Back to Journals » Biologics: Targets and Therapy » Volume 17
Elevated Serum Vinculin in Patients with HBV/HCV-Associated Liver Cirrhosis and Hepatocellular Carcinoma: A Pilot Study
Authors Essa A , Essa ES, El-deeb SM, Seleem HEM, Al Sahlawi M , Al-Omair OA, Shehab-Eldeen S
Received 20 January 2023
Accepted for publication 4 March 2023
Published 19 March 2023 Volume 2023:17 Pages 23—32
DOI https://doi.org/10.2147/BTT.S405500
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Shein-Chung Chow
Abdallah Essa,1,2 Enas Said Essa,3 Sara Mahmoud El-deeb,3 Hossam Eldin Mostafa Seleem,1 Muthana Al Sahlawi,2 Omar Ahmed Al-Omair,2 Somaia Shehab-Eldeen1,2
1Tropical Medicine Department, Faculty of Medicine, Menoufia University, Shebin Elkom, Egypt; 2Internal Medicine Department, College of Medicine, King Faisal University, Al-Ahsa, Kingdom of Saudi Arabia; 3Clinical Pathology Department, Faculty of Medicine, Menoufia University, Shebin Elkom, Egypt
Correspondence: Somaia Shehab-Eldeen, Tropical Medicine Department, Faculty of Medicine, Menoufia University, Yassen Abd Al Ghafar Street, Shebin Elkom, Menoufia Governorate, 32511, Egypt, Tel +201117251523, Email [email protected]
Background: The stiffness of the extracellular matrix (ECM) controls many cellular processes, such as migration and differentiation. Cells detect stiffness through adhesion structures termed focal adhesions (FAs). Vinculin, an actin-binding FA protein, plays a pivotal role in FA-mediated mechanotransduction.
Aim: This study aimed to explore the role of vinculin in the development of HBV/HCV-induced hepatocellular carcinoma (HCC).
Methods: Vinculin levels in a total number of 100 serum samples from patients with HBV/HCV-induced liver cirrhosis and HCC, as well as healthy controls, were analyzed using an enzyme-linked immunosorbent assay (ELISA).
Results: In patients with HCC and liver cirrhosis, the serum vinculin levels were significantly greater than in controls (503.8± 242.2 and 728.4± 1044.8 vs 77.7± 36.1 respectively, p< 0.001). However, results showed no link between serum vinculin and the clinicopathological features of HCC.
Conclusion: Patients with HBVor HCV-induced liver cirrhosis and HCC have significantly higher serum levels of vinculin than do controls. This might point to a potential role for vinculin in the development of HCC. More research into how this protein affects the development of HCC at the molecular level could lead to better clinical treatments and the development of new molecular therapies.
Keywords: vinculin, HCC, extracellular matrix, liver cirrhosis, focal adhesion proteins
Introduction
Hepatocellular carcinoma (HCC) is a kind of liver cancer that mostly affects people with chronic liver disease and cirrhosis. HCC morbidity has increased in recent decades, and it now ranks second only to lung cancer in terms of national mortality. The fact that tumor cells are prone to being invasive and metastatic, which is started by tumor cell migration, is one of the main contributing factors to the high mortality rate. As a result, blocking tumor cell migration and metastasis might pave the way for cancer therapy.1
Researchers in the field of biology have recently discovered that the microenvironment around a tumor is crucial to its development, invasion, and spread.1 Several different cells, cytokines, growth factors, and other proteins make up the complex microenvironment.2 Extracellular matrix (ECM) proteins are the most plenteous non-cellular solid-state component in the microenvironment of a tumor. They keep the three-dimensional shape of tumor cells and tissues, and their biochemical effects on tumor cell invasion and metastasis are documented.2–4 Matrix stiffening is a solid tumor’s most noticeable mechanical and physical feature. This is mostly caused by the excessive amounts of extracellular matrix proteins that are deposited and crosslinked.5,6
Mechanical stiffness has the potential to disturb the equilibrium of cellular surface force, leading to the clustering of integrins and the creation of focal adhesions, that carry signals from the external matrix into the cells and subsequently influence their biological phenotypes and features, like cell shape, growth, and differentiation.7–10 It also influences cell migration by remodeling the cytoskeleton.11
