Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Elevated TPOAb is a Strong Predictor of Autoimmune Development in Patients of Type 2 Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease: A Case–Control Study
Authors Wang C , Niu Q, Lv H , Li Q, Ma Y, Tan J, Liu C
Received 3 September 2020
Accepted for publication 27 October 2020
Published 16 November 2020 Volume 2020:13 Pages 4369—4378
DOI https://doi.org/10.2147/DMSO.S280231
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Chenyi Wang,1,2,* Qianglong Niu,1,* Haihong Lv,1,* Qian Li,1 Yuping Ma,1 Jiaojiao Tan,1 Chunhua Liu1
1Department of Endocrinology, The First Hospital of Lanzhou University, Lanzhou, Gansu 730000, People’s Republic of China; 2Department of Endocrinology, The First Clinical Medical College of Lanzhou University, Lanzhou, Gansu, 730000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Haihong Lv Department of Endocrinology
The First Hospital of Lanzhou University, Lanzhou, Gansu 730000, People’s Republic of China
Email [email protected]
Objective: The aim of this study was to assess the prevalence of thyroid autoimmunity in T2DM with NAFLD, furthermore explore the relationship between elevated TPOAb titer and the severity of NAFLD.
Methods: A total of 400 patients with T2DM were divided into two groups according to NAFLD. Thyroid function and other metabolic indicators were measured.
Results: There were more TPOAb-positive patients in both groups, and the prevalence of TPOAb positive was significantly different in two groups (17% vs 6.9%, p< 0.01). FT4 was significantly lower in patients with T2DM with NAFLD (median FT4 0.89 vs 1.08, p < 0.001), while TSH was increased (median TSH 2.85 vs 2.28, p < 0.05). In patients with T2DM with NAFLD, the proportion of women in the thyroid autoimmune-positive group was significantly higher than the negative (71.1% vs 46%, p < 0.01). Similarly, thyroid autoimmune-positive T2DM and NAFLD patients had lower FT4 levels (median FT4 0.59 vs 0.92, p < 0.001), higher TSH levels (median TSH 3.65 vs 2.67, p < 0.001), and much higher TPOAb/TGAb (median TPOAb/TGAb 6.8 vs 1.46, p < 0.001). The increase of TPOAb was significantly correlated with the severity of fatty liver. HbA1c, TC, TG, TSH, TPOAb/TGAb and severity of fatty liver were risk factors of thyroid autoimmunity.
Conclusion: Autoimmune thyroid disease is more common in patients with T2DM complicated with NAFLD. Elevated TPOAb titer is closely related to fatty liver, suggesting that elevated TPOAb titer is a predictor of autoimmune development in T2DM with NAFLD.
Keywords: type 2 diabetes mellitus, non-alcoholic fatty liver disease, thyroid peroxidase antibody, thyroglobulin antibody, autoimmune thyroid disease
Introduction
Type 2 diabetes mellitus with non-alcoholic fatty liver disease is a common chronic disease in clinical practice,1 and its clinical manifestations are mainly elevated blood glucose and liver damage. Studies suggest that multiple factors are involved in the pathogenesis of T2DM with NAFLD, which may be associated with insulin resistance, oxidative stress, decreased pancreatic β-cell response, C-reactive protein, high level of TNFα and fatty acid-binding protein-4.2–4Although the pathogenesis of these diseases has been deeply understood in recent years, the two diseases are mutually causal and difficult during clinical treatment.5,6 Whether NAFLD presents independently or in combination with metabolic risk factors such as abdominal obesity, hyperlipidemia, hypertension and hyperinsulinemia, growing evidence indicated that NAFLD is associated with extrahepatic complications such as cardiovascular disease, type 2 diabetes, thyroid disease, chronic kidney disease and malignancy.7,8 In addition, it has also been found that patients with NAFLD experience abnormalities in thyroid autoantibodies.9
Both thyroid peroxidase antibody (TPOAb) and thyroglobulin antibody (TGAb) belong to thyroid autoantibodies, and the elevation of thyroid autoantibodies is often accompanied by autoimmune thyroid diseases.10 In autoimmune thyroid diseases, such as the vast majority of patients with Hashimoto’s thyroiditis and some patients with primary hyperthyroidism, a significant increase of thyroid autoantibodies may occur, and the pathogenesis is not fully clarified, which is currently believed to be related to lymphocyte infiltration in the thyroid follicular stroma.11 However, mild to moderate increases can be observed in non-thyroid autoimmune diseases, such as type 1 diabetes, Addison’s disease, pernicious anemia, rheumatoid arthritis and systemic lupus erythematous.12–14 Elevated thyroid autoantibodies are common in patients with T1DM and are generally considered to be possibly associated with autoimmune and inflammatory mechanisms, but they are less in patients with T2DM and NAFLD.
