Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Elevated Serum NOX2 Levels Contribute to Delayed Cerebral Ischemia and a Poor Prognosis After Aneurysmal Subarachnoid Hemorrhage: A Prospective Cohort Study
Authors Wu X, Ji D, Wang Z, Yu W, Du Q, Hu W, Zheng Y, Dong X, Chen F
Received 9 February 2023
Accepted for publication 13 April 2023
Published 29 April 2023 Volume 2023:19 Pages 1027—1042
DOI https://doi.org/10.2147/NDT.S407907
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Xiaoyu Wu,1,* Danfei Ji,1,* Zefan Wang,1,* Wenhua Yu,2 Quan Du,2 Wei Hu,3 Yongke Zheng,3 Xiaoqiao Dong,2 Fanghui Chen4
1The Fourth School of Clinical Medicine, Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 2Department of Neurosurgery, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 3Department of Intensive Care Unit, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 4Emergency Department, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoqiao Dong; Fanghui Chen, Email [email protected]; [email protected]
Background: NADPH oxidase 2 (NOX2) is highly expressed in injured brain tissues. We determined serum NOX2 levels of aneurysmal subarachnoid hemorrhage (aSAH) patients and further investigated correlation of serum NOX2 levels with disease severity, delayed cerebral ischemia (DCI) plus prognosis after aSAH.
Methods: Serum NOX2 levels were measured in 123 aSAH patients and 123 healthy controls. World Federation of Neurological Surgeons scale (WFNS) score and modified Fisher (mFisher) score were utilized to assess disease severity. Modified Rankin scale (mRS) score was used to evaluate the clinical prognosis at 90 days after aSAH. Relations of serum NOX2 levels to DCI and 90-day poor prognosis (mRS score of 3– 6) were analyzed using multivariate analysis. Receiver operating characteristic curve (ROC) was built to evaluate the prognostic predictive capability.
Results: Serum NOX2 levels in aSAH patients, compared with healthy controls, were significantly increased, and were independently correlated with WFNS score, mFisher score and post-stroke 90-day mRS score. Patients with poor prognosis or DCI had significantly higher serum NOX2 levels than other remainders, and serum NOX2 levels independently predicted 90-day poor prognosis and DCI. Serum NOX2 had high prognosis and DCI predictive abilities, and their areas under ROC curve were similar to those of WFNS score and mFisher score.
Conclusion: Serum NOX2 levels are significantly associated with hemorrhage severity, poor 90-day prognosis and DCI in aSAH patients. Hence, complement NOX2 may serve as a potential prognostic biomarker after aSAH.
Keywords: NADPH oxidase 2, biomarker, aneurysmal subarachnoid hemorrhage, disease severity, delayed cerebral ischemia, prognosis
Introduction
Spontaneous subarachnoid hemorrhage (SAH) is a common emergency in neurosurgical science. Aneurysmal SAH (aSAH) approximately accounts for 85% of spontaneous SAH.1 aSAH is characterized by rapid onset, and high rates of disability and death. Delayed cerebral ischemia (DCI) is a serious complication after aSAH and can greatly increase the risk of poor prognosis in such patients.2 Therefore, DCI prediction is of clinical benefit to aSAH management. During decades, biomarkers have been paid extensive attentions with respect to their aidance in severity assessment and prognostic prediction.
The nicotinamide adenine dinucleotide phosphate (NADPH) oxidase family includes 7 subtypes of oxidases, among which NADPH oxidase 2 (NOX2) is one of its main members and is most widely distributed in the central nervous system.3 NOX2, as one of the main pathways for the production of reactive oxygen species (ROS) clusters in brain tissue,4 can reportedly promote inflammatory responses and oxidative stress, thereby disrupting the blood–brain barrier (BBB), exacerbating brain tissue edema and finally aggravating neuronal damage.5–8 NOX2 expression was significantly elevated in brain tissues of mice with traumatic brain injury (TBI) and SAH.5,9 Consistently, elevated expression of NOX2 was revealed in brain tissues from mice model of epileptic ictus or middle cerebral artery occlusion (MCAO).10,11 Also, NOX2 expression by astrocytes was significantly higher in TBI patients than in controls.12 Overall, it is speculated that NOX2 may represent a potential biomarker of acute brain injury.
