Back to Journals » Vascular Health and Risk Management » Volume 19
Eleven-Year Outcomes of a Screening Project for Abdominal Aortic Aneurysm (AAA) in 65-Year-Old Men
Authors Mansoor SM , Rabben T, Hisdal J , Jørgensen JJ
Received 29 March 2023
Accepted for publication 13 June 2023
Published 17 July 2023 Volume 2023:19 Pages 459—467
DOI https://doi.org/10.2147/VHRM.S412954
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Saira Mauland Mansoor,1,2 Toril Rabben,1 Jonny Hisdal,1,2 Jørgen Joakim Jørgensen1– 3
1Department of Vascular Surgery, Oslo University Hospital, Oslo, Norway; 2Institute of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway; 3Department of Traumatology, Oslo University Hospital, Oslo, Norway
Correspondence: Saira Mauland Mansoor, Oslo Universitetssykehus, Ullevål, Karavdelingen, Postboks 4959 Nydalen, Oslo, 0424, Norway, Tel +47 92 82 92 78, Email [email protected]
Objective: Since 2011, the Department of Vascular Surgery at Oslo University Hospital has offered screening for abdominal aortic aneurysm (AAA) to 65-year-old men living in Oslo, Norway. The aim of this study was to evaluate the effect of the screening project on AAA-related mortality and rupture and repair rates in the screened population.
Methods: This cohort study included men that participated in AAA screening at the Department of Vascular Surgery at Oslo University Hospital in the period May 2011 to September 2019. All men with screen-detected AAA (aortic diameter ≥ 30 mm) and subaneurysmal aortic dilatation (aortic diameter 25– 29 mm) were included. A stratified (1:1 with the subaneurysm group), randomized selection of men with normal aortic diameter (< 25 mm) upon screening was also included. The follow-up data on events (ruptures, repairs, and deaths) after screening were collected retrospectively from patient electronic medical records at Oslo University Hospital, the National Population Register and the Norwegian Cause of Death Registry (CoDR).
Results: In total, 2048 men were included, with a median follow-up time of 7.1 years (IQR 3.8). Among men with screen-detected AAA, 0.6% died of AAA-related causes (0.9 AAA-related deaths per 1000 person-years). The rupture rate was 0.3% among men with screen-detected AAA or subaneurysmal aortic dilatation, giving an incidence of 0.5 ruptures per 1000 person-years. The overall repair rate in the AAA group was 20.6% (36.1 repairs per 1000 person-years) and 0.6% (0.9 repairs per 1000 person-years) in the subaneurysm group.
Conclusion: In a population screened for AAA, the incidence of rupture and the AAA-related mortality was very low. Almost one-fifth of the participants with screen-detected AAA underwent elective repair, representing a group that might have presented with rupture if untreated. These results indicate that screening is valuable in preventing AAA rupture and AAA-related mortality.
Keywords: rupture rate, repair rate, subaneurysmal aortic dilatation, AAA-related mortality
Introduction
Abdominal aortic aneurysm (AAA) is usually an asymptomatic condition, often detected incidentally or through screening, and many will not require repair. However, surveillance is indicated to monitor growth and to intercept aneurysms at risk of rupture. AAA rupture is an acute and lethal condition, and intervention (open surgical- or endovascular repair) is required to prevent death. The total mortality for ruptured AAA has been reported to range from 50% to 95%, and the mortality after intervention as 30–43%.1–5 Screening for AAA significantly reduces the risk of rupture and AAA-related mortality.6–10 Some studies have also shown that invitation to screening significantly reduces all-cause mortality.7,8,11
The European Society for Vascular Surgery (ESVS) recommends (Class 1 recommendation, evidence level A) one-time ultrasonographic screening of the abdominal aorta for all men at the age of 65 years.12 The ESVS guidelines recommend surveillance for men with aortic diameter ≥30 millimetres (mm), and men with subaneurysmal aortic dilatation (aortic diameter 25–29 mm) may also be considered for a repeated scan after five to ten years (Class 2b recommendation, evidence level C).
