Back to Journals » Cancer Management and Research » Volume 10
Elevated red blood cell distribution width predicts poor prognosis in patients with oral squamous cell carcinoma
Received 3 June 2018
Accepted for publication 12 July 2018
Published 17 September 2018 Volume 2018:10 Pages 3611—3618
DOI https://doi.org/10.2147/CMAR.S176200
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lu-Zhe Sun
Wenzhang Ge,1,* Jianli Xie,2,* Lianzhen Chang3
1Department of Special Clinic, Jinan Stomatological Hospital, Jinan, Shandong, People’s Republic of China; 2Department of Periodontics and Oral Medicine, Jinan Stomatological Hospital, Jinan, Shandong, People’s Republic of China; 3Medical Department, Jinan Stomatological Hospital, Jinan, Shandong, People’s Republic of China
*These authors contributed equally to this work
Introduction: Although red blood cell distribution width (RDW) has been reported to reflect inflammation and nutritional status and to predict prognosis in several different types of cancer, little is known about how RDW might be related to oral squamous cell carcinoma (OSCC). The present study aimed to investigate the prognostic value of preoperative RDW in OSCC patients.
Materials and methods: We included 236 OSCC patients from Jinan Stomatological Hospital (Shandong, People’s Republic of China) in this retrospective study. All enrolled patients were divided into 2 groups: high RDW (≥15%) and low RDW (<15%) according to the detected RDW values. The correlation of RDW and clinical characteristics was explored, and the prognostic significance of RDW evaluated using Kaplan–Meier curves, log-rank analysis, and the Cox proportional hazards model.
Results: The pretreatment median RDW among all OSCC patients was 14.4%, with a range from 11.6% to 24.5%. The RDW was found to be significantly correlated with node metastasis, tumor length, and TNM stage (P<0.05 for all). As for biochemical parameters, the results showed that higher RDW values were significantly associated with hemoglobin, mean corpuscular volume, white blood cell count, albumin, and C-reactive protein (P<0.01 for all). A significant association of RDW with the tumor marker cytokeratin 19 fragments (CYFRA21-1) and squamous cell carcinoma antigen (SCC-Ag) was also observed (P=0.02, and P=0.03; respectively). Moreover, patients with higher RDW were more likely to receive postoperative therapy (P=0.02). Kaplan–Meier survival curves showed that a high RDW was significantly associated with poor overall survival (OS) (P<0.01), especially in the early stages (I–II). Multivariate analysis revealed that an elevated RDW at diagnosis was an independent prognostic factor for shorter OS (HR =1.46, 95% CI: 1.13–2.86) after adjustment for other cancer-related prognostic factors.
Conclusion: These data suggest that an elevated preoperative RDW (≥15%) at diagnosis may independently predict poorer OS in patients with OSCC, but better-designed studies in the future should be performed to further confirm the value of monitoring RDW.
Keywords: oral squamous cell carcinoma, prognosis, RDW
Introduction
As a routine parameter of a complete blood count (CBC), red blood cell distribution width (RDW) describes the degree of heterogeneity in erythrocyte size; it is often used clinically to identify different types of anemia. Emerging evidence demonstrates an association between a high RDW and increased overall and disease-specific mortality across patients with chronic or progressive inflammatory diseases such as septicemia, cardiovascular disease, cerebrovascular disease, and others.1–3 Although the mechanism by which RDW affects the outcome of these diseases remains largely unknown, its involvement in the systemic release of circulating cytokines probably plays a role. Among the hypothesized underlying factors are IL-6, TNF, and poor nutritional status (eg, iron, folate, and vitamin B12 deficiency) as well as age-associated disease causing changes in erythropoiesis.4–7
It is well known that cancer is both a cause and a result of chronic inflammation and that it can cause intractable malnutrition.8 This has aroused interest in the possible causes of physical debility in individuals with malignancies and the prognostic significance of these causes.9 For example, low performance status and compromised nutritional status account for many patients’ poor quality of life and are obstacles to cancer treatment.10–12 Generally age, performance status, and disease stage are widely used to risk-stratify patients with cancer and guide therapeutic strategy.13 However, the clinical conditions of cancer patients are so complex that investigators have been prompted to search for more appropriate and less expensive biomarkers that would define a patient’s general condition for therapeutic and prognostic purposes. Previous studies have suggested that RDW may have diagnostic and prognostic value in several different types of cancer including lung cancer, hepatocellular carcinoma, prostate cancer, esophageal carcinoma, and others.13–16 Few studies have been aimed specifically at determining the clinical usefulness of RDW in patients with oral squamous cell carcinoma (OSCC), Tangthongkum et al17 did not find any prognostic value of RDW on survival in Thailand OSCC patients. Due to the causal association of region-specific environmental and biological factors with OSCC development, whether RDW also has no association with clinicopathological characteristics in Chinese OSCC patients remained unclear. Therefore, we aimed to investigate RDW levels and to determine the relationships between RDW and various clinical parameters including survival in a Chinese OSCC cohort.