Materials and Methods
Participants
In this study, we prospectively and consecutively enrolled aSAH patients, who were admitted to Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine (Hangzhou, China) from January 2018 to March 2021. We required (1) age between 18 and 80 years; (2) admission within 24 hours after symptom onset; (3) SAH confirmed by computerized tomography (CT) scan; (4) aneurysms diagnosed via CT angiography (CTA) or digital subtraction angiography (DSA); (5) securing aneurysm within 48 hours after admission. Further, we excluded patients with (1) history of neurologic diseases, such as SAH, spontaneous intracerebral hemorrhage, ischemic stroke, arteriovenous malformation, TBI, moyamoya’s disease and intracranial tumors; (2) infection or operational procedures in recent a month; (3) aneurysmal rebleeding; (4) other specific diseases or conditions, eg, pregnancies, malignancies, uremia and cirrhosis; or (5) other conditions, ie, loss to follow-up, refusal to participation, unavailable samples and incomplete information. A group of healthy volunteers constituted controls. The protocol of this study was approved by the Institutional Review Board of Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine (Opinion No: Medical Ethics Review No. 058-01). Owing to the serious conditions and poor consciousness of aSAH patients who were enrolled in this research, all informed consents of aSAH patients were signed by relatives of the patients. The use of relative consent vs patient consent was approved by the Institutional Review Board of Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine. And the control themselves signed informed consent.
Variables
We collected information including age, gender, cigarette smoking, alcohol consumption, previous underlying diseases (including hypertension, diabetes mellitus and hyperlipidemia), admission time and blood-collection time. Modified Fisher (mFisher) score and World Federation of Neurological Surgeons (WFNS) scale were recorded to assess the hemorrhage severity. The location and size of intracranial aneurysm were determined according to CTA or DSA, and intraventricular hemorrhage and acute hydrocephalus were determined by CT scan. The following criteria were considered for DCI: 1) clinical deterioration (namely, a new focal deficit, decrease in concentration of consciousness, or both), and/or 2) a new infarct on head CT scan that was invisible at admission or immediately post-operatively, and cannot be attributed to other causes by means of clinical assessment, imaging of the brain, and appropriate laboratory studies.13 Surgical procedures for aneurysms included endovascular intervention or neurosurgical clipping. Patients were followed for 90 days and functional prognosis was assessed using a modified Rankin (mRS) score. And a poor prognosis was defined as mRS score of 3–6.14
Immune Analysis
Blood samples were collected from patients on admission and those of controls were obtained at entry into study. Laboratory indices, such as blood leukocyte count, blood platelet count, blood hemoglobin levels, blood glucose levels, were measured using the conventional methods. For the determination of NOX2 levels, acquired serum samples were stored at −80 °C until assayed. Serum NOX2 levels were quantified by commercially available enzyme linked immunosorbent assay (FANKEWEI, Qingdao, Shandong Province, China) according to the manufactures’ instructions. All measurements were in duplicate done by the same technician who was blinded to the clinical data and two measurements were averaged for final analysis.
Statistical Analysis
All statistical analyses were performed using SPSS 26.0. Using GraphPad Prism version 9.0 (GraphPad Software Inc., La Jolla, CA, USA) and MedCalc 9.6.4.0 (MedCalc Software, Mariakerke, Belgium), the graphs were drawn. Categorical variables were expressed as frequencies (percentages), and continuous variables with normal and non-normal distribution were expressed as mean ± standard deviation and median (upper and lower quartiles), respectively. χ2 test or Fisher’s Exact test was used to compare various variables between groups for qualitative data, and Mann–Whitney U-test or t-test was used for quantitative data. Spearman correlation coefficient was used to determine bivariate correlation. Linear regression model was established to determine the variables associated with serum NOX2 levels and modified Rankin score. Multivariate linear correlation analysis was performed to determine the variables independently associated with serum NOX2 levels and mRS score after adjusting for other confounding factors. Logistic regression model was built to investigate whether serum NOX2 levels were associated with DCI and 90-day poor prognosis. Variables with significant differences in univariate analysis were included in binary Logistic regression model to determine variables, which were independently associated with DCI and 90-day poor prognosis. Results were presented as odds ratios (ORs) and 95% confidence intervals (CIs). The receiver operating characteristic (ROC) curve was outlined to study the predictive value of serum NOX2 levels on DCI and 90-day poor prognosis in patients with aSAH, and the area under the curve (AUC) was estimated. Cutoff values were determined using the Youden J method, wherein sensitivity and specificity were generated. Differences of P<0.05 were defined as statistically significant. In order to assess bivariate correlation, sample size should be at least 22. And for making intergroup comparison of catergorial variables, least sample size should be 59. Thus, a total of 123 patients were investigated in the current study, and sample size was enough for clinical analysis.
