Back to Journals » Cancer Management and Research » Volume 13
Elevated RBP-Jκ and CXCL11 Expression in Colon Cancer is Associated with an Unfavorable Clinical Outcome
Authors Liu M, Guo H, Jiang L, Jiao M, Wang S, Tian T, Fu X, Wang W
Received 22 December 2020
Accepted for publication 14 April 2021
Published 5 May 2021 Volume 2021:13 Pages 3651—3661
DOI https://doi.org/10.2147/CMAR.S298580
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Meng-jie Liu, Hui Guo, Li-li Jiang, Min Jiao, Shu-hong Wang, Tao Tian, Xiao Fu, Wen-juan Wang
Department of Medical Oncology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, 710061, People’s Republic of China
Correspondence: Wen-juan Wang
Department of Medical Oncology, The First Affiliated Hospital of Xi’an Jiaotong University, No. 277 Yanta West Road, Xi’an, Shaanxi, People’s Republic of China
Tel +86 15829235823
Fax +86 29 85324086
Email [email protected]
Introduction: This study aims at exploring the expression and significance of recombination signal-binding protein for immunoglobulin kappa J region (RBP-Jκ) and C-X-C motif chemokine 11 (CXCL11) in human colon cancer tissues.
Methods: The RBP-Jκ and CXCL11 expression levels were assessed by immunohistochemistry and quantitative real-time polymerase chain reaction (qRT-PCR) in patients with colon cancer, and their prognostic significance was evaluated.
Results: Through analyzing 342 samples of colon cancer patients treated at our institution, increased expression of RBP-Jκ and CXCL11 was found in human colon cancer specimens compared with matched paratumorous normal specimens (P< 0.001). A positive correlation was found between RBP-Jκ expression and CXCL11 expression (P< 0.001). High RBP-Jκ expression was significantly associated with poorly differentiated tumors (P=0.005), invasion beyond propria muscularis (P=0.025), lymph node metastases (P=0.005), distant metastasis (P< 0.001), advanced tumor-node-metastasis (TNM) stage (P=0.004), and a shorter overall survival (P< 0.001). An increase in CXCL11 protein expression was associated with poorly differentiated tumors (P=0.015), invasion beyond propria muscularis (P=0.029), lymph node metastases (P=0.031), distant metastasis (P=0.045), advanced TNM stage (P=0.026), and a shorter overall survival of patients (P< 0.001). In multivariate Cox regression analysis, RBP-Jκ protein expression (P=0.036), CXCL11 protein expression (P=0.001), differentiation (P< 0.001), depth of invasion (P=0.009), distant metastasis (P< 0.001), and TNM stage (P< 0.001) were independent prognostic indicators of colon cancer.
Conclusion: High expression of RBP-Jκ is closely associated with high CXCL11 expression, which represents a risk factor for the poor overall survival of colon cancer patients.
Keywords: RBP-Jκ, CXCL11, prognosis, colon cancer
Introduction
Colon cancer is one of the most common tumor diseases worldwide, whose incidence rate is increasing and ranks the third among malignant tumors.1 Owing to the colon cancer’s unobvious early clinical symptoms, rapid development, and low early diagnosis rate, most patients cannot be diagnosed until the middle and late stages, for which reason they may miss the best treatment period. Therefore, a profound understanding with respect to the molecular events during the development of colon cancer and the metastasis is of crucial priority for early detection and treatment of the disease.
RBP-Jκ is an important transcription factor in the Notch pathway and a DNA-binding protein from CSL (CBF1/Su(H)/Lag-1) family,2 performing not only a Notch-dependent role but also a Notch-independent role.3 Existing studies have discovered that the abnormal expression of Notch signaling can change the normal microenvironment and result in colon cancer.4 Regarding mammals, when the Notch signaling pathway is activated, the Notch intracellular domain (NICD) entering the nucleus mainly depends on its binding with the transcription factor RBP-Jκ to exert its physiological function. RBP-Jκ mediated the transcriptional regulation activity of all four Notch receptors (Notch1-Notch4)5.Yong et al6 found that the proliferation of breast cancer cells and prostate cancer cells was inhibited after RBP-Jκ was knocked down. It has been reported that the inhibition of RBP-Jκ expression suppressed oral cancer cell EMT.7 Therefore, it has been verified that RBP-Jκ plays a key role in the occurrence and development of cancers. However, the specific functions and mechanisms of RBP-Jκ in colon cancer progression remains unclear so far.