Materials and methods
Study design
For our research, we chose to design and implement a case–control study. The study population comprised patients presenting to Jinan Stomatological Hospital (Shandong, People’s Republic of China) between June 2012 and September 2013 for the evaluation and management of OSCC. Patients with a new histologically confirmed diagnosis of primary OSCC were included; all of them underwent surgical resection of the primary tumor. Excluded were patients with inflammatory conditions such as infections or collagen disease, anemia, cardiovascular disease, cerebrovascular disease, or COPD. Also, OSCC patients were excluded if they had had a previous malignancy, tumor metastasis, chemotherapy, or radiotherapy before surgery. The cancer stage was determined in accordance with the American Joint Committee on Cancer classification system and the histopathologic postoperative pTNM categories system (International Union against Cancer). Pretreatment blood samples were collected from all OSCC patients using routine methods at the time of diagnosis. This study was conducted in conformity with the Declaration of Helsinki on medical protocol and ethics, and the regional Ethical Review Board of Jinan Stomatological Hospital approved the study (Approval Number: JNKQYYEC20090102006). Written informed consent was obtained from all participants.
Measurement of variables
Blood parameters from the CBC including white blood cells (WBCs), platelets (PLTs), hemoglobin, mean cell volume (MCV), and RDW were analyzed with an automated hematology analyzer XE-2100 (Sysmex Corp, Kobe, Japan). The normal range of RDW obtained from the CBC in general and in our laboratory was 11.5%–15%. Biochemical parameters, alanine transaminas (ALT), albumin, creatinine, and C-reactive protein (CRP), were analyzed with a Hitachi Modular P800 (Hitachi High-tech Corporation, Tokyo, Japan). Tumor markers including cytokeratin 19 fragments (CYFRA21-1), squamous cell carcinoma antigen (SCC-Ag), and carcinoembryonic antigen (CEA) were measured using a Cobas 6,000 Autoanalyzer (Roche Diagnostics, Pleasanton, CA, USA). The cutoff value for RDW was defined as 15%, as in previous research, simultaneously in combination with the normal range.13,14 Meanwhile, hemoglobin, albumin, CRP, CEA, and cancer antigen 72-4 (CA72-4) were grouped based on the upper or lower limit of the normal reference range, respectively. All included patients were followed at 4-month intervals until death or the last follow-up on November 2017. Overall survival (OS) was defined as the interval from the date of surgery to the date of disease recurrence, death, or last follow-up. The median follow-up time was 42 months, with a range of 4–52 months.
Statistical analysis
The data are reported as number (%) and mean ± standard deviation. Normality was assessed by Shapiro–Wilk goodness-of-fit tests. Differences in continuous values between 2 groups were assessed with Student’s t-test for normally distributed variables and nonparametric Mann–Whitney U tests for nonnormally distributed variables as appropriate. The Pearson’s χ2 test was utilized to calculate the associations between RDW and clinical parameters. Survival probabilities were estimated by using Kaplan–Meier analysis, and significant differences were evaluated by using the log-rank test. The Cox regression analysis model was used to perform multivariate analysis of prognostic factors. All analyses were conducted using the SPSS 24.0 statistical software (IBM Corporation, Armonk, NY, USA) and the GraphPad Prism software 6.0 (GraphPad, La Jolla, CA, USA). P<0.05 was considered statistically significant.
Results
Participants
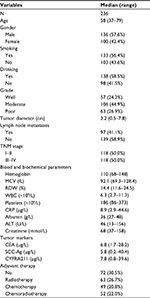
A total of 236 patients with a histopathologically confirmed diagnosis of OSCC were included in the study; their demographic data are shown in Table 1. The pretreatment median RDW of all OSCC patients was 14.4%, with a range from 11.6% to 24.5%. Patients were categorized into 2 groups according to their RDW values: 95 cases with an RDW value ≥15% were defined as the high-RDW group whereas 141 cases with an RDW value <15% were defined as the low-RDW group.