Results
Patient Selection and Characteristics
This prospective cohort study included a total of 162 first-ever aSAH patients hospitalized within 24 hours after the onset of symptoms. Subsequently, 39 patients were excluded because of aneurysm rebleeding (5 cases), suspected pseudoaneurysm (4 cases), previous neurological diseases (7 cases), other special circumstances (11 cases), lost to follow-up (4 cases), refused to participation (3 cases), and unavailable blood samples (5 cases). Finally, a total of 123 patients were enrolled in the study (Figure 1). In addition, a total of 123 healthy volunteers were recruited as controls. There were no statistically significant differences in demographics between patients and controls (Table 1).
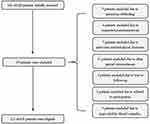 |
Figure 1 Flowing chart of selecting eligible patients with aneurysmal subarachnoid hemorrhage. |
 |
Table 1 Differences of Demographic Data and Vascular Risk Factors Between Initially Enrolled Patients and Finally Eligible Patients, as Well as Between Finally Eligible Patients and Controls |
A total of 68 females and 55 males were included in the study with a female-to-male ratio of 1.24. Their age ranged from 30 to 78 years (mean, 54.5 years; standard deviation, 11.1 years), 25 cases (20.3%) were smokers, 32 cases (26.0%) were alcohol drinkers, 24 cases (19.5%) had hypertension, 15 cases (12.2%) had diabetes mellitus, and 21 cases (17.1%) suffered from hyperlipidemia. With respect to aSAH severity, WFNS score ranged from 1 to 5 (median, 3; upper-lower quartiles, 2–4), and mFisher score ranged from 1 to 4 (median, 2; upper-lower quartiles, 2–3). Aneurysmal characteristics included aneurysm shape (cystic, 102 cases; others, 21 cases), aneurysm location (anterior circulation, 97 cases; posterior circulation, 26 cases), and aneurysm diameter (≥10 mm, 50 cases; <10 mm, 73 cases). There was surgical clipping in 50 cases (40.7%), and endovascular intervention in 73 cases (59.3%) for securing aneurysm. Complications included intraventricular bleeding in 21 cases (17.1%) and acute hydrocephalus in 19 cases (15.4%). An external ventricular drainage was done in 18 cases (14.6%). The range of time between onset of symptom and hospital admission was from 1.0 to 24.0 h (median, 7.0 h; upper-lower quartiles, 5.0–11.0 h), with their blood samples collected from 1.4 to 25.0 h (median, 7.5 h; upper-lower quartiles, 5.0–12.0 h). Arterial systolic blood pressure ranged from 95 to 185 mmHg (median, 149 mmHg; upper-lower quartiles, 133–161 mmHg), with arterial diastolic blood pressure from 53 to 119 mmHg (mean, 86.9 mmHg; standard deviation, 14.3 mmHg). In addition, blood glucose levels ranged from 4.0 to 20.8 mmol/L (median, 7.4 mmol/L; upper-lower quartiles, 5.6–9.7 mmol/L), blood potassium levels ranged from 2.84 to 5.36 mmol/L (mean, 3.79 mmol/L; standard deviation, 0.44 mmol/L) and blood leucocyte count ranged from 4.2 to 17.6×109/L (mean, 9.1×109/L; standard deviation, 2.9×109/L). mRS score ranged from 0 to 6 (median, 2; upper-lower quartiles, 1–3). Ninety-day mRS score of aSAH patients was 0 in 29 patients, 1 in 23 patients, 2 in 24 patients, 3 in 19 patients, 4 in 15 patients, 5 in 7 patients, and 6 in 6 patients. In aggregate, 47 patients had a poor prognosis (mRS score of 3–6).
