Back to Journals » Journal of Inflammation Research » Volume 15
Elevation of Preprocedural Systemic Immune Inflammation Level Increases the Risk of Contrast-Associated Acute Kidney Injury Following Coronary Angiography: A Multicenter Cohort Study
Authors Lai W, Zhao X, Huang Z , Xie Y, Yu S, Tu J, Guo D, Xiu J, Mai Z, Li Q, Huang H, Li H, Xu JY , Lu H, Chen G, Chen S, Liu J, Liu Y
Received 4 March 2022
Accepted for publication 4 May 2022
Published 13 May 2022 Volume 2022:15 Pages 2959—2969
DOI https://doi.org/10.2147/JIR.S364915
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Wenguang Lai,1,2,* Xiaoli Zhao,3,* Zhidong Huang,1,* Yun Xie,1,2,* Sijia Yu,1,4 Jiabin Tu,5 Dachuan Guo,6 Jiaming Xiu,5 Ziling Mai,1,2 Qiang Li,1 Haozhang Huang,1,4 Huanqiang Li,1 Jun-Yan Xu,1,7 Hongyu Lu,1 Guanzhong Chen,1 Shiqun Chen,1 Jin Liu,1 Yong Liu1
1Department of Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, 510080, People’s Republic of China; 2School of Biology and Biological Engineering, South China University of Technology, Guangzhou, People’s Republic of China; 3Department of Cardiology, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China; 4The Second School of Clinical Medicine, Southern Medical University, Guangzhou, People’s Republic of China; 5Department of Cardiology, Longyan First Affiliated Hospital of Fujian Medical University, Longyan, 364000, People’s Republic of China; 6Department of Cardiology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, Guangdong, People’s Republic of China; 7Ministry of Education, Key Laboratory of Hainan Trauma and Disaster Rescue, College of Emergency and Trauma, Hainan Medical University, Haikou, 571199, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yong Liu; Jin Liu, Department of Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, 510080, People’s Republic of China, Email [email protected]; [email protected]
Background: Inflammation and immune responses play an important role in the pathophysiology of contrast-associated acute kidney injury (CA-AKI), and systemic immune inflammation index (SII) has recently emerged as a new parameter for immune and inflammatory response evaluation. However, limited research has been undertaken to explore the relationship between SII and CA-AKI following coronary angiography (CAG).
Patients and Methods: From January 2007 to December 2020, 46,333 patients undergoing CAG were included from 5 Chinese tertiary hospitals. SII was calculated as total peripheral platelets count × neutrophil-to-lymphocyte ratio. Patients were categorized by preprocedural SII quartiles: Q1 ≤ 404.5, Q2 > 404.5 and ≤ 631.7, Q3 > 631.7 and ≤ 1082.8, Q4 > 1082.8. Univariable and multivariable logistic regression were used to reveal the link between preprocedural SII and CA-AKI.
Results: A total of the 46,333 patients (62.9 ± 11.5 years, female 28.1%) were included in the study. The incidence of CA-AKI was 8.4% in Q1 group, 8.7% in Q2 group, 9.4% in Q3 group, 15.1% in Q4 group. In the multivariable model, comparing the highest (Q4 group) to lowest (Q1 group) SII level categories, preprocedural SII was related to a higher risk of CA-AKI after fully adjusting for well-known confounders, and there was no statistically difference in the other two SII level categories (Q2 and Q3 groups) compared with Q1 group (adjusted model 3: Q2 group: OR: 0.98, 95% CI: 0.87– 1.11, P = 0.771; Q3 group: OR: 1.04, 95% CI: 0.92– 1.18, P = 0.553; Q4: OR: 1.65, 95% CI: 1.45– 1.88, p < 0.001; P for trend < 0.001). Similar results were found for all the subgroups analysis except for patients undergoing PCI, and the interaction analyses for age, PCI and AMI were significant. In addition, Kaplan–Meier curves demonstrated that the lowest quartile group showed the worst all-cause mortality in a significant SII level-dependent manner among the four groups (Log rank test; p < 0.0001).
Conclusion: Elevated preprocedural SII level was a significant and independent risk factor for CA-AKI following CAG. Higher-quality prospective studies are needed to validate the predictive value of SII for CA-AKI.