Chemokines are members of the secretory protein family that are of vital necessity in inflammation and leukocyte chemotaxis.8 Recent studies suggested that chemokines and their receptors are also crucial in tumor cell proliferation, migration and antiapoptosis.9 Our sequencing results found that RBP-Jκ can upregulate the expression of CXCL11. CXCL11, also known as interferon-inducible T-cell alpha chemoattractant (I-TAC) or interferon-gamma-inducible protein 9 (IP-9), is a member of the ELR-CXC chemokine family, whose receptors are CXCR3 and CXCR7. Cancer cells can produce CXCL11 by an autocrine mechanism or release CXCL11 by regulating tumor stromal cells in the microenvironment.10 CXCL11 not only regulates the directional movement of cancer cells but also participates in the process of tumor cells entering and leaving blood vessels, immune escape, proliferation and angiogenesis.11 Existing studies have proven that the CXCL9/CXCL10/CXCL11-CXCR3 axis of CXCL11 can promote or inhibit the occurrence and development of tumors according to different tumor types and tumor development stages.12
Considering the above research findings, supporting data from in vitro and murine tumor models have highlighted the key roles of RBP-Jκ and CXCL11 in intestinal tumorigenesis. Nevertheless, the co-expression of these two proteins and their prognostic relevance in human colon cancer need to be established. Therefore, we evaluated the expression of RBP-Jκ and CXCL11 in human colon cancer specimens, and the relationship among these molecules with traditional prognostic indicators and clinical outcome of patients was evaluated.
Materials and Methods
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Conduct of Human Ethics Committee of the First Affiliated Hospital, College of Medicine of Xi’an Jiaotong University. Each patient first provided written informed consent.
Patients and Specimens
Three hundred and forty-two patients with colon cancer being recruited at the First Affiliated Hospital, College of Medicine of Xi’an Jiaotong University from January 2012 to December 2019 were enrolled in this study. None of the patients had undergone cancer-related treatment before curative surgery. Table 1 shows the main clinicopathologic data. The pathological types of all the specimens were confirmed by two independent pathologists under double-blinded conditions. The tumors were classified according to the criteria from the TNM Union for International Cancer Control (UICC), and the tumor cellular differentiation (TCD) was defined using Edmondson’s classification. The follow-up for all cases was terminated in February 2020. In survival analysis, cases were excluded when patients were either lost to follow-up or died of causes other than colon cancer.
 |
Table 1 Association of RBP-Jκ and CXCL11 Protein Expression with Clinicopathological Features in 342 Colon Cancer Patients |
Immunohistochemistry
The immunohistochemistry (IHC) SP method was used for detection. Immunohistochemical analysis of formalin-fixed paraffin-embedded tissue was conducted using avidin biotin complex immunoperoxidase technology. The antibodies used were anti-RBP-Jκ (1:200; ab25949, Abcam, Cambridge, MA, USA) and anti-CXCL11 (1:500; ab9955, Abcam, Cambridge, MA, USA). The positive expression of RBP-Jκ mainly existed in the nucleus, and the positive expression of CXCL11 existed in the cytoplasm. Five visual fields were randomly selected for each section under a high-power microscope, which were scored according to the proportion of tumor-positive cells and to the intensity of staining. The number of positive cells was 0%~5% for 0 points, >5%~25% for 1 point, >25%~50% for 2 points, >50%~75% for 3 points, and >75%~50% for 4 points. The staining intensity was scored as 0 points for colorless, 1 point for pallide-flavens, 2 points for yellow and 3 points for brown. When the product of the two scores was ≥4, it was recorded as positive, whereas <4 as negative.13 All scoring and interpretations of the results were conducted independently by two observers blinded to the other clinicopathological variables.