The Relationship Between Serum NOX2 Levels and Hemorrhage Severity After aSAH
In Figure 2, serum NOX2 levels were significantly higher in patients than in controls (median, 2196.50 pg/mL vs 947.07 pg/mL). Using Spearman analysis, serum NOX2 levels were significantly correlated with WFNS score (r=0.599, P<0.001), mFisher score (r=0.552, P<0.001) and other variables, including intraventricular bleeding (r=0.220, P=0.014), acute hydrocephalus (r=0.190, P=0.035), blood glucose levels (r=0.212, P=0.018) and blood leukocyte count (r=0.324, P<0.001). When the above six variables were incorporated in a multivariate linear regression model, we found that WFNS score (β=0.383, P<0.001) and mFisher score (β=0.252, P=0.019) were independently associated with serum NOX2 levels (Table 2). All patients were dichotomized based on WFNS score or mFisher score, namely, WFNS scores 1–3 and 4–5, or mFisher scores 1–2 and 3–4. It was found that WFNS score and mFisher score, both of which were whether categorical or continuous variables, were significantly associated with serum NOX2 levels (Figure 3).
 |
Table 2 Correlation Between Serum NADPH Oxidase 2 Levels and Other Variables Using Spearman Correlation Coefficient and Linear Regression Analysis in Aneurysmal Subarachnoid Hemorrhage |
Relationship Between Serum NOX2 Levels and mRS Score After aSAH
In Figure 4, mRS score was highly correlated with serum NOX2 levels (r=0.563, P<0.001) and other variables, including WFNS score (r=0.660, P<0.001), mFisher score (r=0.614, P<0.001), blood leukocyte count (r=0.324, P<0.001), intraventricular bleeding (r=0.394, P<0.001), blood glucose level (r=0.222, P=0.014) and acute hydrocephalus (r=0.267, P=0.003) using Spearman analysis. In Table 3, when those preceding variables were forced in a multivariate linear regression model, we found that WFNS score (β=0.293, P=0.004), mFisher score (β=0.200, P=0.040) and serum NOX2 level (β=0.211, P=0.012) were independently associated with mRS score.
 |
Table 3 Correlation Between Modified Rankin Scale and Other Variables Using Spearman Correlation Coefficient and Linear Regression Analysis in Aneurysmal Subarachnoid Hemorrhage |
Relationship Between Serum NOX2 Levels and DCI
A total of 34 patients suffered from DCI. And serum NOX2 levels were significantly higher in patients with the development of DCI than in those who did not (Figure 5). In Figure 6, serum NOX2 levels effectively predicted the occurrence of DCI after aSAH (AUC, 0.730; 95% CI, 0.642–0.806) with a cut-off value of 2603.83 pg/mL being selected using Youden method. Serum NOX2 levels >2603.83 pg/mL discriminated patients at risk of DCI with sensitivity of 67.65% and specificity of 76.40% (maximum Youden index J, 0.4405). Interestingly, serum NOX2 levels had similar predictive ability, as compared to the mFisher score (AUC, 0.708; 95% CI, 0.619–0.786, P=0.715) and WFNS score (AUC, 0.776; 95% CI, 0.692–0.846; P=0.467). Subsequently, we constructed a combined binary logistic regression model using serum NOX2 levels, WFNS score and mFisher score. And it was found that the new combined model (AUC, 0.796, 95% CI 0.714–0.863) displayed significantly higher predictive ability for DCI than mFisher score (P=0.028) (Figure 7).