Keywords: systemic immune inflammation index, contrast-associated acute kidney injury, coronary angiography, preprocedural
Introduction
Contrast-associated acute kidney injury (CA-AKI) is a common complication following coronary angiography (CAG), with an estimated incidence of up to 12%,1 and is a more frequent complication in patients with multiple risk factors like diabetes mellitus (DM) and chronic kidney disease (CKD).2 Meanwhile, previous studies demonstrated that patients with CA-AKI had a poor prognosis.3 Therefore, identifying the patients with high-risk CA-AKI is needed in clinical practice.
It has been demonstrated that the occurrence and progression of CA-AKI is mediated by different mechanisms, of which, inflammation and activation of immune responses may play a key role in this process.4,5 Additionally, experimental studies showed that immune cells including neutrophils, dendritic cells, macrophages and lymphocytes contribute to the pathogenesis and repair of AKI.6 Recently, systemic immune inflammation index (SII; SII = platelet count × neutrophil count/lymphocyte count), a new inflammation parameter, has been proposed to assess the inflammatory and immune status.7 SII is not only a potential prognostic marker in patients with various cancers,8,9 but also related to adverse outcomes in patients with cardiovascular disease.10 However, the association between preprocedural SII and CA-AKI development following CAG has not been tested.
Based on these findings, this multi-center study is designed to clarify the relationship between preprocedural SII and CA-AKI risk in a large Chinese population, which may help clinicians to early evaluate the risk of CA-AKI following CAG and initiate the treatment for prevention.
Methods
Study Population
We collected the baseline information from the registry of Cardiorenal ImprovemeNt II (CIN-II) study from January 2007 to December 2020 in five south Chinese regional central tertiary teaching hospitals (2 urban and 3 rural regions, see in Supplemental Table 1). CAG or PCI was performed in patients with clinical indications. After excluding patients who did not receive CAG, lacked preoperative or postoperative serum creatinine (Scr) data, missed laboratory parameters, a total of 46,333 patients were included in our research. The inclusion criteria were as follows: 1) patients undergoing CAG were included, 2) creatinine was measured before procedure and within 48 hours after procedure, 3) patients with long-term follow-up information, 4) patients with complete clinical data. All of the patients were divided into four groups by preprocedural SII quartiles (Supplementary Figure 1). All personal details were erased to protect patient data confidentiality. This clinical study was approved by the Guangdong Provincial People’s Hospital ethics committee (NO. GDREC2019-555H-2) and complied with the declaration of Helsinki. All participating sites received institutional review board approval from their own ethics committees.
Data Collection
Clinical information after the patient’s first admission was collected from the Electronic Clinical Management System (ECMS) for all participant hospitals, including demographic information, medical history, laboratory test results, medications, as well as procedure. Clinical data was processed and imported into a relational database that can be queried using R, version 4.0.3 software (R Foundation for Statistical Computing, Vienna, Austria). Two senior cardiologists were responsible for the data quality control and periodical database verification. Biochemistry data including platelet, neutrophils and lymphocyte were measured on admission by an automatic biochemical analyser before procedure. The serum creatinine (Scr) concentration was measured at admission and within 24h, 48h, and 72 h after administration of contrast. Scr was measured by a kinetic colorimetric assay (Jaffe) in all centers using the machine (Beckman, AU5800, the United States). Other biochemical indicator measurement was performed on admission. Uniform methods of measurement were used in all centers.
Study Endpoint and Variables
The primary endpoint was CA-AKI, defined as an increase in the Scr level of >0.5 mg/dL or >25% from the baseline level within the first 48h after procedure.11 The secondary endpoints were long-term all-cause mortality after admission (the median follow-up period was 6.1 years [interquartile range: 3.0–8.8]). Systemic immune-inflammation index (SII) was calculated as total platelets count× neutrophil-to-lymphocyte ratio. The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were calculated as follows: absolute neutrophil count/absolute lymphocyte count and total number of platelets/absolute lymphocyte count, respectively. CKD was defined as an estimated glomerular filtration rate (eGFR) calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation <60 mL/min/1.732.12 Congestive heart failure (CHF) was defined as New York Heart Association (NYHA) class >2 or Killip class >1. Acute myocardial infarction (AMI), DM, and hypertension (HT) were diagnosed according to ICD-10. Anemia was defined as a hematocrit ≤39% for males or ≤36% for females. The Mehran scores (MRS) were calculated by eight variables (age > 75 years, hypotension (HT), intra-aortic balloon pump (IABP), CHF, diabetes, anemia, contrast volume (CMV) and eGFR).13
Statistical Analysis
The population was classified into four groups according to preprocedural SII quartiles: Q1, Q2, Q3, and Q4. Descriptive analysis for continuous variables were shown as means ± standard deviation or medians and interquartile ranges according to distribution. Categorical variables were described as proportions. Continuous variables were compared by analysis of variance (ANOVA) and categorical variables by Pearson’s Chi-square test.