Real-Time Reverse Transcription-Polymerase Chain Reaction Analysis
Total RNA was extracted from fresh tissue samples using TRIzol reagent (Invitrogen, Carlsbad, CA), and reverse transcription was conducted using Prime ScriptTM RT Master Mix (Takara, Dalian, China) according to the manufacturer’s instructions. The reaction system was prepared using SYBR Premix Ex Taq TM II (Takara, Dalian, China) and performed using an iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, CA). The primer sequences used for RBP-Jκ and CXCL11 analysis are listed out in Table 2. Using GAPDH as the internal reference, the relative expression of RBP-Jκ and CXCL11 was calculated by the 2−ΔΔCt method. All the samples were run in triplicate.
 |
Table 2 Primer Sequences for RT-PCR |
Statistical Analysis
All statistical analyses were performed using SPSS 22.0 software (SPSS Inc., Chicago, USA). Chi-squared test or Fisher’s exact test was adopted to evaluate the associations between RBP-Jκ and CXCL11 expression and each clinicopathologic parameter. The measurement data were presented as means±SD. The differences between two sample means were analyzed for statistical significance by Student’s t-test (independent samples t-test) or the u-test. One-way analysis of variance (ANOVA) was performed to analyze the difference among three or four sample means, and Spearman’s rank test was used to assess the correlation between RBP-Jκ and CXCL11 expression. The overall survival was analyzed by the Kaplan-Meier method using Cox regression, and the Log rank test was used to analyze differences in survival curves. A two-tailed P-value was used in all the analyses. P<0.05 was considered to indicate statistical significance.
Results
Expression of RBP-Jκ in Colon Cancer Tissues
The RBP-Jκ expression was examined in 342 primary colon cancer tissues and paired paratumorous normal colon tissues. Among these 342 primary colon cancer tissues, high RBP-Jκ expression was observed in 224 (65.50%) cases, whereas only 75 (21.93%) cases of matched paratumorous normal colon tissues had high RBP-Jκ expression. IHC staining indicated that the RBP-Jκ located mainly in the nucleus (Figure 1). Moreover, the RBP-Jκ expression was markedly higher in colon cancer tissues than in paratumorous normal colon tissues (P<0.001; Table 1). The RBP-Jκ expression was significantly correlated with differentiation, depth of invasion, lymph node metastasis, distant metastasis and different TNM stages (P = 0.005, 0.025, 0.005, <0.001, and 0.004, respectively; Table 1). No significant association was observed between RBP-Jκ expression and age, gender of patients, or tumor location (Table 1). Furthermore, we examined RBP-Jκ mRNA expression in the 342 pairs of primary colon cancer tissues and paratumorous normal colon tissues by real-time PCR, and increased RBP-Jκ mRNA expression was observed in most colon cancer tissues compared with that in the paratumorous normal colon tissues (P<0.001). In addition, the association between RBP-Jκ mRNA expression and clinicopathologic factors was similar to the results of immunohistochemical analysis (Table 3).
 |
Table 3 Association of RBP-Jκ and CXCL11 mRNA Expression with Clinicopathological Features in 342 Colon Cancer Patients |
Expression of CXCL11 in Colon Cancer Tissues
Among these 342 primary CRC tissues, 255 (74.56%) cases exhibited elevated CXCL11. CXCL11 staining revealed a predominantly cytoplasmic localization (Figure 1). However, high CXCL11 expression was detected in only 104 (30.41%) of the paratumorous normal colorectal tissues. Among the 342 colon cancer tissues, a significant correlation existed between CXCL11 expression and differentiation, depth of invasion, lymph node metastasis, distant metastasis and different TNM stages (P = 0.015, 0.029, 0.031, 0.045, and 0.026, respectively; Table 1). In addition, real-time PCR suggested that the CXCL11 mRNA levels in colon cancer tissues were greatly increased compared with those in paratumorous normal colon tissues (P<0.001). Through evaluating the clinical significance of CXCL11 mRNA expression, we found that the overexpression of CXCL11 mRNA was strongly associated with differentiation, depth of invasion, lymph node metastasis, distant metastasis and different TNM stages (P<0.001, =0.012, <0.001, <0.001, and =0.001, respectively; Table 3), which is similar to the IHC analysis results.