The close relationship between type 1 diabetes and thyroid autoimmunity has been reported in many previous studies.12,15–17 In recent years, some studies have shown that the elevated levels of thyroid autoantibodies are correlated with T2DM.18,19 Osei Sarfo-Kantanka et al found a higher prevalence of thyroid autoimmunity in T2DM patients compared to healthy people.19 In clinical practice, we found that the serum levels of thyroid autoantibodies in patients with T2DM and NAFLD often increase, however, the correlation between the two has not been reported before. The purpose of this paper is to investigate the correlation between T2DM with NAFLD and the levels of thyroid autoantibodies, and further clarify the pathogenesis of T2DM with NAFLD for providing new ideas for clinical treatments.
Subjects and Methods
Subjects
A total of 400 patients with T2DM aged≥18 years, including 185 males and 215 females, who were hospitalized in the Department of Endocrinology of the First Hospital of Lanzhou University from September 2014 to September 2019 were selected. This study was approved by the Ethics Committee of the First Hospital of Lanzhou University and written informed consent for the study was obligatory for all participants. According to the results of the abdominal ultrasound, the patients were divided into the observation group and the control group according to the presence or absence of NAFLD. There were 212 patients with NAFLD, including 105 males and 107 females, and 188 patients without NAFLD, including 80 males and 108 females.
Inclusion criteria: 1. Aged≥18 years old; 2. The diagnosis of T2DM refers to the Standards of Medical Care in Diabetes issued by the American Diabetes association (ADA) in 2019.
Exclusion criteria: 1. Acute complications of diabetes, such as diabetic ketoacidosis, hyperosmolar hyperglycemia syndrome, acute infection, etc.; 2. History of heavy drinking, viral hepatitis, drug-induced liver disease, autoimmune liver disease, total parenteral nutrition, Wilson’s disease and other specific diseases that can lead to fatty liver; 3. History of thyroid disease or thyroid surgery, history of neck trauma, and taking drugs that affect thyroid function; 4. Severe heart, liver, and kidney dysfunction; 5. History of malignant tumors; 6. Pregnant or lactating women; 7. Children and adolescents aged less than 18 years.
General Clinical Data Collection
Standard medical history was collected for patients who met the above criteria. General data of the two groups were obtained in detail, such as the age, gender, duration of T2DM, history of smoking and drinking, previous medical history and medication history. Height, weight and arterial blood pressure were measured on the first day of admission.
Serology Data Collection
All eligible patients were submitted to venous blood under fasting state in the morning on the second day of admission to determine the levels of laboratory parameters such as total number of white blood cells, lymphocyte percentage, total number of lymphocytes, FBG, FINS, fasting C-peptide, HbA1c, TC, TG, LDL-C, HDL-C, ALT, AST, GGT, TT3, TT4, FT3, FT4, TSH, TPOAb, TGAb, TBG, and TG. The above indexes were determined by standardized high-performance liquid chromatography assay (Bio-Rad Variant II hemoglobin testing autoanalyzer) or chemiluminescent immunoassay (Roche Diagnostics, Cobas e411 automated immunoassay analyzer, Indianapolis, USA).
Homeostasis model assessment-insulin resistance (HOMA-IR) was calculated using the following formula: fasting serum insulin (mU/L)×fasting blood glucose (mmol/L)/22.5, and insulin resistance was defined as HOMA-IR≥2.6×10−6 molU/L2.
Assessment of Thyroid Function
Thyroid function of the enrolled patients was assessed by chemiluminescent immunoassay (Siemens, IMMULITE 2000, Erlangen, Germany). Normal thyroid function was defined as serum TSH and FT4 within the reference range. Subclinical hypothyroidism was defined as serum TSH>4.94uIU/mL and FT4 level within the reference range. Overt hypothyroidism was defined as serum TSH>4.94uIU/mL and FT4 level <0.7 ng/dL. Subclinical hyperthyroidism was defined as serum TSH < 0.35uIU/mL and FT4 level within the reference range. Overt hyperthyroidism was defined as serum TSH < 0.35uIU/mL and FT4 level >1.48 ng/dL. TPOAb positive (serum level > 5.61 IU/mL) and/or TGAb positive (serum level >4.11 IU/mL) were considered as the presence of thyroid autoimmunity.