Correlation of pretreatment RDW and patients characteristics
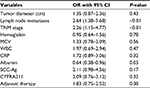
The associations between RDW level and clinical parameters are summarized in Table 2. The RDW was found to be significantly correlated with node metastasis (P<0.01), tumor size (P=0.03), and TNM stage (P<0.01). As for biochemical parameters, the results showed that higher RDW values were significantly associated with hemoglobin, MCV, WBC, albumin, and CRP (P<0.01 for all), but not PLT, ALT, or creatinine. As for serum tumor biomarkers, significant associations between RDW, CYFRA21-1, and SCC-Ag could be observed (P=0.02, P=0.03; respectively) but not with CEA. We also found that patients with higher RDWs were more likely to receive postoperative therapy (P=0.02). Moreover, logistic regression analysis of the relationship between RDW and the demographics of OSCC patients were analyzed. As shown in Table 3, there were significant associations between RDW and nodal metastasis (P<0.01), TNM stage (P<0.01), and albumin (P=0.03).
RDW and prognosis
Kaplan–Meier survival curves confirmed that a high RDW was associated with poor OS, and the difference in survival rate between the high- and low-RDW groups was statistically significant (P<0.01, Figure 1A). A significant difference in OS between the high- and low-RDW groups with early-stage (I–II) OSCC was also observed (P<0.01, Figure 1B). In the late-stage (III–IV) groups, a shorter survival time was observed in OSCC patients with high RDWs, but this was not statistically significant (P=0.07, Figure 1C). As shown in Table 4, univariate analysis indicated that tumor size, nodal metastasis, RDW, hemoglobin, CRP, albumin, SCC-Ag, and adjuvant therapy were significantly associated with OS (P<0.05 for all). To identify the independent prognostic factors, significant factors based on the univariate analysis were included in the multivariate Cox analysis. Analysis using a Cox multivariate hazards regression model revealed that high RDW was an independent prognostic biomarker for predicting poor OS in our patients with OSCC (HR =1.46, 95% CI: 1.13–2.86; Table 4).
Discussion
In the present study, we analyzed the significance of RDW in Chinese patients with OSCC and found that RDW was significantly associated with hemoglobin, albumin, and CRP, in keeping with the findings from previous studies among patients with lung cancer and esophageal cancer, thus supporting the notion that high levels of RDW reflect chronic inflammation and poor nutritional status in patients with these conditions.13,14 Additionally, our results showed a positive association between RDW and clinical cancer stage, nodal metastasis, and particularly tumor size, suggesting a potential association between RDW and increased inflammation/malnutrition induced by the progression of disease. Further, we found that a high preoperative RDW was meaningfully associated with a poor clinical outcome in patients with OSCC.
OSCC is among the most lethal malignancies of the oral cavity; with a variable regional distribution, it accounts for more than 10% of all cancers in some Asian regions.18 OSCC is characterized by frequent metastases, a high rate of recurrence, and a poor prognosis.19 As in other types of cancer, inflammation and malnutrition are hallmarks of OSCC; these frequently cause local inflammatory fester and worsen malnutrition due to inappetence and obstructed feeding.8,20 It is widely acknowledged that tumor-generated inflammatory responses mediated by cytokines and other inflammatory mediators may promote the growth, invasion, and metastasis of cancers.21 In addition, malnutrition makes it more difficult for individuals to bear the burden of their illness. Some hematologic parameters including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and CRP are significantly correlated with host inflammation and nutritional status,22,23 which have been identified as an important biomarker in predicting progression or survival of OSCC patients. For example, Chen et al24 recently reported that preoperative NLR is an independent factor predicting the prognosis of OSCC, especially for patients who have undergone postoperative chemoradiotherapy; these investigators suggest that it could serve as a potential target for improving patients’ prognosis. Acharya et al25 have reported that an elevated CRP may predict lymph node metastasis and that CRP could be added as an extension to known clinicopathologic parameters – such as lymph node metastasis, staging, and histology – to predict the prognosis in OSCC. And Adel et al26 have reported that preoperative CRP is an important biomarker for classifying high-risk lymph node density in patients with OSCC. Similarly, previous studies have reported that preoperative PLR is directly associated with nodal involvement in OSCC. Preoperative PLR is superior to NLR for predicting lymph node metastases in OSCC.27 Similar to NLR and PLR, our results also showed that RDW could reflect enhanced inflammation, metastasis, and advanced stage in OSCC patients.