Clinical evidence has shown that most cases of hepatocellular carcinoma originate in fibrotic or cirrhotic livers and that individuals with more advanced cirrhosis are more likely to have lower median survival times.12,13 Increased hepatic stiffness promotes the growth and progression of hepatocellular carcinoma and also predicts a considerable risk of negative outcomes. So, the stiffness of the liver matrix may be a key factor in how and where a tumor grows.14,15 With the help of actomyosin-generated forces, cells pull on the ECM and sense stiffness through structures called focal adhesions (FAs) that hold the cell to the ECM.16
Vinculin, which is an actin-binding FA protein, has recently been identified as a key participant in FA-mediated mechanotransduction. Vinculin is widely expressed and concentrates on cell-ECM and cell-cell interactions. It has an N-terminal head (Vh), a C-terminal tail (Vt), and hinge domains. Vinculin’s ability to bind to and crosslink F-actin makes it essential for cytoskeleton remodeling. When bound to F-actin, Vt reveals cryptic homodimerization sites and bundles actin filaments.17–19 Vinculin gene expression and protein expression in non-muscle cells are correlated, and higher matrix stiffness promotes the binding of Vt and F-actin, which influences cells’ mechanotransduction and stimulates cytoskeleton remodeling.16
Many studies have shown that cells with less vinculin expression are more likely to spread to other parts of the body and are strongly linked to how aggressive a tumor is.20–22
CD147 is implicated in vinculin-mediated focal adhesion creation, which then promotes cytoskeleton reorganization to allow human HCC cells to invade and migrate.23 It has been found that patients with HCC who had increased CD147 levels in their serum and/or tumor tissue had a worse prognosis.24 However, measurement of serum vinculin has not been done in HCC patients.
The present study aimed to explore the role of vinculin in the development of HBV/HCV-induced HCC by assessing its concentrations in the blood of patients who had liver cirrhosis and/or HCC. Also, to look for any link between the levels of vinculin in the blood and the clinicopathological features of HCC.
Subjects and Methods
Subjects
The study was conducted in the period from May 2021 to March 2022 and included 100 participants, distributed as 51 hepatitis virus-related hepatocellular carcinoma (HCC), 24 hepatitis virus-related cirrhosis patients, and 25 healthy control subjects. Patients were selected from inpatients and outpatients of Menoufia University Hospital.
Patients over the age of 18 who had liver cirrhosis and/or a primary HCC diagnosis from either a histology exam or two dynamic imaging studies were eligible for the study. Patients who had other types of cancer, as well as patients who had previously been treated for chronic hepatitis or HCC, were excluded from participation in the study.
Methods
HCC diagnosis was documented by radiological findings in ultrasonography (US), computerized tomography (CT), magnetic resonance imaging (MRI), and serum alpha-fetoprotein (AFP).25 The diagnosis of liver cirrhosis was made based on the patient’s medical history, as well as the results of a clinical exam, blood tests, and radiological imaging. All subjects in the study had tests for HBV and HCV infections, liver function tests, a full blood picture, and AFP. The clinical and laboratory data for each patient were looked at, and the Child-Pugh score and BCLC stage were figured out.
Serum Vinculin (VCL) Level
Serum VCL level was assayed using Elabscience Human VCL (Vinculin) ELISA Kit, Cta No: E-EL-H1795, Elabscience Biotechnology Inc. USA.
The kit utilizes the Sandwich-ELISA idea. A Human VCL-specific antibody is pre-coated on the ELISA microplate included in this kit. Following the addition of the standards and samples, the specific antibody was mixed into the wells of the microplate. The next step was to add a biotinylated detection antibody specific for Human VCL and an Avidin-Horseradish Peroxidase (HRP) conjugate to each microplate well and then incubate the plate, then wash to remove free components. In each well, the substrate solution was thereafter added. The only wells that displayed blue coloration were those that included Human VCL, biotinylated detection antibody, and Avidin-HRP conjugate. By adding a stop solution, the enzyme-substrate reaction was stopped, and the color changed to yellow. At a wavelength of 450 nm, the optical density (OD) was measured spectrophotometrically. The value of the optical density (OD) is directly related to the amount of human VCL present. By comparing the OD of the samples to the standard curve, the concentration of Human VCL in the samples was determined.