Diagnosis of Non-Alcoholic Fatty Liver Disease
The diagnosis of NAFLD is referred to The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance From the American Association for the Study of Liver Diseases, issued by American Association for the Study of Liver Diseases (AASLD), American College of Gastroenterology (ACG) and American Gastroenterology Association (AGA) in 2018. An experienced sonographer performed abdominal ultrasound for each patient, and diffuse fatty liver was present in two of the following three abdominal ultrasound findings: ①diffuse enhancement of the near-field echoes of the liver, with stronger echoes than the kidneys; ②unclear display of the intrahepatic tubular structure; ③gradual attenuation of the far-field echoes of the liver.
The severity of fatty liver was graded as follows: ①mild fatty liver: the size and shape of the liver were normal, the echo in the front field was enhanced, the echo attenuation in the back field was not obvious, and the tubular structure of the liver was clear; ②moderate fatty liver: the size and shape of the liver were normal, or mild to moderate enlargement, the echo in the front field was enhanced, the echo in the back field was attenuated, and the tubular structure was vague but still identifiable; ③severe fatty liver: the liver was significantly enlarged, the shape was full, the echo in the front field was significantly enhanced, the echo attenuation in the back field was obvious, and even there was an anechoic area, the contour was unclear, and the tubular structure was illegible.
Statistical Analysis
All data were statistically analyzed using the SPSS 26.0 software package. For quantitative variables, results were expressed as mean ± standard deviation for parametric data and as median (interquartile range) for non-parametric data. Categorical variables were expressed as the number of patients and percentage (%). Significant differences between quantitative variables according to whether they followed a normal distribution were evaluated by independent sample t-test and independent sample non-parametric test, respectively. The chi-square test was used to compare the significance of categorical variables. Independent risk factors associated with thyroid autoimmunity were determined by binary logistic regression model. All statistical tests were bilateral, and p < 0.05 was considered statistically significant.
Results
Clinical Baseline Data and Thyroid Function According to the Presence or Absence of NAFLD
The clinical baseline data of patients in the two groups are shown in detail in Table 1. Compared with the T2DM alone group, indicators such as age, duration of diabetes, fasting insulin, insulin resistance index, glycosylated hemoglobin, hyperlipidemia, and transaminases of patients in the T2DM combined with NAFLD group were significantly increased, and the differences were significant (p < 0.05). Other clinical data such as FBG, smoking history and GGT were not significantly different although T2DM with NAFLD group was higher than that in the control group with T2DM alone (p > 0.05). Although there was no significant statistical difference in the prevalence of thyroid autoimmunity between the two groups, the prevalence of thyroid autoimmunity was higher in the T2DM with NAFLD group compared with the T2DM alone group (6.6% vs 3.7%, p > 0.05). There were more TPOAb-positive patients than TGAb-positive patients in either the T2DM with NAFLD group or the T2DM alone group (17% vs 8%, 6.9% vs 4.8%), and the prevalence of TPOAb-positive was statistically significantly different between the two groups (17% vs 6.9%, p < 0.01). FT4 was significantly lower in patients with T2DM complicated with NAFLD than in the T2DM group (median FT4 0.89 vs 1.08, p < 0.001), while TSH was significantly increased compared with the T2DM alone group (median TSH 2.85 vs 2.28, p < 0.05) (shown in Table 2).
 |
Table 1 Comparison of Clinical Baseline Data Between T2DM with NAFLD and T2DM Patients |
 |
Table 2 Comparison of Thyroid Function Between T2DM with NAFLD and T2DM Patients |
General Conditions of T2DM with NAFLD Patients According to Thyroid Antibodies
Among the population with T2DM and NAFLD, 38 (17.9%) were thyroid autoimmune positive and 174 (82.1%) were thyroid autoimmune negative (as shown in Table 3). Compared with the thyroid autoimmune-negative group, the proportion of women in the thyroid autoimmune-positive group was significantly higher (71.1% vs 46%, p < 0.01), the duration of T2DM was relatively longer (median duration of T2DM 12.5 years vs 6.5 years, p < 0.001), and the total number of lymphocytes was also significantly higher (median number of lymphocytes 2.34×109/L vs 1.96×109/L, p < 0.01). In addition, metabolic parameters such as blood glucose, blood lipids, and transaminases were also significantly higher than those in people with negative thyroid autoimmunity (p < 0.05). Similarly, T2DM patients with NAFLD who had positive thyroid autoimmunity had lower FT4 levels (median FT4 0.59 vs 0.92, p < 0.001) and higher TSH levels (median TSH 3.65 vs 2.67, p < 0.001). TPOAb/TGAb was also much higher in those with positive thyroid autoimmunity than in negative patients (median TPOAb/TGAb 6.8vs1.46, p < 0.001). In the thyroid autoimmune-positive group, the majority of patients were severe fatty liver (42.1%), while in the thyroid autoimmune-negative group, a large proportion (59.2%) of patients were mild fatty liver. No significant difference was found between the two groups in the number of patients with moderate fatty liver disease (p > 0.05).