We also observed a significant association between high RDW and poor prognosis in all of our patients, whether they had early- or late-stage OSCC. Although RDW values were positively associated with cancer stage, it is not surprising that all patients with higher RDW values had shorter survival times. However, there was a great difference in OS between high- and low-RDW values in patients with stage I–II disease as compared with those with stage III–IV disease, since the prognosis for the latter was worse. This phenomenon has also been observed in patients with lung cancer, in that statistically significant differences were observed only in those with early lung cancer rather than those in the later stages of disease.13 One potential explanation for this difference may be the stronger association of RDW value with performance status (PS), albumin, and CRP in the early stages of cancer. In later stages, more confounding factors play a role in inflammation, poor nutritional status, finally prognosis. Consistent with previous studies, our OSCC patients with poor PS, lower albumin, and higher CRP had significantly poorer survival; the multivariate Cox analysis indicated similar results with regard to these independent prognostic factors. When these confounding factors were excluded, RDW remained as an independent prognostic factor. However, Tangthongkum et al17 have discussed the association of RDW with prognosis in Thailand OSCC patients, and they did not observe any prognostic value of RDW on survival outcome. One possibility for this discrepancy is the different RDW cutoff value. Tangthongkum et al17 used the RDW cutoff point of 14.05, while the present study used the upper limited of the normal reference range of RDW (15.0). OSCC patients could be divided into totally different subgroup according to different RDW cutoff value, so it makes sense that the set of RDW cutoff value could affect the results. The other possibility is the difference in clinicopathological and treatment variables of OSCC patients between these 2 studies. The majority of OSCC patients from Tangthongkum et al’s17 study were male (64.2%), had III–IV stage (66.8%), tumors were well differentiated (59.2%), as well as did not undergo chemotherapy. Besides, the environmental factors between these 2 cohorts may also be the potential cause of this discrepancy.
Obviously, there are several limitations to this study. First, there may be potential bias and inaccuracy in data collection, as in most retrospectively designed studies, and our sample size was comparatively small. Also, we could not divide patients into 2 cohorts including an exploration set and a validation set. We could not evaluate the association of RDW with other clinicopathological characteristics due to lack of enough information such as histological characteristics (Ki67, p53, basaloid feature, etc). Second, the treatment regimens and therapy strategy varied among individuals. This led to differences difference in therapeutic response and accounts for discrepant prognoses. We did not analyze the role of compounding factors including different treatment regimens and therapy strategies in the association between RDW and the survival of patients, which is likely a considerable limitation. Overall, we hope that our findings will be validated in further multicenter investigations with larger sample sizes and less heterogeneity.
Conclusion
In summary, our study revealed a potential association between RDW and hemoglobin, MCV, albumin, family cancer history, CRP, WBC, NLR, clinical cancer stage, node metastasis, and tumor size in OSCC patients. A significant correlation between higher RDW and poor prognosis was also suggested. Because RDW values can be routinely examined by CBC, this relatively inexpensive and easily available parameter may be used as a new marker to aid in the treatment and prognosis of OSCC. Better-designed studies in the future should be performed to further confirm the value of monitoring RDW.
Disclosure
The authors report no conflicts of interest in this work.