Time-to-event data were shown in graphs using Kaplan–Meier curves, and the differences between groups were assessed by Log rank test. Multivariable Cox proportional hazard regression models adjusting for potential confounders were performed to analyze hazard ratios (HRs) with 95% confidence interval (CI) for comparing non-CA-AKI patients with CA-AKI patients (adjusted confounders including demographic characteristics, medical history and medicine). Univariable and multivariable logistic regression analyses tested the association between preprocedural SII and CA-AKI. Any variable with a p-value <0.10 in the univariable analysis and variables based on clinical plausibility were included in the model 3, which were fully adjusted for angiotensin-converting enzyme inhibitors and angiotensin receptor blockers (ACEI/ARB), age, AMI, anemia, coronary artery disease (CAD), CMV, CHF, DM, eGFR, gender, HT, high-density lipoprotein cholesterol (HDL-C), IABP, low-density lipoprotein cholesterol (LDL-C), PCI, statins.5,14 We used the variance inflation (VIF) as the criterion for multicollinearity. When VIF ≥5, it can be considered that there is a multicollinearity between independent variables.15 Collinearity analysis demonstrated no collinearity among the variables. The association between preprocedural SII and the risk of CA-AKI was displayed in forest plot, and restricted cubic splines were used to evaluate the level-dependent relationship between the natural log of SII and OR. In addition, SII, NLR or PLR were included in the MRS to compare the predictive power before and after the addition, respectively. Missing values in the candidate predictor variables were imputed using multivariate imputations by the chained equation method.
We further explored heterogeneity in subgroup analyses (age, gender, PCI, AMI and CHF). Stratification and interaction analysis were used to ensure robustness of the results. All statistical analyses were performed using R (ver. 4.0.3). A 2-tailed P value <0.05 was considered statistically significant.
Results
Patient Characteristics
A total of 46,333 patients undergoing CAG were enrolled, including 33,315 males and 13,018 females (mean age 62.9 ± 11.5 years). All patients were split into the following quartiles based on preprocedural SII levels subsequently: quartile (Q)1 group (n = 11,583), Q2 group (n = 11,583), Q3 group (n = 11,584) and Q4 group (n = 11,583). With the increasing level of preprocedural SII, the elderly population was gradually increasing, and patients had higher levels of neutrophil counts, platelet counts, WBC and LDL-C, more likely to be combined with AMI, HT, DM, CHF and anemia, as well as more likely to receive statins and ACEI/ARB. On the contrary, as preprocedural SII level increased, the lymphocyte counts, albumin (ALB), hemoglobin (HGB), left ventricular ejection fraction (LVEF), and eGFR were significantly lower. More details are shown in Table 1.
 |
Table 1 Baseline Characteristics According to Preprocedural SII Level Categories |
Prevalence of CA-AKI and Clinical Outcomes
CA-AKI occurred in 4815 (10.4%) patients. The incidence of CA-AKI was only 8.4% (n = 973) in Q1 group, 8.7% (n = 1010) in Q2 group, and 9.4% (n = 1086) in Q3 group, while the incidence significantly increased to 15.1% (n = 1746) in Q4 group (p for trend <0.001) (Figure 1). As preprocedural SII level increased, the 1-year mortality (p for trend <0.001) (Figure 2). Kaplan–Meier curves demonstrated that the lowest quartile group showed the worst long-term all-cause mortality in a significant SII level-dependent manner among the four groups (Log rank test; p < 0.001) (Figure 3). Moreover, cox proportional hazard regression analysis showed that the risk of mortality was higher in patients with increased SII level (Q2 group: HR: 1.15, 95% CI: 1.05–1.26, P < 0.001; Q3 group: HR: 1.31, 95% CI: 1.19–1.43, P < 0.001; Q4 group: HR: 1.59, 95% CI: 1.45–1.75, P < 0.001; P for trend <0.001) (Supplementary Table 2).