Correlation Between RBP-Jκ Expression and CXCL11 Expression in Colon Cancer Tissues
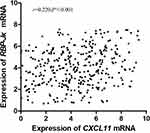
The correlation between the expression of RBP-Jκ and CXCL11 in colon cancer tissues was analyzed at the protein and mRNA levels. In RBP-Jκ IHC analysis, 118 (34.50%) colon cancer samples were RBP-Jκ-low tumors, and 224 (65.50%) were RBP-Jκ-high tumors. In CXCL11 IHC analysis, 255 (74.56%) tumors exhibited high-level CXCL11, whereas 87 (23.44%) exhibited low-level CXCL11 (Table 1). Spearman’s rank test revealed a significant positive correlation between RBP-Jκ expression and CXCL11 expression in colon cancer tissues (r=0.245; P<0.001; Table 4). We also observed a similar correlation between RBP-Jκ and CXCL11 mRNA expression in colon cancer samples (r=0.220; P<0.001; Figure 2).
 |
Table 4 Association Between RBP-Jκ and CXCL11 in 342 Colon Cancer Patients |
Survival Analysis
Of the 342 patients with colon cancer, none were lost to follow-up. The median survival time was 42 months. Kaplan-Meier survival curves were generated (Figure 3A and B) to evaluate the prognostic significance of RBP-Jκ and CXCL11 protein expression. The analysis of prognostic factors for survival was summarized in Table 5. The results of univariate analysis revealed that age, differentiation, depth of invasion, lymph node metastasis, distant metastasis, TNM stage, RBP-Jκ expression, and CXCL11 expression were critical prognostic factors for patients’ overall survival time (Table 5). No significant correlation was observed between gender (P=0.574) or tumor location (P=0.103) and colon cancer patient prognosis.
 |
Table 5 Univariate and Multivariate Analyses of 342 Colon Cancer Patients |
Cox proportional hazards model was adjusted for age, differentiation status, depth of invasion, lymph node metastasis, distant metastasis, TNM stage, RBP-Jκ expression and CXCL11 expression in multivariate analysis. The analytical results suggested that the differentiation status (P<0.001), depth of invasion (P=0.009), distant metastasis (P<0.001), TNM stage (P<0.001), RBP-Jκ expression (P=0.036), and CXCL11 expression (P=0.001) were independent predictors of survival for patients with colon cancer, indicating that high RBP-Jκ expression and high CXCL11 expression were significantly correlated with the risk of a poor prognosis (Table 5). No significant correlation was observed between the other analyzed clinicopathological factors and colon cancer patients’ overall survival (Table 5).
To explore the prognostic significance of the combined RBP-Jκ protein expression and CXCL11 protein expression profile, Kaplan-Meier survival curves were therefore depicted. We obtained the following four combination subgroups: RBP-Jκ-high and CXCL11-high, RBP-Jκ-high and CXCL11-low, RBP-Jκ-low and CXCL11-high and RBP-Jκ-low and CXCL11-low. Our measurement data revealed that the overall survival time of colon cancer patients with high RBP-Jκ expression was obviously shorter than that of colon cancer patients with low RBP-Jκ expression (Figure 3A). Patients with CXCL11-high colon cancers also had apparently poorer outcomes than those with CXCL11-low tumors (Figure 3B). Further analysis showed that the subgroup of patients with high RBP-Jκ and CXCL11 co-expression had a shorter overall survival time than those with other combinations (Figure 3C and D).