References
Patel KV, Ferrucci L, Ershler WB, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med. 2009;169(5):515–523. | ||
Förhécz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohászka Z, Jánoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009;158(4):659–666. | ||
Kim J, Kim YD, Song TJ, et al. Red blood cell distribution width is associated with poor clinical outcome in acute cerebral infarction. Thromb Haemost. 2012;108(2):349–356. | ||
de Gonzalo-Calvo D, de Luxán-Delgado B, Rodríguez-González S, et al. Interleukin 6, soluble tumor necrosis factor receptor I and red blood cell distribution width as biological markers of functional dependence in an elderly population: a translational approach. Cytokine. 2012;58(2):193–198. | ||
Rhodes CJ, Howard LS, Busbridge M, et al. Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: clinical prevalence, outcomes, and mechanistic insights. J Am Coll Cardiol. 2011;58(3):300–309. | ||
Douglas SW, Adamson JW. The anemia of chronic disorders: studies of marrow regulation and iron metabolism. Blood. 1975;45(1):55–65. | ||
Ferrucci L, Guralnik JM, Woodman RC, et al. Proinflammatory state and circulating erythropoietin in persons with and without anemia. Am J Med. 2005;118(11):1288. | ||
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. | ||
Nicolini A, Ferrari P, Masoni MC, et al. Malnutrition, anorexia and cachexia in cancer patients: a mini-review on pathogenesis and treatment. Biomed Pharmacother. 2013;67(8):807–817. | ||
Kiss N. Nutrition support and dietary interventions for patients with lung cancer: current insights. Lung Cancer. 2016;7:1–9. | ||
Kovarik M, Hronek M, Zadak Z. Clinically relevant determinants of body composition, function and nutritional status as mortality predictors in lung cancer patients. Lung Cancer. 2014;84(1):1–6. | ||
Boniface MM, Wani SB, Schefter TE, et al. Multidisciplinary management for esophageal and gastric cancer. Cancer Manag Res. 2016;8:39–44. | ||
Koma Y, Onishi A, Matsuoka H, et al. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS One. 2013;8(11):e80240. | ||
Wan GX, Chen P, Cai XJ, et al. Elevated red cell distribution width contributes to a poor prognosis in patients with esophageal carcinoma. Clin Chim Acta. 2016;452:199–203. | ||
Albayrak S, Zengin K, Tanik S, Bakirtas H, Imamoglu A, Gurdal M. Red cell distribution width as a predictor of prostate cancer progression. Asian Pac J Cancer Prev. 2014;15(18):7781–7784. | ||
Smirne C, Grossi G, Pinato DJ, et al. Evaluation of the red cell distribution width as a biomarker of early mortality in hepatocellular carcinoma. Dig Liver Dis. 2015;47(6):488–494. | ||
Tangthongkum M, Tiyanuchit S, Kirtsreesakul V, Supanimitjaroenporn P, Sinkitjaroenchai W. Platelet to lymphocyte ratio and red cell distribution width as prognostic factors for survival and recurrence in patients with oral cancer. Eur Arch Otorhinolaryngol. 2017;274(11):3985–3992. | ||
Petersen PE. Oral cancer prevention and control – the approach of the World Health Organization. Oral Oncol. 2009;45(4–5):454–460. | ||
Tsantoulis PK, Kastrinakis NG, Tourvas AD, Laskaris G, Gorgoulis VG. Advances in the biology of oral cancer. Oral Oncol. 2007;43(6):523–534. | ||
Tetè S, Nicoletti M, Saggini A, et al. Nutrition and cancer prevention. Int J Immunopathol Pharmacol. 2012;25(3):573–581. | ||
Eltohami YI, Kao HK, Lao WW, et al. The prediction value of the systemic inflammation score for oral cavity squamous cell carcinoma. Otolaryngol Head Neck Surg. 2018;158(6):1042–1050. | ||
Chen ZY, Raghav K, Lieu CH, et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br J Cancer. 2015;112(6):1088–1097. | ||
Shau HY, Kim A. Suppression of lymphokine-activated killer induction by neutrophils. J Immunol. 1988;141(12):4395–4402. | ||
Chen F, Lin L, Yan L, Qiu Y, Cai L, He B. Preoperative neutrophil-to-lymphocyte ratio predicts the prognosis of oral squamous cell carcinoma: a large-sample prospective study. J Oral Maxillofac Surg. 2017;75(6):1275–1282. | ||
Acharya S, Kale J, Hallikeri K, Anehosur V, Arnold D. Clinical significance of preoperative serum C-reactive protein in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2018;47(1):16–23. | ||
Adel M, Tsao CK, Wei FC, et al. Preoperative SCC antigen, CRP serum levels, and lymph node density in oral squamous cell carcinoma. Medicine. 2016;95(14):e3149. | ||
Acharya S, Rai P, Hallikeri K, Anehosur V, Kale J. Preoperative platelet lymphocyte ratio is superior to neutrophil lymphocyte ratio to be used as predictive marker for lymph node metastasis in oral squamous cell carcinoma. J Investig Clin Dent. 2017;8(3):e12219. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.