To our knowledge, only four randomized trials have been conducted to evaluate AAA screening, dating back to the 1980s and 1990s.13–16 Since then, several observational follow-up studies of individuals with screen-detected AAA have demonstrated the benefits of screening.17–19 Epidemiological variations in screened populations, a decreasing prevalence of AAA, and differences in the organization of AAA screening and surveillance, all warrant continued evaluation of AAA screening.20
Since 2011, the Department of Vascular Surgery at Oslo University Hospital has conducted a research project in which AAA screening is offered to 65-year-old men living in Oslo, Norway.21 The aim of this study was to evaluate the effect of the screening project on AAA-related mortality, AAA rupture rate and AAA repair rate.
Materials and Methods
This observational follow-up study included 65-year-old men that participated in AAA screening at Oslo University Hospital, in the period May 2011 to September 2019. The study period spanned from May 2011 until July 2022. Data on participants were collected retrospectively. The primary endpoints were AAA-related mortality and AAA rupture- and repair rates among men with screen-detected AAA or subaneurysmal dilatation. The secondary endpoint was all-cause mortality for all the participants.
The included participants were allocated to three groups according to aortic diameter upon screening: AAA (aortic diameter ≥30 mm), subaneurysmal aortic dilatation (aortic diameter 25–29 mm) and normal aortic diameter (<25 mm). We included all men with screen-detected AAA and subaneurysmal aortic dilatation, and a stratified (1:1 with the subaneurysm group), randomized selection of men with normal aortic diameter (Figure 1).
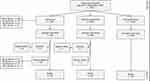 |
Figure 1 The inclusion process and the registered events after screening, at Oslo University Hospital. Abbreviation: AD, aortic diameter. |
The screening project is described in detail, by Rabben et al, in previously published work on AAA prevalence and risk factors in the same study population.21 Annually, 65-year-old men living in Oslo are invited to AAA screening once by letter. The AAA screening is conducted at the Department of Vascular Surgery at Oslo University Hospital by specially trained nurses and radiographers. Upon screening, oral and written information is given to all screening participants with an aortic diameter ≥25 mm, including recommendations on surveillance through their general practitioners (GPs) at specific intervals. This written information is also sent to the participants’ GPs. AAA surveillance in Oslo is managed by GPs until aortic diameters reach 45 mm, after which the participants are referred to Oslo University Hospital for further evaluation, follow-up, and treatment. Oslo University Hospital is the only center for AAA repair in Oslo. If participants have moved to another city during the follow-up period or found themselves in another city upon rupture, they might have received treatment in other hospitals.
By September 2019, 13,215 men had been screened, revealing an AAA prevalence of 2.6% in this population.21 The attendance rate varied from 63% to 78%. Demographics for this screened population, such as smoking and comorbidities, have been reported in a previous study, in which four independent risk factors for AAA were identified – smoking, hypertension, BMI >30 and diabetes mellitus, of which the latter had an inverse relation.21
All deaths during the study period were accounted for through the National Population Register. Causes of death were obtained from patient electronic medical records at Oslo University Hospital and the Norwegian Cause of Death Registry (CoDR). In the CoDR, causes of death have not yet been made available for deaths that have occurred after 31 December 2021.
Follow-up data on rupture and repair for men with AAA and subaneurysmal aortic dilatation were collected retrospectively from the patient electronic medical records at Oslo University Hospital. Indication for AAA repair was evaluated according to ESVS guidelines.12 All patients considered for AAA repair at Oslo University Hospital were discussed at multidisciplinary meetings, with vascular surgeons and interventional radiologists, before they received treatment. Statistical analyses were performed with Stata/SE (ver. 17.0). Continuous variables are presented as medians with interquartile ranges (IQR) and categorical variables are presented as values and percentages. Incidence rates are presented per 1000 person-years with 95% confidence intervals (CI). Kaplan–Meier survival analysis was performed using the screening date, and the date of death or the censored date (the latest being 31 July 2022). The Log rank test was used to test for differences in mortality risk between groups, and the significance level was set to p < 0.05. Causes of death were compared between groups using contingency tables and Pearson’s chi-squared test when all expected counts were above five, and Fisher’s exact test when the expected count criterion was not fulfilled.