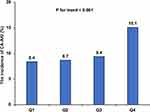 |
Figure 1 The incidence of CA-AKI in the four groups. |
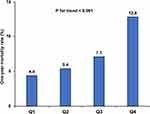 |
Figure 2 The incidence of one-year mortality in the four groups. |
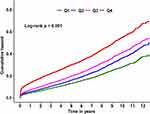 |
Figure 3 Kaplan–Meier curve in terms of all-cause mortality according to the preprocedural SII quartiles. |
Association Between Preprocedural SII and CA-AKI
When preprocedural SII as a continuous variable, it was significantly associated with CA-AKI (per 100 increase: adjusted odds ratio [aOR], 1.02, 95% confidence interval (CI): 1.01–183 1.02, P < 0.001), whereas the result of log (SII) was similar (aOR, 1.25, 95% CI: 1.17–133, P < 0.001). Subsequently, to further analyze the relationship between the range of preprocedural SII and the increased risk of CA-AKI, we performed univariable and multivariable regression analysis using preprocedural SII as a categorical variable. In the model 3, comparing the highest (Q4 group) to lowest (Q1 group) SII level categories, preprocedural SII was related to a higher risk of CA-AKI after fully adjusting for well-known confounders, and there was no statistical difference in the other two SII level categories (Q2 and Q3 groups) compared with Q1 group (adjusted model 3: Q2 group: OR: 0.98, 95% CI: 0.87–1.11, P = 0.771; Q3 group: OR: 1.04, 95% CI: 0.92–1.18, P = 0.553; Q4 group: OR: 1.65, 95% CI: 1.45–1.88, p < 0.001, p-for trend <0.001) (Table 2, Figure 4). Restricted cubic splines showed that the risk of CA-AKI had a non-linear relationship with continuous preprocedural SII even after fully adjusting for confounding factors (nonlinear P < 0.001): the risk of CA-AKI rose steeply when SII was in a relatively higher level (Figure 5).
 |
Table 2 Univariable and Multivariable Logistic Regression Analysis of the Association Between Preprocedural SII Level and CA-AKI Following CAG |
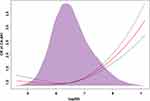 |
Figure 5 Restricted spline curve for the CA-AKI adjusted odds ratio. |
Discrimination and Calibration Analysis of Preprocedural SII Added to Models
Continuous variable SII, PLR or NLR added to MRS did contribute to a certain increase in C-statistics (Mehran: C-statistic = 0.694, 95% CI: 0.680–0.707; Mehran + PLR: C-statistic = 0.699, 95% CI: 685–0.713; Mehran + NLR: C-statistic = 0.702, 95% CI: 0.692–0.719; Mehran + Sll: C-statistic = 0.706, 95% CI: 0.688–0.715) (Figure 6).
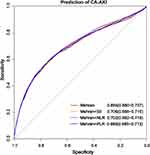 |
Figure 6 Receiver operator characteristics curves of Mehran risk score, Mehran+SII, Mehran+NLR and Mehran+PLR model. |
Subgroup Analysis
Similar results were found for the subgroup analysis regardless of age [age >65 years or ≤65 years], gender, AMI, or CHF, but the SII in Q3 and Q4 quartiles increased the risk of CA-AKI among patients undergoing PCI. Stratified and interaction analyses indicated that the relationship between CA-AKI and preprocedural SII levels was evident in patients undergoing PCI (P for interaction <0.001), age (P for interaction = 0.023), or AMI (P for interaction = 0.021) (Supplementary Figure 2).
Discussion
To our knowledge, this is the first multicenter study with a large sample to investigate the relationship between preprocedural SII and CA-AKI risk following CAG. In our study, preprocedural elevated SII level is an independent risk factor. It is further discovered that when SII is in high-level state (SII >1082.8), it has a significant impact on the CA-AKI. Our findings point to the importance of accounting for SII level in assessing the risk of CA-AKI following CAG. Monitoring SII levels may be considered as one focus of CA-AKI prevention efforts among patients who will be undergoing CAG.