Statistical Analysis
The mean and standard deviation were used to report the quantitative data. For normally distributed variables, data were compared by the Student t-test (between every two groups) and the Anova test (among the three groups). For abnormally distributed variables, data were compared using the Mann–Whitney test. Qualitative data were compared using the chi-square test. Correlation studies were done using the Spearman coefficient. P < 0.05 was considered significant.
Ethics
This study was approved by the ethical committee of the Faculty of Medicine, Menoufia University, and conducted in the context of the Helsinki Declaration 1975 as revised in 1983. All participants provided verbal informed consent, which was acceptable and approved by the ethical committee of the Faculty of Medicine, Menoufia University.
Results
Assessment data for 51 viral-related HCC, 24 viral-related liver cirrhosis,and 25 controls were presented in Table 1
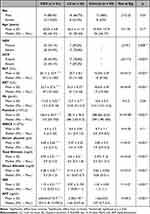 |
Table 1 Comparison Between the Three Studied Groups According to Different Parameters |
There was a significant difference among the three study groups regarding AST, ALT, serum albumen, total bilirubin, direct bilirubin, Hb concentration, platelet count, INR, and AFP. The WBCs count did not differ significantly among the three groups (Table 1).
Serum vinculin levels were significantly higher in HCC patients (503.8±242.2) and cirrhosis patients (728.4±1044.8) than in controls (77.7±36.1) (p<0.001) (Figure 1).
 |
Figure 1 Serum vinculin in the studied groups. Abbreviation: n.s, non-significant. Note: ***; <0.001. |
In HBV and HCV patients, serum VCL levels did not differ significantly between HCC and cirrhotic patients (Table 2).
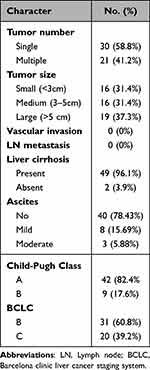 |
Table 2 Tumor Characteristics in HCC Group (n = 51) |
Tumor characteristics are shown in Table 3.
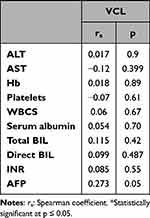 |
Table 3 Correlation Between Serum Vinculin (VCL) Level with the Laboratory Parameters in the HCC Group (n = 51) |
In the HCC patient group, there was no significant correlation between serum VCL level and any of the studied laboratory parameters (AST, ALT, serum albumen, total bilirubin, direct bilirubin, Hb concentration, WBC count, platelet count, INR, or AFP) (Table 4).
 |
Table 4 Relation Between Serum VCL Level and the Clinicopathological Characters Among the HCC Group |
In the HCC patient group, no significant relation was observed between VCL level and patient age, viral etiology, presence or absence of liver cirrhosis, number of tumor masses, tumor size, Child-Pugh classification, or BCLC classification (Table 5).
 |
Table 5 Difference in VCL Levels Between HCC and LC Groups as Regard the Viral Etiology |
Discussion
Hepatocellular carcinoma (HCC) is a frequent malignancy that often develops in the context of cirrhosis and chronic hepatitis virus infections. Approximately 80% of all incidences of HCC are caused by hepatitis B and C.26 HCC represents a major public health concern due to its high incidence and mortality. Inoperable tumors or tumors that return or metastasize often after surgical removal are blamed for the high death rate.27 Understanding the molecular factors influencing the emergence and spread of HCC is crucial to finding targets for efficient medical treatment.28
Matrix stiffness is essential for the development and progression of several forms of cancer. Tumors in solid malignancies, such as breast and pancreatic cancers, often include excessively stiff tissues, which are primarily formed by stiff extracellular matrices created by accumulation, contraction, and cross-linking. Mechanotransduction, the process by which mechanical cues such as the stiffness of the matrix are converted into biochemical signaling in the cells, is triggered when extracellular matrices are rigid. As a result, the cellular phenotypes of cancer cells and stromal cells in tumors are determined.29
It is well known that cancerous tissue is solid and desmoplastic. Cancerous tissues are more rigid in several kinds of organs than normal or neighboring tissues. Normal liver tissue is defined as having a stiffness below 6 kPa, while disease states like cirrhosis and fibrosis, which can lead to hepatocellular carcinoma, are defined as having a stiffness beyond 8–12 kPa (HCC).30
Cancer cells multiply and cluster to form tumors. Cancer cells, like normal cells, may adjust their growth rate in response to changes in the matrix’s stiffness. The ability of cancer cells in HCC to proliferate is enhanced by the presence of a stiff matrix.31
Cancer-related biophysical consequences of ECM stiffness may compromise medication transport and anticancer agent sensitivity. As a result, detecting tumor stiffness may help in treatment stratification.32
Vinculin, an adhesion protein, connects filamentous (F)-actin to the integrin-binding protein talin at cell-cell junctions and focal adhesions (FAs) to mediate force transmission between the actin cytoskeleton and extracellular matrix (ECM).33 Vinculin plays a crucial role in detecting and responding to external mechanical stimuli, such as the stiffness of the extracellular matrix.34 So, a high serum vinculin level in hepatitis virus-caused liver cirrhosis and HCC could affect how HCC patients are stratified for treatment.