 |
Table 3 Comparison of the General Conditions of Patients with T2DM and NAFLD According to Thyroid Antibody |
Relationship Between Different TPOAb Titers and Multiple Possible Influencing Factors
The prevalence of TPOAb-positive was significantly higher than that of TGAb in T2DM with NAFLD (Table 2). TPOAb was divided into four groups according to the degree of TPOAb titer elevation, it was not difficult to find a significant correlation between TPOAb titer and the severity of NAFLD (p < 0.001). When TPOAb titer was within the normal range, the number of patients with mild fatty liver was the highest (59.1%), followed by moderate fatty liver (29%), and severe fatty liver was the least (11.9%). With the increase of TPOAb titer, the proportion of people with severe fatty liver also gradually increased. When TPOAb titer was greater than 50 times the upper limit of normal, there was only 1 case of moderate fatty liver, the number of people with severe fatty liver was 7, accounting for 87.5% (as shown in Table 4). In addition, as TPOAb titer increased, HbA1c, TC, TG, ALT, and TSH also increased. Conversely, FT4 gradually decreased. Although there was no significant statistical difference between the levels of HbA1c in the four groups, there was also an obvious trend of increase.
 |
Table 4 Relationship Between Different TPOAb Titer and Severity of Fatty Liver |
Among the multiple possible influencing factors, the increase of cholesterol was the most significant, and there were significant differences between groups 2, 3, and 4 when compared with group 1, respectively (p < 0.05). Other indicators, such as transaminases and thyroid function, were mostly manifested as significant differences between group 2 and group 1 (Table 5).
 |
Table 5 Relationship Between Different TPOAb Titer and Multiple Possible Influencing Factors |
Binary Logistic Regression Analysis in T2DM with NAFLD and Thyroid Autoimmunity Patients
We performed binary logistic regression analysis of the factors that may affect thyroid autoimmunity in T2DM patients with NAFLD. In addition to male gender and elevated FT4 as independent protective factors for autoimmunity, diabetes duration, HbA1c, TC, TG, TSH, TPOAb/TGAb, and NAFLD severity were all independent risk factors for the development of thyroid autoimmunity (as shown in Table 6). For every 1 mmol/L increase in cholesterol and triglyceride, the risk of autoimmunity in patients with T2DM and NAFLD increased 4.294-fold and 3.217-fold, respectively, and for every 1% increase in HbA1c, the risk of autoimmunity increased 1.312-fold. In addition, every 1 uIU/mL increase in TSH increased the risk of autoimmunity by 2.456-fold, and for a 1-unit increase in TPOAb/TGAb, the risk increased 1.265-fold. Compared with T2DM patients without fatty liver, the risk of autoimmunity was increased by 0.258-fold, 0.059-fold, and 0.232-fold in patients with different degrees of fatty liver, respectively.
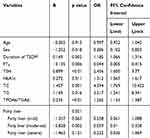 |
Table 6 Binary Logistic Regression Analysis of Thyroid Autoimmunity in T2DM and NAFLD Patients |
Multivariate Logistic Regression Analysis of Different Levels of TPOAb with Patients
Multiple regression analysis illustrated that different TPOAb titers were associated with NAFLD (P < 0.05) (Table 7). The prevalence of NAFLD in patients with high TPOAb titer was 0.045-fold higher than in those with low and moderate TPOAB titer.
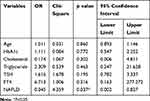 |
Table 7 Multivariate Logistic Regression Analysis of Different Levels of TPOAb with Patients |
Discussion
High prevalence of thyroid disease is more common in type 1 diabetes compared to T2DM due to the associated autoimmunity and common genetic basis.17 Latent autoimmune diabetes in adults (LADA) is a special type of diabetes characterized by disease-specific autoantibodies (anti-glutamic decarboxylase GAD-Ab, IA-2Ab), with great heterogeneity in clinical manifestations. However, the autoimmunity of LADA is not only targeted at pancreatic cells. Previous studies have found that LADA patients express high TPOAb activity.20 However, it has also been found that there are many people with T2DM complicated with autoimmune thyroid disease, and the intrinsic mechanism may be more complex. Sarfo-Kantanka, O. and Chen, Y. et al reported the presence of serum thyroid autoantibodies in patients with T2DM and NAFLD, respectively,9,19 but it remains unknown whether thyroid autoimmunity exists in patients with both chronic endocrine diseases and the intrinsic association. However, in recent years there have been reports on the association of thyroid autoimmunity and thyroid cancer, ischemic stroke, cognitive and emotional disorders and adverse pregnancy outcomes.21–24 Therefore, it is necessary to explore the level of serum thyroid autoantibodies and their potential relationship in patients with T2DM complicated with NAFLD.