CA-AKI is a complex and multi-factorial process. Although the definite mechanisms of CA-AKI are not completely elucidated, increased inflammatory response has been proved to play a vital role in the development and progress of CA-AKI.16 Previous researches found that some inflammatory indicators have a predictive value for the incidence of CIN.17,18 Kurtul et al found a positive correlation between the development of contrast-induced nephropathy (CIN) and neutrophil–lymphocyte ratio (NLR) in non-ST segment elevation myocardial infarction (NSTEMI) patients who underwent CAG.14 Our previous study also suggested that high sensitivity C-reactive protein (Hs-CRP) was a predictor of CIN following PCI among STEMI patients.5 SII, a new inflammation parameter, not only had a better prediction of poor outcomes in patients with cancer compared with NLR and platelet lymphocyte ratio (PLR)19 but also was associated with major cardiovascular events in CAD patients.10 Recently, Gok et al reported that SII had good accuracy in predicting more severe disease in patients with acute pulmonary embolism.20 In addition, higher SII levels were independently associated with the presence of isolated coronary artery ectasia, which demonstrated that a more severe inflammatory process may involve in the development of this variant of CAD.21 Besides, our study demonstrated that the preprocedural SII could improve predictive performance of MRS, which was similar to the NLR and PLR in predicting the risk of CA-AKI following CAG. It is meaningful to pay attention to the SII variation in CAG population, especially when SII >1082.8, which would significantly increase the risk of CA-AKI.
Similar to previous studies, our results showed that CHF and IABP were associated with a 1-fold and 2-fold increased risk of CA-AKI, respectively. Impairment in kidney function is common in HF patients, a variety of processes are involved including hemodynamic, hormonal, inflammatory.22 In addition, IABP can be used in AMI patients with cardiogenic shock for hemodynamic support, those patients with reduced effective blood volume and high level of inflammation are susceptible to developing AKI.23 Previous researches had demonstrated that different pathophysiological mechanisms may involve in the occurrence of CA-AKI, including renal vasoconstriction, direct cytotoxic effects of contrast agents, apoptosis, oxidative stress, and tubular obstruction.24 Meanwhile, the relationship between immune and inflammatory response and CA-AKI has been studied in both patients and animal models. Systemic inflammation may increase kidneys’ local inflammatory processes elicited by contrast medium reabsorption favoring the development of CI-AKI.25,26 In addition, contrast injection can lead to a significantly elevated release of inflammatory cytokines, including IL-6, TNF-α, NF-Κb, and NLRP3, which were accompanied by renal dysfunction and tubular injury.27–30 Moreover, in the AKI animal models, neutrophils may participate in renal injury by obstructing the renal microvasculature, secreting oxygen free radicals, proteases, and pro-inflammatory cytokines.6,31 Lymphocytes are key cells of the adaptive immune system, and many studies have revealed the substantial role of a diverse subset of lymphocytes in AKI. Of which, CD4+ T cells contribute to the establishment of renal injury in the early phase of renal injury, but regulatory T cells are likely to have a role in renoprotection due to their anti-inflammatory effect.6
In our study, the elevated preprocedural SII is associated with an increased risk of CA-AKI following CAG, especially when SII is in high-level state (SII >1082.8). Therefore, SII can be used as a tool for risk stratification following CAG to estimate patients’ susceptibility to CA-AKI and help clinicians recognize high-risk patients. Moreover, personalized perioperative hydration and reduction of contrast volume should be recommended in patients with higher level of SII, and avoiding angiography if possible.24 Besides, it is critical to managing risk factors, including HT, DM, CHF and anemia, which increase the occurrence of CA-AKI.32 Finally, more high-quality studies in the future are needed to verify the prediction of SII for CA-AKI and immune inflammation may be a novel therapeutic target for CA-AKI.
Limitation
This research has some limitations. First, this was a retrospective study, which cannot reflect direct causation. However, our study was derived from a large multicenter cohort, and we have made extensively adjusted for confounding factors. Second, the incidence of CA-AKI will be slightly fluctuation based on the different CA-AKI definitions, but it will not affect the relative occurrence tendency of CA-AKI in each group, and the definition of CA-AKI in our research has been widely cited in previous studies. Third, there may be some deviation when we screened patients, whereas our study included a large patient population and our results were stable in subgroup analysis. Fourth, cause-specific death was not available in this study, and it is difficult to examine the significant association between preprocedural SII and cause-specific death. Finally, we lacked a high-quality external validation cohort with which to verify our results.