In this work, we showed for the first time that the serum level of vinculin was significantly increased in patients with HBV/HCVinduced liver cirrhosis and HCC than in controls. But the results showed that there was no link between serum vinculin and the clinical variables that were studied in HCC patients.
From another aspect, reduced cell adhesion, reorganization of the cytoskeleton, breakdown of the extracellular matrix, and the development of protrusions are all part of the migration process, which plays a crucial role in tumor invasion and metastasis. Different junctional proteins combine to generate macromolecular structures called focal adhesions (FAs). They are found in junctional locations for integrin-mediated cell-matrix attachment and function in cell adhesion, migration, and survival.35,36 Researchers have shown over and over again that the way some focal adhesion proteins are expressed can be used to predict the future and help with treatment.28
The focal adhesion protein vinculin connects adhesion plaques to F-actin fibers by either beginning the development of bundled actin fibers or altering existing microfilaments.37 When vinculin is taken away, mouse embryonic fibroblasts move around more freely, make less focal adhesions (FAs), and stick to the extracellular matrix (ECM) less.38
Vinculin slows down cell migration.39,40 This is supported by the observation that vinculin expression is downregulated in numerous human cancers that metastasize and that injected nude mice with cancer cells overexpressing vinculin have a reduced ability to metastatically spread,41,42 indicating that vinculin impedes cell migration in vivo as well. However, since tumor metastasis includes additional vinculin-dependent processes such as cell survival, apoptosis, and proliferation, the exact mechanism by which vinculin inhibits tumor metastasis in the animal model is unclear.33 Vinculin does appear to influence cell migration; it enhances, rather than inhibits, fibroblast invasion into 3D collagen gels and cancer cell invasion in vitro and in vivo in an ECM-dependent way.40,43 The invasiveness of collagen in various cancer cells corresponds with the magnitude and anisotropy of contractile forces produced by the cells, as well as the anisotropy of cell shape, suggesting that increasing contractile force generation and cell shape anisotropy are two additional ways in which vinculin facilitates 3D collagen invasion.44 Other research has shown that vinculin promotes invasion by increasing the regulation or improvement of the transmission of traction forces in a three-dimensional condition.40
Our research may have limitations owing to the limited number of patients, the fact that the study did not include patients in all different stages of HCC, and the lack of correlation with tissue expression levels. So, more large-scale research with correlations to histopathology in different HCC stages is needed to find out how vinculin works at the molecular level and find possible treatment targets for HCC.
Conclusions
The current study demonstrated that the serum level of vinculin is significantly increased in patients with HBV or HCV-induced liver cirrhosis and HCC than in controls, but the results showed no correlation between serum vinculin levels and the studied clinical variables in HCC patients. However, this could highlight the possible role of vinculin in the development of liver cirrhosis and HCC. More research on the molecular pathways through which this protein contributes to the development of HCC could lead to better clinical care and the creation of new molecular therapeutics.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Disclosure
The authors declare that they have no competing interests.
References
1. Yu H, Shen Y, Jin J, Zhang Y, Feng T, Liu X. Fluid shear stress regulates HepG2 cell migration though time-dependent integrin signaling cascade. Cell Adh Migr. 2018;12:56–68. doi:10.1080/19336918.2017.1319042
2. Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. In: Seminars in Cancer Biology. Elsevier; 2011.