Previous studies have found that the prevalence of thyroid dysfunction is significantly higher in patients with T2DM than in the normal population.18,25 In patients with T2DM, hypothyroidism is the most common type of thyroid dysfunction, with the incidence ranging from 18.3% to 45.2%.18,25,26 The differences in the above results may be related to different detection methods, different races, and sample sizes. The present study showed that FT4 level in T2DM combined with NAFLD was significantly lower than control group (median FT4 0.89 vs 1.08, p < 0.001), while TSH level was significantly higher compared with the T2DM group, and the difference was significant (median TSH 2.85 vs 2.28, p < 0.05) (Table 2). Thus, patients with T2DM and NAFLD have a higher prevalence of thyroid dysfunction, which is a further extension of previous findings. Low iodine intake is also a common cause of thyroid disease, which will undoubtedly affect our results. However, this study was conducted in areas with long-term iodine supplementation in salt,27 and iodine deficiency was very unlikely to cause hypothyroidism. Maria E. Barmpari et al believed that hypothyroidism in T2DM is also caused by autoimmunity, similar to that of type 1 diabetes.28 Another prospective cohort study found that hypothyroidism was associated with an increased risk of NAFLD. Hypothyroid patients had a 1.24-fold higher risk of developing NAFLD than euthyroid patients. In addition, the risk of NAFLD gradually decreases from hypothyroidism to hyperthyroidism.8 This may partly explain why the incidence of thyroid dysfunction is higher in patients with T2DM and NAFLD than in those with diabetes alone.
As can be seen from Table 2, the prevalence of thyroid antibodies positive in the T2DM combined NAFLD group was significantly higher than that in the T2DM group alone, and TPOAb positive are more than TGAb-positive patients, the prevalence of TPOAb-positive was significantly different between the two groups. Other studies have also shown that elevated TPOAb is predominant in patients with positive thyroid autoimmunity whether in patients with T2DM alone and NAFLD alone,9,25,29 consistent with our findings. This study found that the prevalence of thyroid autoimmunity in patients with T2DM complicated with NAFLD was 6.6%, which was not statistically significant compared with T2DM alone group (3.7%), but it also showed a significant upward trend. In addition, we used TPOAb/TGAb, which has not been previously reported, as an outcome indicator for the first time, and found that TPOAb/TGAb was significantly different between the two groups (p<0.05), which we speculated both groups of patients had a predominance of elevated TPOAb titer. These may confirm the involvement of autoimmune factors in the pathogenesis of diabetes with fatty liver.
Women predominate in all autoimmune diseases. Patients with T2DM combined with NAFLD were divided into two groups according to thyroid autoimmunity in Table 3, and it can be clearly seen that the majority (71.1%) were female in the thyroid autoimmune-positive group. This may be because B cells can produce more antibodies, and women have stronger humoral and cellular immune responses and higher levels of immune CD4+ T cells. Estrogen also has a significant immunomodulatory effect, it can reduce CD4+/CD8+ T cells ratio and TNF-α cytotoxicity, and increase immunoglobulin secretion, B-cell survival and B-cell polyclonal activation, as well as IgG and IgM production in peripheral blood monocytes.10 It is well known that immune thyroiditis is the leading cause of hypothyroidism. Current studies concluded that the increase of TPOAb and TGAb levels would promote the development of hypothyroidism.10 We also confirmed that, lower FT4 levels (median FT4 0.59 vs 0.92, p < 0.001) and higher TSH levels (median TSH 3.65 vs 2.67, p < 0.001) in thyroid autoimmune-positive patients.
Table 3 shows that patients with moderate to severe fatty liver in the thyroid autoimmune-positive group account for the majority (42.1%), while patients with mild fatty liver in the thyroid autoimmune negative group account for the larger proportion (59.2%). We therefore boldly hypothesized that the presence of thyroid autoimmunity was significantly associated with the severity of NAFLD and confirmed this hypothesis using statistical methods. The severity of fatty liver is graded in Table 4, and there was a significant correlation between the increased TPOAb titer and the severity of NAFLD (p < 0.001).