Conclusion
Elevated preprocedural SII is an independent risk factor of CA-AKI following CAG, and helpful for risk stratification. Our research demonstrated that preprocedural SII level may be an optimal monitoring indicator for the prevention of CA-AKI and prognosis, which provided further evidence to help clinicians judge the CA-AKI risk among patients who will be undergoing CAG.
Data Sharing Statement
The datasets generated and analyzed during the current study are not publicly available due to the institution policy but are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
All traceable personal identifiers were removed from the analytic dataset to protect patients’ privacy. The study protocol was approved by Guangdong Provincial People’s Hospital ethics committee, and the study was performed according to the declaration of Helsinki.
Author Contributions
The authors’ responsibilities were as follows—Research idea and study design: Wenguang Lai, Xiaoli Zhao, Zhidong Huang, Yun Xie; Data acquisition: Sijia Yu, Qiang Li, Haozhang Huang, Huanqiang Li, Ziling Mai, Dachuan Guo, Jiabin Xiu, Hongyu Lu, Jiaming Xiu; Data analysis/interpretation: Guanzhong Chen; Statistical analysis: Shiqun Chen; Supervision and mentorship: Yong Liu, Jiyan Chen; Writing guidance: Jun-Yan Xu, Jin Liu, Shiqun Chen and Yong Liu. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions on the accuracy or integrity of any portion of the work are appropriately investigated and resolved. The authors declare that there is no competing interest. All authors read and approved the final version.
Funding
This research was funded and supported by the Beijing Lisheng Cardiovascular Health Foundation Pilot Fund (No. LHJJ20141751). The National Science Foundation of China (No.81970311 and No.82070360). Study on the function and mechanism of the potential target for early warning of cardiorenal syndrome after acute myocardial infarction based on transformism (DFJH201919, DFJH2020026). Clinical Medicine Research Fund of Guangdong Province (2019ZX01). Key Laboratory of Emergency and Trauma (Hainan Medical University), Ministry of Education (Grant. KLET-202116). NSFC Incubation Project of Guangdong Provincial People’s Hospital (KY0120220041). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript; the work was not funded by any industry sponsors.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial/non-financial relationships that could be construed as a potential conflict of interest.
References
1. Lun Z, Liu L, Chen G, et al. The global incidence and mortality of contrast-associated acute kidney injury following coronary angiography: a meta-analysis of 1.2 million patients. J Nephrol. 2021;34(5):1479–1489. doi:10.1007/s40620-021-01021-1
2. Amin AP, Salisbury AC, McCullough PA, et al. Trends in the incidence of acute kidney injury in patients hospitalized with acute myocardial infarction. Arch Intern Med. 2012;172(3):246–253. doi:10.1001/archinternmed.2011.1202
3. Sato A, Aonuma K, Watanabe M, et al. Association of contrast-induced nephropathy with risk of adverse clinical outcomes in patients with cardiac catheterization: from the CINC-J study. Int J Cardiol. 2017;227:424–429.
4. McCullough PA, Choi JP, Feghali GA, et al. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2016;68(13):1465–1473.
5. Liu Y, Tan N, Zhou YL, et al. High-sensitivity C-reactive protein predicts contrast-induced nephropathy after primary percutaneous coronary intervention. J Nephrol. 2012;25(3):332–340.
6. Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol. 2015;11(2):88–101.
7. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222.
8. Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of Systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. 2018;9(18):3295–3302.
9. Zheng K, Liu X, Ji W, Lu J, Cui J, Li W. The efficacy of different inflammatory markers for the prognosis of patients with malignant tumors. J Inflamm Res. 2021;14:5769–5785.