3. Oskarsson T. Extracellular matrix components in breast cancer progression and metastasis. Breast. 2013;22(Suppl 2):S66–72. doi:10.1016/j.breast.2013.07.012
4. Dong Y, Zheng Q, Wang Z, et al. Higher matrix stiffness as an independent initiator triggers epithelial-mesenchymal transition and facilitates HCC metastasis. J Hematol Oncol. 2019;12:112. doi:10.1186/s13045-019-0795-5
5. Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi:10.1016/j.cell.2009.10.027
6. Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS. Matrix rigidity regulates a switch between TGF-β1–induced apoptosis and epithelial–mesenchymal transition. Mol Biol Cell. 2012;23:781–791. doi:10.1091/mbc.e11-06-0537
7. Tilghman RW, Cowan CR, Mih JD, et al. Matrix rigidity regulates cancer cell growth and cellular phenotype. PLoS One. 2010;5:e12905. doi:10.1371/journal.pone.0012905
8. Tilghman RW, Blais EM, Cowan CR, et al. Matrix rigidity regulates cancer cell growth by modulating cellular metabolism and protein synthesis. PLoS One. 2012;7:e37231. doi:10.1371/journal.pone.0037231
9. Kshitiz P, Kim P, Helen W, Engler A, Levchenko A, Kim D. Control of stem cell fate and function by engineering physical microenvironments. Integr Biol UK. 2012;4:1008–1018. doi:10.1039/c2ib20080e
10. Keely PJ. Mechanisms by which the extracellular matrix and integrin signaling act to regulate the switch between tumor suppression and tumor promotion. J Mammary Gland Biol Neoplasia. 2011;16:205–219. doi:10.1007/s10911-011-9226-0
11. Yeung T, Georges PC, Flanagan LA, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi:10.1002/cm.20041
12. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi:10.1053/j.gastro.2004.09.014
13. Greten T, Papendorf F, Bleck J, et al. Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer. 2005;92:1862–1868. doi:10.1038/sj.bjc.6602590
14. Jung KS, Kim SU, Choi GH, et al. Prediction of recurrence after curative resection of hepatocellular carcinoma using liver stiffness measurement (FibroScan®). Ann Surg Oncol. 2012;19:4278–4286. doi:10.1245/s10434-012-2422-3
15. Cescon M, Colecchia A, Cucchetti A, et al. Value of transient elastography measured with FibroScan in predicting the outcome of hepatic resection for hepatocellular carcinoma. Ann Surg. 2012;256:706–713. doi:10.1097/SLA.0b013e3182724ce8
16. Omachi T, Ichikawa T, Kimura Y, Ueda K, Kioka N. Vinculin association with actin cytoskeleton is necessary for stiffness-dependent regulation of vinculin behavior. PLoS One. 2017;12:e0175324. doi:10.1371/journal.pone.0175324
17. Bakolitsa C, Cohen DM, Bankston LA, et al. Structural basis for vinculin activation at sites of cell adhesion. Nature. 2004;430:583–586. doi:10.1038/nature02610
18. Borgon RA, Vonrhein C, Bricogne G, Bois PR, Izard T. Crystal structure of human vinculin. Structure. 2004;12:1189–1197. doi:10.1016/j.str.2004.05.009
19. Johnson RP, Craig SW. Actin activates a cryptic dimerization potential of the vinculin tail domain. J Biol Chem. 2000;275:95–105. doi:10.1074/jbc.275.1.95
20. Shi X, Guo X, Li X, Wang M, Qin R. Loss of Linc01060 induces pancreatic cancer progression through vinculin-mediated focal adhesion turnover. Cancer Lett. 2018;433:76–85. doi:10.1016/j.canlet.2018.06.015
21. Thakur RK, Yadav VK, Kumar A, et al. Non-metastatic 2 (NME2)-mediated suppression of lung cancer metastasis involves transcriptional regulation of key cell adhesion factor vinculin. Nucleic Acids Res. 2014;42:11589–11600. doi:10.1093/nar/gku860
22. Sun Z, Liu F. Association of Nox1 and vinculin with colon cancer progression. Cancer Invest. 2013;31:273–278. doi:10.3109/07357907.2013.789897
23. Liang Q, Han Q, Huang W, et al. HAb18G/CD147 regulates vinculin-mediated focal adhesion and cytoskeleton organization in cultured human hepatocellular carcinoma cells. PLoS One. 2014;9:e102496. doi:10.1371/journal.pone.0102496
24. Xiao W, Zhao S, Shen F, Liang J, Chen J. Overexpression of CD147 is associated with poor prognosis, tumor cell migration and ERK signaling pathway activation in hepatocellular carcinoma. Exp Ther Med. 2017;14:2637–2642. doi:10.3892/etm.2017.4818
25. EASL Clinical Practice. Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
26. Davis GL, Dempster J, Meler JD, et al. Hepatocellular carcinoma: management of an increasingly common problem. In: Baylor University Medical Center Proceedings. Taylor Francis; 2008.
27. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer. 2020;9:682–720. doi:10.1159/000509424
28. Yam JWP, Tse EYT, Ng IO-L. Role and significance of focal adhesion proteins in hepatocellular carcinoma. J Gastroenterol Hepatol. 2009;24:520–530.
29. Ishihara S, Haga H. Matrix stiffness contributes to cancer progression by regulating transcription factors. Cancers. 2022;14:1049. doi:10.3390/cancers14041049
30. Mueller S, Sandrin L. Liver stiffness: a novel parameter for the diagnosis of liver disease. Hepat Med. 2010;2:49–67. doi:10.2147/HMER.S7394
31. Schrader J, Gordon-Walker TT, Aucott RL, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53:1192–1205. doi:10.1002/hep.24108
32. Jiang Y, Zhang H, Wang J, Liu Y, Luo T, Hua H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J Hematol Oncol. 2022;15:34. doi:10.1186/s13045-022-01252-0
33. Thievessen I, Fakhri N, Steinwachs J, et al. Vinculin is required for cell polarization, migration, and extracellular matrix remodeling in 3D collagen. FASEB J. 2015;29:4555. doi:10.1096/fj.14-268235
34. Shen K, Kenche H, Zhao H, Li J, Stone J. The role of extracellular matrix stiffness in regulating cytoskeletal remodeling via vinculin in synthetic smooth muscle cells. Biochem Biophys Res Commun. 2019;508:302–307. doi:10.1016/j.bbrc.2018.11.142
35. Petit V, Thiery J-P. Focal adhesions: structure and dynamics. Biol Cell. 2000;92:477–494. doi:10.1016/S0248-4900(00)01101-1
36. Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi:10.1038/ncb0807-858
37. Wen -K-K, Rubenstein PA, DeMali KA. Vinculin nucleates actin polymerization and modifies actin filament structure. J Biol Chem. 2009;284:30463–30473. doi:10.1074/jbc.M109.021295
38. Xu W, Baribault H, Adamson ED. Vinculin knockout results in heart and brain defects during embryonic development. Development. 1998;125:327–337. doi:10.1242/dev.125.2.327
39. Thievessen I, Thompson PM, Berlemont S, et al. Vinculin–actin interaction couples actin retrograde flow to focal adhesions, but is dispensable for focal adhesion growth. J Cell Biol. 2013;202:163–177. doi:10.1083/jcb.201303129
40. Mierke CT, Kollmannsberger P, Zitterbart DP, et al. Vinculin facilitates cell invasion into three-dimensional collagen matrices. J Biol Chem. 2010;285:13121–13130. doi:10.1074/jbc.M109.087171
41. Rodriguez Fernandez J, Geiger B, Salomon D, Sabanay I, Zöller M, Ben-Ze’ev A. Suppression of tumorigenicity in transformed cells after transfection with vinculin cDNA. J Cell Biol. 1992;119:427–438. doi:10.1083/jcb.119.2.427
42. Peng X, Nelson ES, Maiers JL, DeMali KA. New insights into vinculin function and regulation. Int Rev Cell Mol Biol. 2011;287:191–231.
43. Rubashkin MG, Cassereau L, Bainer R, et al. Force engages vinculin and promotes tumor progression by enhancing PI3K activation of phosphatidylinositol (3, 4, 5)-triphosphate. Cancer Res. 2014;74:4597–4611. doi:10.1158/0008-5472.CAN-13-3698
44. Koch TM, Münster S, Bonakdar N, Butler JP, Fabry B. 3D traction forces in cancer cell invasion. PLoS One. 2012;7:e33476. doi:10.1371/journal.pone.0033476
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