Moreover, as TPOAb titer increased, HbA1c, TC, TG, ALT and TSH levels also increased, while FT4 gradually decreased, especially, the increase in cholesterol was the most significant (Table 5). On the one hand, thyroid hormone affects glucose metabolism and lipid metabolism by regulating insulin secretion and insulin sensitivity in different tissues. Thyroid autoimmunity often eventually leads to varying degrees of hypothyroidism. Hypothyroidism will alter blood glucose and lipid levels in patients with diabetes and NAFLD and will aggravate the patients’ condition.30 On the other hand, any interference to the immune system may lead to autoimmune diseases. Some studies have found that the presence of immunoreactive corticotropin-releasing hormone in patients with autoimmune thyroid diseases increases cortisol secretion, while glucocorticoids, well-known immunosuppressive agents, have the effect of down-regulating immune function.31 In other words, the presence of thyroid autoimmunity aggravates immune imbalance and may contribute to the development of diabetes and NAFLD.
We concluded that in addition to male gender and elevated FT4 as independent protective factors for autoimmunity, duration of diabetes, HbA1c, TC, TG, TSH, TPOAb/TGAb, and severity of NAFLD are all independent risk factors for the thyroid autoimmunity through logistic regression analysis (as shown in Table 6). Among them, blood lipids had the most significant effect on thyroid autoimmunity, followed by hypothyroidism and blood glucose. For every 1 mmol/L increase in cholesterol and triglyceride, patients with T2DM and NAFLD had a 4.294-fold and 3.217-fold increased risk of autoimmunity, respectively. However, for every 1uIU/mL increase in TSH, the risk of developing autoimmunity increased by 2.456-fold, and for a 1-unit increase in TPOAb/TGAb, the risk increased by 1.265-fold. For each 1% increase in HbA1c, the autoimmune risk was 1.312-fold higher. Therefore, for patients with diabetes and fatty liver, good control of blood glucose and blood lipids and correction of hypothyroidism are particularly important, which can not only reduce insulin resistance, and then reduce the occurrence of long-term complications of diabetes and slow down the progression of liver disease, but also effectively alleviate thyroid autoimmunity, avoid the deterioration of the disease, and affect the quality of life.
There are some limitations to our study. Firstly, it is well known that fine-needle biopsy of the liver is the gold standard for NAFLD diagnosis. In contrast, liver ultrasound (US) is cheaper and more suitable for clinical and epidemiological studies, and has become the most commonly used imaging modality for the diagnosis of NAFLD in clinical practice.32 Secondly, all patients included in this paper were Chinese and the sample size is limited, which should be taken into account in order to apply our conclusions to other races or other regions of the world. Finally, due to the cross-sectional study design, we still unable to determine the temporal relationship between thyroid autoimmunity and T2DM, NAFLD. The potential clinical implications of our study require further prospective studies.
The advantage of this study is that it is the first time to report the presence of high levels of thyroid autoantibodies in patients with T2DM complicated with NAFLD and to analyze their associated risk factors, and finally find the role of TPOAb in the progression of autoimmunity in diabetic and NAFLD patients. The liver plays a central role in the metabolism, transport, and clearance of thyroid hormones, so the normal function of the liver is necessary to maintain normal thyroid hormone levels and functions.33 Our study did not include patients who progressed to cirrhosis and it was not possible to determine the relationship between TPOAb and cirrhosis, while data on thyroid function in cirrhotic patients are scarce and the results are mostly inconsistent. Some studies have found an association between decreased FT3 levels and the severity of liver disease,33,34 while others have suggested that the risk of cirrhosis increases with increased TSH concentration.35 In the study of cirrhotic patients by Vincken, S. et al, the prevalence of thyroid autoimmunity was no difference from healthy controls.33 Therefore, future studies with a larger sample are needed to verify the relationship between cirrhosis and thyroid function.
Conclusion
Elevated thyroid autoantibodies, especially TPOAb, were positively correlated with the severity of fatty liver, suggesting that elevated TPOAb is a strong predictor of autoimmune development in type 2 diabetic patients with NAFLD, and elevated TPOAb/TGAb may also be associated with the severity of fatty liver, and then these conclusions partially elucidate the common pathogenesis of autoimmune diseases. Moreover, we suggest that routine screening for thyroid disease in patients with T2DM and NAFLD should be considered for early detection and reasonable intervention, which may otherwise accelerate the progress of T2DM and NAFLD.