The “Strengthening the reporting of observational studies in epidemiology” (STROBE) guidelines were followed for the reporting of this observational study.
The study was approved by the Regional Committees for Medical and Health Research Ethics (REC reference number 2009/866) and complies with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Results
During the screening period, from May 2011 to September 2019, a total of 13,215 men were screened.21 In total, 330 men with AAA and 859 men with subaneurysmal aortic dilatation were detected and included in this study (Figure 1). In addition, 859 men with aortic diameter <25 mm upon screening were included, leaving a total number of 2048 participants for the final analysis. The median follow-up time was 7.1 years (IQR 3.8), and the last observed exit was after 11 years. At the end of the study period, in July 2022, 11.6% of the participants had moved to other cities in Norway, but the exact moving dates were unknown. Thus, with respect to the outcomes of AAA rupture and repair, these participants may have been lost to follow-up. The data on mortality and cause of death, however, were collected from national registers and were therefore not affected by participants moving.
AAA-Related and All-Cause Mortality
In the AAA group, there were two AAA-related deaths (0.6%) (Table 1). The AAA-related mortality was thus 0.9 deaths per 1000 person-years (95% CI 0.2, 3.7) among men with screen-detected AAA and 0.1 deaths per 1000 person-years (95% CI 0.04, 0.6) in the total study population. One participant died within 30 days following elective AAA repair and one participant, who had been considered not eligible for elective AAA repair, died from a ruptured AAA.
 |
Table 1 Causes of Death for the Three Aortic Diameter Groups |
At the end of the study period, 14.9% of the men in the AAA group had died, 7.5% had died in the subaneurysm group and 6.8% had died in the group of men with normal aortic diameter upon screening (Figure 1). The all-cause mortality was 22.4 deaths per 1000 person-years (95% CI 16.9, 29.7) in the AAA group. In the subaneurysm group, all-cause mortality was 11.2 deaths per 1000 person-years (95% CI 8.8, 14.3), and in the group with normal aortic diameter the all-cause mortality was 10.2 deaths per 1000 person-years (95% CI 7.7, 13.1).
All-cause mortality was significantly higher in the AAA group compared to the subaneurysm group (p < 0.001) and the group with normal aortic diameter (p < 0.001), but the difference was not statistically significant when comparing the subaneurysm group to the group with normal aortic diameter (p = 0.58) (Figure 2).
Information on the cause of death was available for 159 (93.0%) of the 171 deceased participants. Autopsy was performed in 18 cases (10.5%). The major causes of death in all three groups were malignant disease and cardiac-/cerebrovascular events. Lung cancer was the major malignant disease in the AAA- and subaneurysm group (Table 1).
AAA Rupture
In the AAA group, three participants were admitted to Oslo University Hospital due to rupture during the study period (Figure 1). One participant underwent non-emergency endovascular aneurysm repair (EVAR) due to a contained rupture discovered upon screening. The aneurysm diameter was 38 mm, and a mycotic ethology was a possible cause. A second participant underwent emergency open surgical repair for a CT-verified ruptured AAA with a diameter of 65 mm. The rupture occurred 8.5 years after screening. This patient had a follow-up ultrasonographic scan of the abdominal aorta, at the hospital, around one year after the initial screening (screening diameter 35 mm), the diameter was then 36 mm. After this, the patient was under surveillance, at the hospital, for an aneurysm of the ascending aorta with aortic valvular regurgitation, but surveillance for his AAA seems to have fallen out. A third participant died 6.5 years after screening of an untreated ruptured AAA, confirmed upon autopsy. The last known aortic diameter for this man was 57 mm, five months prior to rupture, and he was considered not eligible for elective AAA repair due to advanced age and comorbidities. In total, the rupture rate in the AAA group was 0.9% giving an incidence rate of 1.6 ruptures per 1000 person-years (95% CI 0.5, 4.9).
In the subaneurysm group, one participant was treated for ruptured AAA in another hospital, and further information was unavailable. This gave a total rupture rate of 0.1% in the subaneurysm group, equivalent to an incidence of 0.2 ruptures per 1000 person-years (95% CI 0.02, 1.2).