In Table 4, the proportion of patients with serum NOX2 levels >2603.83 pg/mL was significantly higher in DCI subgroup than in non-DCI subgroup (χ2=20.779, P<0.001). Alternatively, the WFNS score (Z=−4.830, P<0.001), mFisher score (Z=−3.687, P<0.001), blood leucocyte count (Z=−2.161, P=0.031), blood glucose levels (Z=−2.033, P=0.042), percentage of acute hydrocephalus (χ2=7.016, P=0.008) and percentage of intraventricular bleeding (χ2=7.479, P=0.005) were significantly increased in patients who experienced DCI, compared with those who did not (Table 4). Consistently, the above-mentioned associations were confirmed using univariate logistic regression model. Moreover, the preceding significant variables in univariate analysis were entered in the multivariable logistic regression model and subsequently it was revealed WFNS score (OR=1.850; 95% CI, 1.061–3.226; P=0.030) and serum NOX2 levels >2603.83 pg/mL (OR=2.978; 95% CI, 1.008–8.803; P=0.048) emerged as the two independent predictors of DCI (Table 5).
 |
Table 4 Factors Associated with Delayed Cerebral Ischemia in Aneurysmal Subarachnoid Hemorrhage |
 |
Table 5 Univariate and Multivariate Logistic Regression Analysis of Predictors for Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage |
Relationship Between Serum NOX2 Levels and 90-Day Functional Outcome After aSAH
A total of 47 patients had a poor prognosis (mRS score 3–6) at 90 days after aSAH. Interestingly, serum NOX2 levels were significantly higher in patients with poor prognosis than those with good prognosis (P<0.001). In addition to this, serum NOX2 levels substantially distinguished the occurrence of 90-day poor prognosis after aSAH (AUC, 0.760; 95% CI, 0.675–0.833). Furthermore, using Youden method, a cut-off value of 2364.86 pg/mL was chosen. And serum NOX2 levels >2364.86 pg/mL discriminated patients at risk of a poor prognosis at 90 days after stroke, with the sensitivity of 74.47%, and the specificity of 78.95% (maximum Youden index J, 0.5342). The predictive ability of serum NOX2 levels was similar to those of the mFisher score (AUC, 0.802; 95% CI, 0.721–0.869, P=0.387) and WFNS score (AUC, 0.785; 95% CI, 0.702–0.854, P=0.590) (Figure 8). Interestingly, AUC of serum NOX2 levels combined with WFNS score and mFisher score (AUC, 0.834, 95% CI 0.757–0.895) significantly exceeded that of WFNS score (P=0.383) or serum NOX2 levels (P=0.363) alone (Figure 9).
As compared to good prognosis patients, those with poor prognosis had significantly elevated percentage of serum NOX2 levels >2364.86 pg/mL (χ2=34.139, P<0.001). Alternatively, the WFNS score (Z=−5.420, P<0.001), mFisher score (Z=−5.836, P<0.001), blood white leucocyte count (Z=−3.394, P=0.001), blood glucose levels (Z=−2.788, P=0.005), percentage of acute hydrocephalus (χ2=5.923, P=0.015) and percentage of intraventricular bleeding (χ2=8.685, P=0.003) were significantly higher in patients with poor prognosis than in those with good prognosis (Table 6). The above-mentioned substantial associations were also demonstrated using univariate logistic regression analysis. Moreover, those significant variables were forced in the binary logistic regression model, and subsequently mFisher score (OR=2.088; 95% CI, 1.092–3.992; P=0.026) and serum NOX2 levels >2364.86 pg/mL (OR=5.133; 95% CI, 1.723–15.291; P=0.003) retained to be independently associated with poor prognosis at 90 days after stroke (Table 7).