Abbreviations
T2DM, type 2 diabetes mellitus; NAFLD, non-alcoholic fatty liver disease; TPOAb, thyroid peroxidase antibody; TGAb, thyroglobulin antibody; HbA1c, glycosylated hemoglobin; TC, cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase; TT3, total triiodothyronine; TT4, total thyroxine; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Clearance and Patient Consent
The study was conducted in accordance with the Declaration of Helsinki. This study was approved by the Ethics Committee of the First Hospital of Lanzhou University.
Acknowledgments
The authors gratefully acknowledge the participation and cooperation of all patients in this study as well as the Department of Endocrinology of the First Hospital of Lanzhou University.
Funding
This study was supported by the Scientific Research Project of Health Industry of Gansu Province, China (No. GSWSKY2018-47).
Disclosure
The authors report no conflicts of interest for this work.
References
1. Cusi K, Sanyal AJ, Zhang S, et al. Non-alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes Metab. 2017;19(11):1630–1634. doi:10.1111/dom.12973
2. Hazim A, Purnamasari D, Kalista K, Lesmana C, Nugroho P. The influence of insulin resistance in the occurrence of non-alcoholic fatty liver disease among first degree relatives of type 2 diabetes. Diabetes Metab Syndr. 2019;13(2):1431–1435. doi:10.1016/j.dsx.2019.01.058
3. Rhee EJ. Nonalcoholic fatty liver disease and diabetes: an epidemiological perspective. Endocrinol Metab. 2019;34(3):226–233. doi:10.3803/EnM.2019.34.3.226
4. VanWagner LB, Ning H, Allen NB, et al. Twenty-five-year trajectories of insulin resistance and pancreatic beta-cell response and diabetes risk in nonalcoholic fatty liver disease. Liver Int. 2018;38(11):2069–2081.
5. Lee CH, Lam KS. Managing non-alcoholic fatty liver disease in diabetes: challenges and opportunities. J Diabetes Investig. 2017;8(2):131–133. doi:10.1111/jdi.12534
6. Streba LA, Vere CC, Rogoveanu I, Streba CT. Nonalcoholic fatty liver disease, metabolic risk factors, and hepatocellular carcinoma: an open question. World J Gastroenterol. 2015;21(14):4103–4110. doi:10.3748/wjg.v21.i14.4103
7. Armstrong MJ, Adams LA, Canbay A, Syn WK. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology. 2014;59(3):1174–1197. doi:10.1002/hep.26717
8. Bano A, Chaker L, Plompen EP, et al. Thyroid function and the risk of nonalcoholic fatty liver disease: the rotterdam study. J Clin Endocrinol Metab. 2016;101(8):3204–3211. doi:10.1210/jc.2016-1300
9. Chen Y, Wang N, Chen Y, et al. The association of non-alcoholic fatty liver disease with thyroid peroxidase and thyroglobulin antibody: a new insight from SPECT-China study. Autoimmunity. 2018;51(5):238–244. doi:10.1080/08916934.2018.1488968
10. Frohlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
11. Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev. 2014;13(4–5):391–397. doi:10.1016/j.autrev.2014.01.007
12. Sharifi F, Ghasemi L, Mousavinasab N. Thyroid function and anti-thyroid antibodies in Iranian patients with type 1 diabetes mellitus: influences of age and sex. Iran J Allergy Asthma Immunol. 2008;7(1):31–36.
13. Cardenas Roldan J, Amaya-Amaya J, Castellanos-de la Hoz J. Autoimmune thyroid disease in rheumatoid arthritis: a global perspective. Arthritis. 2012;2012:864907. doi:10.1155/2012/864907
14. Kumar K, Kole AK, Karmakar PS, Ghosh A. The spectrum of thyroid disorders in systemic lupus erythematosus. Rheumatol Int. 2012;32(1):73–78. doi:10.1007/s00296-010-1556-5
15. Al-Khawari M, Shaltout A, Qabazard M, Al-Sane H, Elkum N. Prevalence of thyroid autoantibodies in children, adolescents and young adults with type 1 diabetes in Kuwait. Med Princ Pract. 2015;24(3):280–284. doi:10.1159/000381547
16. Muhame RM, Mworozi EA, McAssey K, Lubega I. Thyroid autoimmunity and function among Ugandan children and adolescents with type-1 diabetes mellitus. Pan Afr Med J. 2014;19:137. doi:10.11604/pamj.2014.19.137.5115
17. Shun CB, Donaghue KC, Phelan H, Twigg SM, Craig ME. Thyroid autoimmunity in type 1 diabetes: systematic review and meta-analysis. Diabet Med. 2014;31(2):126–135.
