the Relationship Between Male Sex and Severe COVID-19 Disease |
Discussion
In a large single-site EMR-based cohort study, we find that male sex is not only associated with higher rates of incident COVID-19, but also severe COVID-19, hospitalization, and death. Therefore, at all steps of the continuum of COVID-19 illness, males appear to be at higher risk. More importantly, our work goes further to help elucidate potential pathways by which male sex may be influencing this increased risk of severe COVID-19, and we find that, after adjusting for age and race/ethnicity, the strongest signal for this association likely is based on inflammatory pathways. SES and comorbidities themselves do not appear to account for the pathways through which sex may be influencing severe COVID-19 outcomes.
The strongest signal we detected for a mediator in the associations between sex and severe COVID-19, albeit non-significant, is for the inflammatory pathway. This is consistent with previous reports,18 but our analysis furthers the evidence through use of causal mediation methods. Higher inflammation, as a reaction to COVID-19 infection and particularly severe COVID-19 infection, in males versus females has been previously documented.19–21 Higher concentrations of IL-10, TNF-alpha, CRP, LDH, ferritin, and IgG were detected among males. Certain interferon and toll-like receptor signaling genes are encoded on X chromosomes only, leading to potentially differential expression between males and females of these important players in viral infections and host control of the infections. Since our ascertainment of the inflammatory markers was after the first COVID-19 test, we strongly advise interpreting our results as exploratory and hypothesis-generating. Alternative explanations for the signal we observe may exist. First, other biologic processes that are more upstream from the host response to the viral infection could be differential between males and females. For example, differences exist in expression of ACE-2 (located on X chromosome) and other receptors that SARS-CoV-2 binds to in humans.22–24 Reduced B-cell and NK-cell transcripts and an increase in inhibitors of nuclear factor kappa-B (NF-κB) signaling have also been observed in males relative to females.11 Another element may be differences in sex hormones and inflammation, such as testosterone concentrations decreasing with age being correlated with pro-inflammatory states, and estrogen playing a role in potentially down-regulating inflammation.23,25 This may seem contradictory to sex-based disparities observed with some autoimmune disease, like systemic lupus erythematosus, which is more prevalent among females and has been linked with higher circulating estrogen levels.26 However, the inflammatory mechanisms by which sex hormones mediate autoimmune disease risk are unclear and may vastly differ from those that modulate risk of severe COVID-19. Therefore, the overlap of SARS-CoV-2 infection and autoimmune disease will be an interesting area to investigate epidemiologically and immunologically going forward. Additionally, the more robust COVID-19 vaccine responses among females than males would also counter these sex hormones-based arguments.27,28 Second, some have suggested that males are more likely to present with more symptoms, and, therefore, have more severe outcomes; this could be confounded by males presenting later than females in the natural progression of their infection.2 Natural language processing studies with EMR data can help better ascertain, for example, if such a phenomenon exists among the individuals we analyzed in this dataset. Third, though others have postulated that higher risk of comorbidities among males than females, except for in older age groups, may be driving this association,9,19,29 our findings from the mediation analysis suggest that comorbidities alone are unlikely to account for the associations we observe between male sex and COVID-19 outcomes.
None of the mediation pathways, SES, comorbidities, or the inflammatory markers, accounted for most of the excess risk associated with male sex and severe COVID-19. Thus, other pathways or factors that we have not accounted for, or not well enough, may account for this association. First, our measures of the inflammatory pathway are severely limited, and studies that can profile these markers in detail prior to and during a COVID-19 infection will shed light on this issue. Second, behavioral or gendered norms could be playing a role. Our EMR-based dataset was limited in its ability to ascertain various such norms. Gebhard et al have suggested several patterns in behavioral and gendered norms that vary between males and females.9 For example, risk factors, such as smoking or drinking rates are generally higher among males than females worldwide. Others have documented lower rates of handwashing, masking, or healthcare-seeking behaviors among males. Others have argued that gendered norms around caregiving, such as more females than males acting as caregivers informally (within their homes, especially for intergenerational family members) and formally (in healthcare settings) may put females at greater risk for COVID-19; however, signals throughout the world indicate males are at greater risk for various COVID-19 outcomes. It is possible that greater social connectedness, higher overall quality of life, or other behavioral factors may be protective for females,9 but this is yet to be documented.
This work has several limitations. First, as an EMR-based analysis, data may be incomplete or susceptible to misclassification. For example, deaths reported in the EMR are likely to be undercounts of deaths that may occur in the community, and using de-identified data prevented us from matching individuals with existing death registries. However, we do not suspect a large amount of misclassification in our primary exposure (sex) and outcomes of interest, and certainly not any differential misclassification by exposure; thus, any non-differential misclassification would only bias our results towards the null. Second, again, because we relied on routinely collected EMR data, we are not able to thoroughly explore the potential signals we detected with inflammatory processes and sex/COVID-19. For instance, ideally, our inflammatory markers of interest would be recorded well before the COVID-19 diagnosis for all participants; however, most are only measured after the first COVID-19 test for some participants. We did attempt to mitigate this tautomeric element of the inflammatory markers being predictive of severe COVID-19 by taking the first value closest to the first COVID-19 test within a 14-day window. Additionally, there is marked bias in that individuals who are most at-risk for severe COVID-19 are more likely to have these inflammatory markers checked in the first place. For this reason, we did not pursue examining the inflammatory markers for the outcome of COVID-19 test positivity. Third, the single site analysis limits generalizability, though our overall findings have been demonstrated in most settings around the world.