Discussion
Dysregulation and abnormal activation of the Notch pathway occur frequently in many human tumors.14 RBP-Jκ is the main transcription factor of the Notch signaling pathway, whose target genes are usually controlled by two active states of RBP-Jκ.15 RBP-Jκ, being referred to as CBF-1 (C promoter-binding factor 1), is a DNA binding protein and a key transcription factor of the Notch signaling pathway. Dysfunction of RBP-Jκ is associated with the loss of epithelial barrier integrity and aberrant conversion of proliferative crypt cells into goblet cells.5,16 Therefore, we considered that changes in the expression of RBP-Jκ-related molecules will probably induce the disorder of colon tissue structure and function and even tumors. Unfortunately, the directionality of RBP-Jκ dysregulation in tumorigenesis remains unclear. Currently, researchers have found that the Notch/RBP-Jκ pathway is changed in various solid tumors including breast cancer,17 ovarian cancer,18 melanoma,19 malignant glioma,20 lung cancer,21 and pancreatic cancer.22 Nagao et al found that RBP-Jκ can regulate the growth of rhabdomyosarcoma cells, and inhibiting RBP-Jκ may be an effective treatment target for rhabdomyosarcoma patients.23 Studies also discovered that the RBP-Jκ expression was increased in lung cancer. Inhibition of RBP-Jκ expression significantly inhibited the proliferation of lung cancer cells.21 Overall, RBP-Jκ is involved in carcinogenesis and the progression of human tumors. Our study suggested that RBP-Jκ expression is increased in colon cancer and closely correlates with poor differentiation, depth of invasion, lymph node metastasis, distant metastasis, and advanced TNM stage of tumors, thereby revealing that RBP-Jκ plays an oncogenic role in the development of colon cancer.
Chemokines and their receptors closely correlates with tumors, functioning critically in lymphocyte migration and tumor metastasis.24,25 CXCL11 is a chemokine promoting cell proliferation and migration after binding to its receptor, and plays an extremely critical role in directional cell migration. The CXCL11 also induces inflammation and its development. Tumor cells produce mild inflammatory reactions in patients during occurrence and development, thus promoting the expression of CXCL11, increasing its chemotaxis induction, inducing the corresponding immune response of the body, as well as promoting inflammation, whose functions and performances in inducing tumor cell proliferation and regulating angiogenesis26 must not be neglected. CXCL11 possesses potent tumor-promoting activity in colorectal cancer27 and ovarian carcinomas,28 however, in contrast to the above two studies, in the study conducted by Qun Gao, CXCL11 expression positively correlated with a prolonged overall survival of lung cancer patients.11 Our unpublished sequencing results showed that CXCL11 is a novel target for the RBP-Jκ. In our study, we examined CXCL11 expression in both primary colon cancer tissues and paired paratumorous normal colon tissues. Real-time PCR and IHC analyses revealed that high CXCL11 mRNA and protein expression correlate with the differentiation status, depth of invasion, lymph node metastasis, distant metastasis, and advanced TNM stage in colon cancer. In the study conducted by Gao et al, CXCL11 was highly expressed in CRC compared with that in control tissue, which is in good consistency with our obtained data.27 Therefore, our study suggests that CXCL11 expression in colon cancer patient tissues can serve as a useful biomarker for colon cancer, and that high CXCL11 expression plays an indispensable role during colon cancer progression, indicating similar potential roles of RBP-Jκ and CXCL11 in colon cancer.
The relationship between the combination of RBP-Jκ status and CXCL11 status and their prognostic relevance in CRC has yet to be determined. Several studies have demonstrated that the significances of Notch signaling pathways and chemokines must be emphasized in tumor progression and subsequent metastases of numerous cancers.29–31 There is also evidence of their involvement in colon cancer,32 however, the possible crosstalk mechanism between Notch pathways and chemokine pathways remains uncertain, for which reason we focus on these two markers (RBP-Jκ and CXCL11) to investigate the expression and clinical significance in colon cancer with a preliminary study.