18. Elebrashy IN, El Meligi A, Rashed L, Salam RF, Youssef E, Fathy SA. Thyroid dysfunction among type 2 diabetic female Egyptian subjects. Ther Clin Risk Manag. 2016;12:1757–1762. doi:10.2147/TCRM.S112302
19. Sarfo-Kantanka O, Sarfo FS, Ansah EO, et al. Frequency and determinants of thyroid autoimmunity in Ghanaian type 2 diabetes patients: a case-control study. BMC Endocr Disord. 2017;17(1):2. doi:10.1186/s12902-016-0152-4
20. Kucera P, Nováková D, Behanová M, Novak J, Tlaskalová-Hogenová H, Andel M. Gliadin, endomysial and thyroid antibodies in patients with latent autoimmune diabetes of adults (LADA). Clin Exp Immunol. 2003;133(1):139–143. doi:10.1046/j.1365-2249.2003.02205.x
21. Adhami M, Michail P, Rao A, et al. Anti-thyroid antibodies and TSH as potential markers of thyroid carcinoma and aggressive behavior in patients with indeterminate fine-needle aspiration cytology. World J Surg. 2019;15(2):203–208.
22. Shi Z, Zhang X, Chen Z, Liebeskind DS, Lou M. Elevated thyroid autoantibodies and intracranial stenosis in stroke at an early age. Int J Stroke. 2014;9(6):735–740. doi:10.1111/ijs.12177
23. Leyhe T, Müssig K. Cognitive and affective dysfunctions in autoimmune thyroiditis. Brain Behav Immun. 2014;41:261–266. doi:10.1016/j.bbi.2014.03.008
24. Tim K, Derakhshan A, Taylor PN. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019;322(7):632–641. doi:10.1001/jama.2019.10931
25. Sotak S, Felsoci M, Lazurova I. Type 2 diabetes mellitus and thyroid disease: a two-sided analysis. Bratisl Lek Listy. 2018;119(6):361–365.
26. Díez JJ, Iglesias P. An analysis of the relative risk for hypothyroidism in patients with type 2 diabetes. Diabet Med. 2012;29(12):1510–1514. doi:10.1111/j.1464-5491.2012.03687.x
27. Li Y, Teng D, Ba J, et al. Efficacy and safety of long-term universal salt iodization on thyroid disorders: epidemiological evidence from 31 provinces of mainland China. Thyroid. 2020;30(4):568–579. doi:10.1089/thy.2019.0067
28. Barmpari ME, Kokkorou M, Micheli A, et al. Thyroid dysfunction among greek patients with type 1 and type 2 diabetes mellitus as a disregarded comorbidity. J Diabetes Res. 2017;2017:6505814. doi:10.1155/2017/6505814
29. Fleiner HF, Bjøro T, Midthjell K, Grill V, Åsvold BO. Prevalence of thyroid dysfunction in autoimmune and type 2 diabetes: the population-based HUNT study in Norway. J Clin Endocrinol Metab. 2016;101(2):669–677. doi:10.1210/jc.2015-3235
30. Chaker L, Ligthart S, Korevaar TI, et al. Thyroid function and risk of type 2 diabetes: a population-based prospective cohort study. BMC Med. 2016;14(1):150. doi:10.1186/s12916-016-0693-4
31. Agha-Hosseini F, Shirzad N, Moosavi MS. The association of elevated plasma cortisol and Hashimoto’s Thyroiditis, a neglected part of immune response. Acta Clin Belg. 2016;71(2):81–85. doi:10.1080/17843286.2015.1116152
32. Ballestri S, Romagnoli D, Nascimbeni F, Francica G, Lonardo A. Role of ultrasound in the diagnosis and treatment of nonalcoholic fatty liver disease and its complications. Expert Rev Gastroenterol Hepatol. 2015;9(5):603–627. doi:10.1586/17474124.2015.1007955
33. Vincken S, Reynaert H, Schiettecatte J, Kaufman L, Velkeniers B. Liver cirrhosis and thyroid function: friend or foe? Acta Clin Belg. 2017;72(2):85–90. doi:10.1080/17843286.2016.1215641
34. Manka P, Bechmann L, Best J, et al. Low free triiodothyronine is associated with advanced fibrosis in patients at high risk for nonalcoholic steatohepatitis. Dig Dis Sci. 2019;64(8):2351–2358. doi:10.1007/s10620-019-05687-3
35. Kim D, Kim W, Joo SK, Bae JM, Kim JH, Ahmed A. Subclinical hypothyroidism and low-normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin Gastroenterol Hepatol. 2018;16(1):123–131e121. doi:10.1016/j.cgh.2017.08.014
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