In total, the rupture rate was 0.3% among men with aortic diameter ≥25 mm upon screening, giving an incidence of 0.5 ruptures per 1000 person-years (95% CI 0.2, 1.4).
AAA Repair
In the AAA group, a total of 68 men (20.6%) received AAA repair after a median time of 1.8 years (IQR 4.1), corresponding to an incidence of 36.1 repairs per 1000 person-years (95% CI 28.5, 45.8) (Figure 1). The diameters upon repair and aortic diameter growth rates are given in Table 2. Upon screening, 21 men already had an aortic diameter ≥55 mm, and all received elective AAA repair shortly after the screening.
 |
Table 2 Aortic Diameter Upon Repair and Aortic Growth Rate for Five Groups Defined by Aortic Diameter Upon Screening |
In the subaneurysm group, a total of five men (0.6%) received AAA repair after a median time of 8.2 years (IQR 2.4). This gave an incidence of 0.9 repairs per 1000 person-years (95% CI 0.4, 2.1) in the subaneurysm group.
Excluding three participants that underwent rupture repairs (Figure 1), a total of 70 participants underwent elective AAA repair, where 63 (90.0%) were open surgical repairs, 5 (7.1%) were EVAR procedures and 2 (2.9%) were unknown (they had been treated in other cities than Oslo). Among the 70 elective repairs, one participant died within 30 days of repair (open surgical repair), giving an overall 30-day mortality rate of 1.4% for elective AAA repairs.
Discussion
The main finding in this study was an AAA-related mortality incidence of 0.1 per 1000 person-years in a screened population of 65-year-old men. The incidence of rupture among men with screen-detected AAA and subaneurysmal aortic dilatation was 0.5 per 1000 person-years. This indicates that AAA screening in 65-year-old men is still a relevant and valuable effort.
Our results are in coherence with other observational studies revealing low AAA-related mortality and rupture rates in screened populations. Hultgren et al detected two AAA-related deaths in the 5-year follow-up 662 individuals with screen-detected AAA.17 Haque et al found that 7.5% of deaths with recorded cause (35.8% of the deaths lacked recorded causes) were due to AAA rupture, in a population under surveillance over 19 years.22 Chun et al present an overall AAA-related mortality of 0.03% in a screened population with a follow-up time of 10 years, and 2.1% suspected deaths from rupture among individuals with screen-detected AAA.18 Previous studies report rupture rates in different screened populations with varying follow-up times, ranging from 0.004% to 2.1%.17,18,23 The feasibility of comparing AAA-related mortality and rupture rates across studies can, however, be questioned due to the varying ways of reporting these outcomes and the small outcome numbers.
The discrepancies in AAA-related mortality and rupture rates across studies may reflect differences in the distribution of risk factors in the screened populations and in the distribution of aneurysm size upon screening. It may also reflect variations in the organization of surveillance regimes and the length of follow-up in the reporting of studies. Taking these factors into account, it may not be feasible to evaluate screening programs based exclusively on AAA-related mortality and rupture rates. As a point in case, Hultgren et al found a higher rupture rate among non-participants, defined as men who declined their invitation to AAA screening.17 Ahmad et al examined self-reported reasons for non-participation in AAA screening, in which the main reply was “did not want screening”.24 Non-participants in screening may be an important group to focus on in the effort to reduce AAA-related mortality.
An important aspect of AAA-related mortality is the potential for undetected AAA-related deaths. All deceased participants in this study population were accounted for through the National Population Register and their causes of death were obtained from the CoDR, decreasing the apparent risk of undetected AAA-related deaths. However, the stated causes of death were given without an autopsy in 90% of the cases, and there were in total six cases categorised as sudden death/unknown cause.