10. Yang YL, Wu CH, Hsu PF, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 2020;50(5):e13230. doi:10.1111/eci.13230
11. Briguori C, D’Amore C, De Micco F, et al. Left ventricular end-diastolic pressure versus urine flow rate-guided hydration in preventing contrast-associated acute kidney injury. JACC Cardiovasc Interv. 2020;13(17):2065–2074. doi:10.1016/j.jcin.2020.04.051
12. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006
13. Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44(7):1393–1399. doi:10.1016/j.jacc.2004.06.068
14. Kurtul A, Yarlioglues M, Duran M, Murat SN. Association of neutrophil-to-lymphocyte ratio with contrast-induced nephropathy in patients with non-ST-elevation acute coronary syndrome treated with percutaneous coronary intervention. Heart Lung Circ. 2016;25(7):683–690. doi:10.1016/j.hlc.2016.01.007
15. O’Brien R. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41(5):673–690. doi:10.1007/s11135-006-9018-6
16. Mehran R, Dangas GD, Weisbord SD. Contrast-associated acute kidney injury. N Engl J Med. 2019;380(22):2146–2155. doi:10.1056/NEJMra1805256
17. Kwasa EA, Vinayak S, Armstrong R. The role of inflammation in contrast-induced nephropathy. Br J Radiol. 2014;87(1041):20130738. doi:10.1259/bjr.20130738
18. Tanık VO, Çınar T, Velibey Y, et al. Neutrophil-to-lymphocyte ratio predicts contrast-induced acute kidney injury in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. J Tehran Heart Cent. 2019;14(2):59–66.
19. Chen JH, Zhai ET, Yuan YJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23(34):6261–6272. doi:10.3748/wjg.v23.i34.6261
20. Gok M, Kurtul A. A novel marker for predicting severity of acute pulmonary embolism: systemic immune-inflammation index. Scand Cardiovasc J. 2021;55(2):91–96. doi:10.1080/14017431.2020.1846774
21. Esenboğa K, Kurtul A, Yamantürk YY, Akbulut İM, Tutar DE. Comparison of systemic immune-inflammation index levels in patients with isolated coronary artery ectasia versus patients with obstructive coronary artery disease and normal coronary angiogram. Scand J Clin Lab Invest. 2022;82(2):132–137. doi:10.1080/00365513.2022.2034034
22. Kumar U, Wettersten N, Garimella PS. Cardiorenal syndrome: pathophysiology. Cardiol Clin. 2019;37(3):251–265. doi:10.1016/j.ccl.2019.04.001
23. Fuernau G, Poenisch C, Eitel I, et al. Prognostic impact of established and novel renal function biomarkers in myocardial infarction with cardiogenic shock: a biomarker substudy of the IABP-SHOCK II-trial. Int J Cardiol. 2015;191:159–166. doi:10.1016/j.ijcard.2015.04.242
24. Zhang F, Lu Z, Wang F. Advances in the pathogenesis and prevention of contrast-induced nephropathy. Life Sci. 2020;259:118379. doi:10.1016/j.lfs.2020.118379
25. Toso A, Leoncini M, Maioli M, et al. Relationship between inflammation and benefits of early high-dose rosuvastatin on contrast-induced nephropathy in patients with acute coronary syndrome: the pathophysiological link in the PRATO-ACS study (protective effect of rosuvastatin and antiplatelet therapy on contrast-induced nephropathy and myocardial damage in patients with acute coronary syndrome undergoing coronary intervention). JACC Cardiovasc Interv. 2014;7(12):1421–1429.
26. Quintavalle C, Fiore D, De Micco F, et al. Impact of a high loading dose of atorvastatin on contrast-induced acute kidney injury. Circulation. 2012;126(25):3008–3016.
27. Laskey WK, Gellman J. Inflammatory markers increase following exposure to radiographic contrast media. Acta Radiol. 2003;44(5):498–503.
28. Lu Z, Cheng D, Yin J, et al. Antithrombin III protects against contrast-induced nephropathy. EBioMedicine. 2017;17:101–107.
29. Machado RA, Constantino Lde S, Tomasi CD, et al. Sodium butyrate decreases the activation of NF-κB reducing inflammation and oxidative damage in the kidney of rats subjected to contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27(8):3136–3140.
30. Liu X, Song W, Zhang X, et al. Downregulating LncRNA XIST attenuated contrast-induced nephropathy injury via regulating miR-133a-3p/NLRP3 axis. J Thromb Thrombolysis. 2021;52(2):440–453.
31. Kinsey GR, Okusa MD. Role of leukocytes in the pathogenesis of acute kidney injury. Crit Care. 2012;16(2):214.
32. Ozkok S, Ozkok A. Contrast-induced acute kidney injury: a review of practical points. World J Nephrol. 2017;6(3):86–99.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