Conclusion
This works furthers the growing body of evidence that shows that males are at higher risk of severe COVID-19 disease outcomes and goes the next step by assessing effect modification and mediation by key sociodemographic, clinical and biologic factors. While no significant mediating effect was observed through SES, comorbidities, and inflammatory status, we saw the greatest mediating effect through inflammation. More work, with granular measures, is recommended to better understand the individual and combined inflammatory factors that may modulate severe disease risk as well as understand other behavioral and clinical factors that may play a role in this relationship.
Acknowledgments
This work was supported by the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) (grant number K23AI120855). The funding source was not involved in the study design, the collection, analysis and interpretation of data, writing of the report or the decision to submit the article for publication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr Alexander L Greninger is part of the central testing contract for Abbott and has grants from Merck and Gilead unrelated to the submitted work. The authors have no other competing interests to declare.
References
1. Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):1–10. doi:10.1038/s41467-020-19741-6
2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
3. Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145. doi:10.3760/cma.j.issn.0254-6450.2020.02.003
4. Jin J-M, Bai P, He W, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public health. 2020;8:152. doi:10.3389/fpubh.2020.00152
5. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032
6. Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020;73:11.
7. The COVID-19 sex-disaggregated data tracker; 2021. Available from: https://globalhealth5050.org/the-sex-gender-and-covid-19-project/dataset/.
8. Conti P, Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents. 2020;34(2):339–343. doi:10.23812/Editorial-Conti-3
9. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11:1–13. doi:10.1186/s13293-020-00304-9
10. Wenham C, Smith J, Morgan R. COVID-19: the gendered impacts of the outbreak. Lancet. 2020;395(10227):846–848. doi:10.1016/S0140-6736(20)30526-2
11. Lieberman NA, Peddu V, Xie H, et al. In vivo antiviral host transcriptional response to SARS-CoV-2 by viral load, sex, and age. PLoS Biol. 2020;18(9):e3000849. doi:10.1371/journal.pbio.3000849
12. Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588(7837):315–320. doi:10.1038/s41586-020-2700-3
13. Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192–e197. doi:10.1016/S1473-3099(20)30483-7
14. Kind AJ, Buckingham WR. Making neighborhood-disadvantage metrics accessible—the neighborhood atlas. N Engl J Med. 2018;378(26):2456. doi:10.1056/NEJMp1802313
15. Area deprivation index, 2018 version; 2020. Available from: https://www.neighborhoodatlas.medicine.wisc.edu/.
16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
17. UW laboratory test guide; 2021. Available from: https://testguide.labmed.uw.edu/public/.
18. Scully EP, Schumock G, Fu M, et al. Sex and gender differences in testing, hospital admission, clinical presentation, and drivers of severe outcomes from COVID-19. Open Forum Infect Dis. 2021;8(9):ofab448. doi:10.1093/ofid/ofab448
19. Meng Y, Wu P, Lu W, et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. 2020;16(4):e1008520. doi:10.1371/journal.ppat.1008520
20. Qin L, Li X, Shi J, et al. Gendered effects on inflammation reaction and outcome of COVID‐19 patients in Wuhan. J Med Virol. 2020;92(11):2684–2692. doi:10.1002/jmv.26137
21. Zeng F, Dai C, Cai P, et al. A comparison study of SARS‐CoV‐2 IgG antibody between male and female COVID‐19 patients: a possible reason underlying different outcome between sex. J Med Virol. 2020;92(10):2050–2054. doi:10.1002/jmv.25989
22. Asselta R, Paraboschi EM, Mantovani A, Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID-19 severity in Italy. 2020;12:10087.
23. Dana PM, Sadoughi F, Hallajzadeh J, et al. An insight into the sex differences in COVID-19 patients: what are the possible causes? Prehosp Disaster Med. 2020;35(4):438–441. doi:10.1017/S1049023X20000837
24. Lazartigues E, Qadir MMF, Mauvais-Jarvis F. Endocrine significance of SARS-CoV-2ʹs reliance on ACE2. Endocrinology. 2020;161(9):bqaa108. doi:10.1210/endocr/bqaa108
25. Li Y, Jerkic M, Slutsky AS, Zhang H. Molecular mechanisms of sex bias differences in COVID-19 mortality. Critical Care. 2020;24(1):1–6. doi:10.1186/s13054-020-03118-8
26. Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35(3):347–369. doi:10.1016/j.yfrne.2014.04.004
27. Gee J, Marquez P, Su J. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:283–288. doi:10.15585/mmwr.mm7008e3
28. Shimabukuro T, Cole M, Su J. Reports of Anaphylaxis After Receipt of mRNA COVID-19—United States, December 14, 2020–January 18, 2021. JAMA. 2021;325(11):1101. doi:10.1001/jama.2021.1967
29. Palaiodimos L, Kokkinidis DG, Li W, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi:10.1016/j.metabol.2020.154262
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