As expected, and in coherence with other studies, there was a significantly higher all-cause mortality among participants with screen-detected AAA, compared to participants with aortic diameter <30 mm.18,23 Duncan et al found that the mortality risk in men with subaneurysmal aortic dilatation was significantly higher than in men with aortic diameters <25 mm.25 In our study, there was no significant difference in all-cause mortality between these two groups. The cohort in this study was relatively large, but few patients died, leading to a potential lack of statistical power to detect differences in all-cause mortality. Causes of death were not significantly different across the three groups. The major causes of death were malignant disease and cardiac-/cerebrovascular events, and this finding is in agreement with those in other studies.22,23,25
One-fifth of men with screen-detected AAA underwent AAA repair in this study. Data from the national screening program in Sweden have shown a repair rate of 22% among screen-detected AAAs during a follow-up period of 1–7 years.17 The high repair rate among men with screen-detected AAA in our study suggests that screening is beneficial in preventing potential ruptures. Another important aspect is that Lindholt et al found that the 30-day mortality after elective AAA repair was significantly lower in patients with screen-detected AAA, compared to that in patients with incidentally detected AAA.26
In the subaneurysm group, we found a repair rate of 0.6%. Only 50% of the participants in this study had a follow-up time of more than seven years. With longer observation time, we could expect a higher repair rate in the subaneurysm group. Other observational studies have demonstrated that 58–68% of men with screen-detected subaneurysmal aortic dilatation develop AAA within five years of surveillance, and 26–30% reach an aortic diameter ≥55 mm within 10–15 years.27–29
In this study, only 7.1% of elective repairs were performed using EVAR. The Department of Vascular Surgery at Oslo University Hospital opts for open surgical repair in young and eligible/low-risk patients, in accordance with the National Institute for Health and Care Excellence (NICE) guidelines.30 Since screening was performed in 65-year-old men, after a median follow-up time of 7 years, they are still considered to be young patients.
The 30-day mortality after elective AAA repair of 1.4% in this study, representing a single patient who underwent open surgical repair, naturally lacks statistical power. A recent meta-analysis reported a 30-day mortality of 1.2% and 3.1% for elective EVAR and open surgical repair, respectively.31 The Norwegian Vascular Surgery Registry (NORKAR) reports a decrease in 30-day mortality after elective AAA repair from 2.5% to 1.8% during the last five years.32 EVAR procedures constitute 49–57% of all AAA repairs in Norway. The Department of Vascular Surgery at Oslo University Hospital is currently the only center in Norway offering AAA screening.
Limitations
The follow-up data were obtained exclusively from electronic medical records at Oslo University Hospital. Two issues present themselves. Firstly, Oslo University Hospital is the only treatment center for AAA in Oslo, but at the end of the study period 11.6% of the participants had a registered home address in another city. Secondly, AAA surveillance is managed by GPs until the aortic diameters reach 45 mm, and the number of participants lost to follow-up is unknown. Chun et al found a follow-up rate of 65% for men with screen-detected AAA, while men with small aneurysms (30–39 mm) were less likely to be followed up than men with larger aneurysms.33 In their study, follow-ups were initiated by electronic medical record reminders sent to the primary care physicians. In the Multicentre Aneurysm Screening Study (MASS) in England, the seven-year follow-up rate for men with screen-detected AAA was 76%, and follow-ups were organized by the screening facilities during the first five years.9 In view of the organization of the healthcare system in Norway, it would not be feasible to manage all AAA surveillance in hospitals. However, the primary healthcare system in Norway is reliable in systematic surveillance of medical conditions, and several other surveillance regimes are managed by GPs, for example surveillance in regard to relapse after treatment of colon cancer.
Conclusion
The follow-up of 65-year-old men screened for AAA showed low AAA-related mortality, constituting of one participant with AAA rupture found unsuited for repair due to advanced age and comorbidities and one participant who died after elective AAA repair. The circumstances around these deaths did not indicate any insufficiency in the AAA screening or the AAA surveillance. Almost one-fifth of the men with screen-detected AAA underwent elective AAA repair. Untreated, some of these participants may have developed rupture, indicating that AAA screening may prevent several potential ruptures. These results show that screening is still a relevant effort in reducing AAA-related mortality. A few men with screen-detected subaneurysmal aortic dilatation grew to an aortic diameter indicating AAA repair and this supports current ESVS guidelines suggesting a repeated ultrasonographic scan after five to ten years for this subgroup.