 |
Table 6 Factors Associated with 90-Day Poor Prognosis in Aneurysmal Subarachnoid Hemorrhage |
 |
Table 7 Univariate and Multivariate Logistic Regression Analysis of Predictors for 90-Day Poor Prognosis After Aneurysmal Subarachnoid Hemorrhage |
Discussion
aSAH is a life-threatening critical illness in neurologic field, wherein DCI secondary to cerebral vasospasm is the most common and serious complication. Clinically, the WFNS score and mFisher score are very frequently used to assess disease severity and predict functional outcome and DCI occurrence after aSAH.15 During decades, researchers have focused on the prognostic role of circulating biomarkers in aSAH. In the current study, besides an interesting finding that there was a significantly higher serum NOX2 levels in aSAH patients than in healthy controls, we found that (1) serum NOX2 levels showed a significant positive correlation with mFisher score and WFNS score; (2) serum NOX2 levels were independently associated with DCI and 90-day poor prognosis after aSAH; (3) serum NOX2 levels had a significantly high predictive ability for DCI and poor 90-day prognosis, and its predictive ability was similar to those of mFisher score and WFNS score. In conclusion, elevated serum NOX2 levels were strongly correlated with hemorrhage severity and independently associated with functional outcome and DCI, suggesting that serum NOX2 may be a potential prognostic biomarker for aSAH. Compelling evidence has shown that inflammatory response and oxidative stress play important roles in disease progression of aSAH and are also associated with functional outcome and DCI. The pathophysiological process of DCI involves microcirculatory constriction, microthrombosis, cortical diffusion ischemia and delayed apoptosis, which result from neuroinflammation and oxidative damage after aSAH.16,17
NOX2, as a major source of ROS, is mainly expressed in normal cerebral cortex and hippocampal neurons of animals. After experimental brain injury, including acute ischemic stroke, traumatic brain injury and aSAH, NOX2 levels in brain tissues were significantly elevated.18 In several clinical studies enrolling a small sample of patients with Nasu-Hakola disease, TBI or aSAH, NOX2 expression by neuron and glia were significantly up-regulated in injured brain tissues.9,12,19,20 In peripheral blood of humans with brain injury, NOX2 levels were substantially elevated. Specifically, serum NOX2 levels were significantly higher in patients with neurodegenerative disease compared to healthy controls.21 Our study included more than 100 patients and found a significant elevation of serum NOX2 levels after aSAH. Clearly, blood–brain barrier is greatly disrupted after acute brain injury. Thus, it is deduced that NOX2 in the peripheral blood may be derived from the injured brain tissues. Nevertheless, in a controlled study of 22 patients with TBI, NOX2 expression was significantly elevated in blood leukocytes compared to controls.20 Hence, it is possible that NOX2 in the peripheral blood of patients with acute brain injury may be partially released from peripheral blood cells.
Up to now, data have been supportive of the notion that NOX2 may exacerbate brain injury via aggravating oxidative stress.22,23 In an in vitro ischemic stroke model, NOX2 promoted the generation and accumulation of ROS in neurons, as well as the death of reoxidized neurons. In mice with acute cerebral ischemia, NOX2 knockout substantially reduced infarct size and lessened blood–brain barrier damage.24 Similarly, NOX2 knockout mice showed a significant decrease in hematoma volume, brain edema, neurological deficits, and mortality after intracerebral hemorrhage.25,26 Also, application of NOX2 inhibitors to head-traumatized mice obviously resulted in a significant decrease in ROS expression of brain tissues, improved motor and cognitive function, and reduced damage volume.27 Thus, NOX2 may exacerbate brain tissue and nerve damage after brain injury by producing excess ROS.
Since a previous clinical investigation showed that raised NOX2 expressions in brain tissues had a significant negative correlation with GCS scores and GOS scores of TBI patients,12 no studies have analyzed the relationship between circulating NOX2 levels and severity plus prognosis of humans with acute brain injury. Using multivariate analysis, we found that serum NOX2 levels after aSAH were independently correlated with WFNS score, mFisher score, 90-day mRS scores; and independently discriminated patients at risk of DCI and poor prognosis (mRS score of 3–6). In addition, serum NOX2 level, WFNS score and mFisher score had similar predictive ability for DCI as well as poor prognosis. Intriguingly, serum NOX2 level combined with WFNS score and mFisher score showed significantly elevated prognostic ability, in comparison to WFNS score or mFisher score. In conclusion, serum NOX2 may be a potential biomarker to predict DCI and functional outcome, and assess disease severity after aSAH.
The current study has several limitations. First, although we enrolled 123 aSAH patients in this study, this was a single-center study, which is characterized by a small sample size and therefore, a larger cohort study is required to validate the current conclusions. Second, serum NOX2 levels were only tested at admission time. Hence, investigation of its dynamic change in future may be of clinical significance.