Acknowledgments
Professor and vascular surgeon Jørgen Jørgensen (1947–2017) is gratefully acknowledged for establishing the aorta screening project at Oslo University Hospital in May 2011. We also thank the screening team for their efforts in organizing and managing the AAA screening. Lastly, we are grateful to Professor Manuela Zucknick at the Oslo Centre of Biostatistics and Epidemiology (OCBE), Institute of Basic Medical Sciences, University of Oslo, and the OCBE Statistical Advising Service for helpful consultations on the statistical analyses.
Disclosure
The authors received no financial support and have no conflicts of interest to report.
References
1. Reimerink JJ, van der Laan MJ, Koelemay MJ, Balm R, Legemate DA. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br J Surg. 2013;100(11):1405–1413. doi:10.1002/bjs.9235
2. Karthikesalingam A, Wanhainen A, Holt PJ, et al. Comparison of long-term mortality after ruptured abdominal aortic aneurysm in England and Sweden. Br J Surg. 2016;103(3):199–206. doi:10.1002/bjs.10049
3. Karthikesalingam A, Holt PJ, Vidal-Diez A, et al. Mortality from ruptured abdominal aortic aneurysms: clinical lessons from a comparison of outcomes in England and the USA. Lancet. 2014;383(9921):963–969. doi:10.1016/S0140-6736(14)60109-4
4. Roosendaal LC, Kramer GM, Wiersema AM, Wisselink W, Jongkind V. Outcome of ruptured abdominal aortic aneurysm repair in octogenarians: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2020;59(1):16–22. doi:10.1016/j.ejvs.2019.07.014
5. Laine MT, Laukontaus SJ, Kantonen I, Venermo M. Population-based study of ruptured abdominal aortic aneurysm. Br J Surg. 2016;103(12):1634–1639. doi:10.1002/bjs.10200
6. Cosford PA, Leng GC. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev. 2007;2:Cd002945. doi:10.1002/14651858.CD002945.pub2
7. Ali MU, Fitzpatrick-Lewis D, Miller J, et al. Screening for abdominal aortic aneurysm in asymptomatic adults. J Vasc Surg. 2016;64(6):1855–1868. doi:10.1016/j.jvs.2016.05.101
8. Takagi H, Ando T, Umemoto T. Abdominal aortic aneurysm screening reduces all-cause mortality: make screening great again. Angiology. 2018;69(3):205–211. doi:10.1177/0003319717693107
9. Thompson SG, Ashton HA, Gao L, Scott RA. Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomised multicentre Aneurysm screening study. BMJ. 2009;338:b2307. doi:10.1136/bmj.b2307
10. Guirguis-Blake JM, Beil TL, Senger CA, Coppola EL. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US preventive services task force. JAMA. 2019;322(22):2219–2238. doi:10.1001/jama.2019.17021
11. Thompson SG, Ashton HA, Gao L, Buxton MJ, Scott RA. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99(12):1649–1656. doi:10.1002/bjs.8897
12. Wanhainen A, Verzini F, Van Herzeele I, et al. Editor’s choice - European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57(1):8–93. doi:10.1016/j.ejvs.2018.09.020
13. Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ. 2005;330(7494):750. doi:10.1136/bmj.38369.620162.82
14. Ashton HA, Buxton MJ, Day NE, et al. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360(9345):1531–1539. doi:10.1016/S0140-6736(02)11522-4
15. Norman PE, Jamrozik K, Lawrence-Brown MM, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ. 2004;329(7477):1259. doi:10.1136/bmj.38272.478438.55
16. Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br J Surg. 1995;82(8):1066–1070. doi:10.1002/bjs.1800820821
17. Hultgren R, Elfström KM, Öhman D, Linné A. Long-term follow-up of men invited to participate in a population-based abdominal aortic aneurysm screening program. Angiology. 2020;71(7):641–649. doi:10.1177/0003319720921741
18. Chun KC, Dolan KJ, Smothers HC, et al. The 10-year outcomes of a regional abdominal aortic aneurysm screening program. J Vasc Surg. 2019;70(4):1123–1129. doi:10.1016/j.jvs.2019.01.053
19. Wanhainen A, Hultgren R, Linné A, et al. Outcome of the Swedish nationwide abdominal aortic aneurysm screening program. Circulation. 2016;134(16):1141–1148. doi:10.1161/CIRCULATIONAHA.116.022305
20. Lilja F, Wanhainen A, Mani K. Changes in abdominal aortic aneurysm epidemiology. J Cardiovasc Surg. 2017;58(6):848–853. doi:10.23736/S0021-9509.17.10064-9
21. Rabben T, Mansoor SM, Bay D, Sundhagen JO, Guevara C, Jorgensen JJ. Screening for abdominal aortic aneurysms and risk factors in 65-year-old men in Oslo, Norway. Vasc Health Risk Manag. 2021;17:561–570. doi:10.2147/VHRM.S310358
22. Haque A, McCollum C. Patients on AAA surveillance are at greater threat of cardiovascular events or malignancy than their AAA: outcomes of AAA surveillance over 19 years at a tertiary vascular centre. Ann Vasc Surg. 2022;83:158–167. doi:10.1016/j.avsg.2021.11.013
23. Oliver-Williams C, Sweeting MJ, Jacomelli J, et al. Safety of men with small and medium abdominal aortic aneurysms under surveillance in the NAAASP. Circulation. 2019;139(11):1371–1380. doi:10.1161/CIRCULATIONAHA.118.036966
24. Ahmad M, Reading K, Gannon MX. Improving Abdominal Aortic Aneurysm (AAA) screening uptake through patient engagement-analysis and outcomes of strategies to improve uptake at a regional program level. Ann Vasc Surg. 2021;72:488–497. doi:10.1016/j.avsg.2020.08.146
25. Duncan JL, Harrild KA, Iversen L, Lee AJ, Godden DJ. Long term outcomes in men screened for abdominal aortic aneurysm: prospective cohort study. BMJ. 2012;344:e2958. doi:10.1136/bmj.e2958
26. Lindholt JS, Norman PE. Meta-analysis of postoperative mortality after elective repair of abdominal aortic aneurysms detected by screening. Br J Surg. 2011;98(5):619–622. doi:10.1002/bjs.7464
27. Oliver-Williams C, Sweeting MJ, Turton G, et al. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg. 2018;105(1):68–74. doi:10.1002/bjs.10715
28. Thorbjørnsen K, Svensjö S, Gilgen NP, Wanhainen A. Long term outcome of screen detected sub-aneurysmal aortas in 65 year old men: a single scan after five years identifies those at Risk of needing AAA Repair. Eur J Vasc Endovasc Surg. 2021;62(3):380–386. doi:10.1016/j.ejvs.2021.05.039
29. Wild JB, Stather PW, Biancari F, et al. A multicentre observational study of the outcomes of screening detected sub-aneurysmal aortic dilatation. Eur J Vasc Endovasc Surg. 2013;45(2):128–134. doi:10.1016/j.ejvs.2012.11.024
30. National Institute for Health and Care Excellence. Abdominal aortic aneurysm: diagnosis and management. NICE guideline NG156; 2020. Available from: https://www.nice.org.uk/guidance/NG156.
31. Antoniou GA, Antoniou SA, Torella F. Editor’s choice - Endovascular vs. open repair for abdominal aortic aneurysm: systematic review and meta-analysis of updated peri-operative and long term data of randomised controlled trials. Eur J Vasc Endovasc Surg. 2020;59(3):385–397. doi:10.1016/j.ejvs.2019.11.030
32. The Norwegian Vascular Surgery Registry (NORKAR). Annual report 2021; 2021. Available from: https://stolav.no/seksjon/NORKAR/Documents/%c3%85rsrapport%20NORKAR%20for%202021%20-%20til%20SKDE%2025.08.pdf.
33. Chun KC, Schmidt AS, Bains S, et al. Surveillance outcomes of small abdominal aortic aneurysms identified from a large screening program. J Vasc Surg. 2016;63(1):55–61. doi:10.1016/j.jvs.2015.08.059
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