Conclusion
To the best of our knowledge, this is the first series for using multifactorial analysis to discern the relationship between serum NOX2 levels and hemorrhage severity, DCI, and long-term functional outcome after aSAH. In the current study, serum NOX2 levels are independently associated with WFNS score, mFisher score, DCI, and 90-day poor prognosis, suggesting that serum NOX2 levels may be highly correlated with hemorrhage severity, DCI, and long-term functional outcome. Thus, serum NOX2 may be a potential biomarker for assessing hemorrhage severity and predicting DCI and 90-day functional outcome after aSAH.
Abbreviations
aSAH, aneurysmal subarachnoid hemorrhage; WFNS, World Federation of Neurological Surgeons scale; mFisher, modified Fisher; mRS, Modified Rankin scale; CT, computerized tomography; ROC, receiver operating characteristic; AUC, area under curve; 95% CI, 95% confidence interval; ROS, reactive oxygen species.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethics Statement
This study was carried out in accordance with the ethical guidelines of the Helsinki Declaration, and its protocol was approved by the Institutional Review Committee of the Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine (Opinion number: Medical Ethics Review No. (058)-01). Relatives of patients and controls themselves gave written informed consent to participate in this study.
Acknowledgments
The authors thank all staffs in Department of Neurosurgery, the Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine (Hangzhou, China) for their technical support.
Funding
This work is financially supported by Construction Fund of Medical Key Disciplines of Hangzhou (No. OO20200485; No. OO20200055), Health Science and Technology Project of Zhejiang Province (No. 2021427523; No. 2022510185), Health Science and Technology Project of Hangzhou (No. A20200514) and Zhejiang Provincial Public Welfare Research Project (No. LGD20H090004).
Disclosure
The authors declare that they have no competing interests.
References
1. Weiland J, Beez A, Westermaier T, Kunze E, Sirén AL, Lilla N. Neuroprotective strategies in aneurysmal subarachnoid hemorrhage (aSAH). Int J Mol Sci. 2021;22(11):5442. doi:10.3390/ijms22115442
2. Chen X, Jiang Y, Liu J, et al. DCI after aneurysmal subarachnoid hemorrhage is related to the expression of MFG-E8. Biomed Res Int. 2021;2021:6568477. doi:10.1155/2021/6568477
3. Schiavone S, Neri M, Trabace L, Turillazzi E. The NADPH oxidase NOX2 mediates loss of parvalbumin interneurons in traumatic brain injury: human autoptic immunohistochemical evidence. Sci Rep. 2017;7(1):8752. doi:10.1038/s41598-017-09202-4
4. Higashi M, Shimokawa H, Hattori T, et al. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res. 2003;93(8):767–775. doi:10.1161/01.RES.0000096650.91688.28
5. Zhang QG, Laird MD, Han D, et al. Critical role of NADPH oxidase in neuronal oxidative damage and microglia activation following traumatic brain injury. PLoS One. 2012;7(4):e34504. doi:10.1371/journal.pone.0034504
6. Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281(9):5657–5667. doi:10.1074/jbc.M506172200
7. Lu XY, Wang HD, Xu JG, Ding K, Li T. NADPH oxidase inhibition improves neurological outcome in experimental traumatic brain injury. Neurochem Int. 2014;69:14–19. doi:10.1016/j.neuint.2014.02.006
8. Lu XY, Wang HD, Xu JG, Ding K, Li T. Pretreatment with tert-butylhydroquinone attenuates cerebral oxidative stress in mice after traumatic brain injury. J Surg Res. 2014;188(1):206–212. doi:10.1016/j.jss.2013.11.1106
9. Zhang L, Li Z, Feng D, et al. Involvement of Nox2 and Nox4 NADPH oxidases in early brain injury after subarachnoid hemorrhage. Free Radic Res. 2017;51(3):316–328. doi:10.1080/10715762.2017.1311015
10. Tannich F, Tlili A, Pintard C, et al. Activation of the phagocyte NADPH oxidase/NOX2 and myeloperoxidase in the mouse brain during pilocarpine-induced temporal lobe epilepsy and inhibition by ketamine. Inflammopharmacology. 2020;28(2):487–497. doi:10.1007/s10787-019-00655-9
11. De Silva TM, Brait VH, Drummond GR, Sobey CG, Miller AA, Kleinschnitz C. Nox2 oxidase activity accounts for the oxidative stress and vasomotor dysfunction in mouse cerebral arteries following ischemic stroke. PLoS One. 2011;6(12):e28393. doi:10.1371/journal.pone.0028393
12. Li Z, Tian F, Shao Z, et al. Expression and clinical significance of non-phagocytic cell oxidase 2 and 4 after human traumatic brain injury. Neurol Sci. 2015;36(1):61–71. doi:10.1007/s10072-014-1909-z
13. Frontera JA, Fernandez A, Schmidt JM, et al. Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke. 2009;40(6):1963–1968. doi:10.1161/STROKEAHA.108.544700
14. Takase H, Chou SH, Hamanaka G, et al. Soluble vascular endothelial-cadherin in CSF after subarachnoid hemorrhage. Neurology. 2020;94(12):e1281–e1293. doi:10.1212/WNL.0000000000008868
15. Zhang X, Liu Y, Zhang S, Wang C, Zou C, Li A. Neutrophil-to-albumin ratio as a biomarker of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2021;147:e453–e458. doi:10.1016/j.wneu.2020.12.084
16. Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10(1):44–58. doi:10.1038/nrneurol.2013.246
17. Li J, Wang J, Wang YL, et al. NOX2 activation contributes to cobalt nanoparticles-induced inflammatory responses and Tau phosphorylation in mice and microglia. Ecotoxicol Environ Saf. 2021;225:112725. doi:10.1016/j.ecoenv.2021.112725
18. Wang J, Liu Y, Shen H, Li H, Wang Z, Chen G. Nox2 and Nox4 participate in ROS-induced neuronal apoptosis and brain injury during ischemia-reperfusion in rats. Acta Neurochir Suppl. 2020;127:47–54. doi:10.1007/978-3-030-04615-6_8
19. Satoh JI, Kino Y, Yanaizu M, et al. Expression of gp91phox and p22phox, catalytic subunits of NADPH oxidase, on microglia in Nasu-Hakola disease brains. Intractable Rare Dis Res. 2016;5(4):275–279. doi:10.5582/irdr.2016.01086
20. Liao Y, Liu P, Guo F, Zhang ZY, Zhang Z, De Re V. Oxidative burst of circulating neutrophils following traumatic brain injury in human. PLoS One. 2013;8(7):e68963. doi:10.1371/journal.pone.0068963
21. Loffredo L, Ettorre E, Zicari AM, et al. Oxidative stress and gut-derived lipopolysaccharides in neurodegenerative disease: role of NOX2. Oxid Med Cell Longev. 2020;2020:8630275. doi:10.1155/2020/8630275
22. Liu H, Zhao L, Yue L, et al. Pterostilbene attenuates early brain injury following subarachnoid hemorrhage via inhibition of the NLRP3 inflammasome and Nox2-related oxidative stress. Mol Neurobiol. 2017;54(8):5928–5940. doi:10.1007/s12035-016-0108-8
23. Malkov A, Popova I, Ivanov A, et al. Aβ initiates brain hypometabolism, network dysfunction and behavioral abnormalities via NOX2-induced oxidative stress in mice. Commun Biol. 2021;4(1):1054. doi:10.1038/s42003-021-02551-x
24. Chen H, Song YS, Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J Cereb Blood Flow Metab. 2009;29(7):1262–1272. doi:10.1038/jcbfm.2009.47
25. Tang J, Liu J, Zhou C, et al. Role of NADPH oxidase in the brain injury of intracerebral hemorrhage. J Neurochem. 2005;94(5):1342–1350. doi:10.1111/j.1471-4159.2005.03292.x
26. Gao L, Shi H, Sherchan P, et al. Inhibition of lysophosphatidic acid receptor 1 attenuates neuroinflammation via PGE2/EP2/NOX2 signalling and improves the outcome of intracerebral haemorrhage in mice. Brain Behav Immun. 2021;91:615–626. doi:10.1016/j.bbi.2020.09.032
27. Chandran R, Kim T, Mehta SL, et al. A combination antioxidant therapy to inhibit NOX2 and activate Nrf2 decreases secondary brain damage and improves functional recovery after traumatic brain injury. J Cereb Blood Flow Metab. 2018;38(10):1818–1827. doi:10.1177/0271678X17738701
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.