An existing study showed that CXCL11 is of great significance in the relationship between neuroendocrine differentiation and TAMs, indicating its possible association with a poor prognosis in colorectal cancer.27 Since RBP-Jκ and CXCL11 are involved in the formation and metastasis of malignant tumors, we therefore examined the expression of RBP-Jκ and CXCL11 in colon cancer. In this study, we demonstrated that RBP-Jκ mRNA and protein expression were highly expressed in colon cancer tissue and were correlated with the differentiation status, depth of invasion, lymph node metastasis, distant metastasis and TNM stage of tumors. Our clinicopathological study revealed that patients with RBP-Jκ-high expression colon cancer had an apparently shorter survival time than those with RBP-Jκ-low colon cancer, which suggests that RBP-Jκ is a vital prognostic marker in colon cancer patients. Despite that certain functional studies have investigated the influence of RBP-Jκ expression and activation, only a few of which have reported higher RBP-Jκ expression in human malignant tumors.6,7,33,34 In response to the study conducted by Maciaczyk et al, the mean RBP-Jκ activation in bulk tumor samples was reported to perform as a clinical predictive biomarker in brain cancers.35 Al Labban et al reported that Notch-effector CSL promotes squamous cell carcinoma.36 Furthermore, it has been discovered that CXCL11 is overexpressed in CRC tissues and cell lines,27 which is in good consistency with our obtained data. However, few studies have compared RBP-Jκ expression in colon cancer. An important finding in our study was the prognostic predictive value of high or low RBP-Jκ and CXCL11 expression based on different survival outcomes of patients with colon cancer, upon which theoretical foundation we therefore suggest that RBP-Jκ and CXCL11 expression may function as potential therapeutic targets in treating colon cancer.
In this study, we analyzed the RBP-Jκ and CXCL11 mRNA and protein expression levels in 342 colon cancer patients classified according to their outcomes. To our knowledge, our study being conducted at present is the first one that investigates the association between RBP-Jκ and CXCL11 expression in colon cancer, as well as high RBP-Jκ and CXCL11 co-expression in relation to malignant colon cancer transformation. Our research findings suggest that the expression of two of the studied markers (RBP-Jκ and CXCL11) are strongly correlated with a poorer prognosis of colon cancer, and that RBP-Jκ and CXCL11 play oncogenic roles. Based on our results, we therefore hypothesize that RBP-Jκ may positively regulate the expression of CXCL11, yet further study is required to explore the possible molecular mechanism between these two markers.
In accordance with the abovementioned results, Kaplan-Meier analysis showed an obviously lower survival time for patients with higher RBP-Jκ and CXCL11 expression. Cox univariate regression analysis showed that RBP-Jκ-high and CXCL11-high patients had a markedly higher risk of relapse and a lower probability of survival. In the Cox multivariate regression model, RBP-Jκ and CXCL11 were independent prognostic factors, imposing the same effects on survival. High RBP-Jκ expression was associated with poor overall survival, which is similar to CXCL11. Additionally, the combination of RBP-Jκ and CXCL11 performed as a more efficient predictor than RBP-Jκ expression or CXCL11 expression alone. Patients with high expression of both RBP-Jκ and CXCL11 had poorer outcomes than those with other combinations of RBP-Jκ and CXCL11 expression. Therefore, the combination of RBP-Jκ and CXCL11 could be an effective prognostic marker. To our best knowledge, this study is the first one analyzing the expression of both RBP-Jκ and CXCL11 in colon cancer.
In summary, we have demonstrated that the co-expression of RBP-Jκ and CXCL11 proteins closely correlates with poor prognosis in colon cancer patients, both proteins perform as independent prognostic factors. These findings have certain significant clinical implications that targeting RBP-Jκ and CXCL11 may provide a new therapeutic modality in treating colon cancer.
Acknowledgments
This work was financially supported by grants from the National Natural Science Foundation of China (No. 81502099), Scientific Research Fund of the First Affiliated Hospital of Xi’an Jiaotong University (2018MS-06), Natural Science Foundation of Shaanxi Province (2019JQ-960), and Key Laboratory of Tumor Precision Medicine Open Project of Shaanxi Province (No. KLTPM-SX2018-B3).
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
2. Tanigaki K, Honjo T. Two opposing roles of RBP-J in Notch signaling. Curr Top Dev Biol. 2010;92:231–252.
3. Raafat A, Bargo S, McCurdy D, Callahan R. The ANK repeats of Notch-4/Int3 activate NF-κB canonical pathway in the absence of Rbpj and causes mammary tumorigenesis. Sci Rep. 2017;7(1):13690. doi:10.1038/s41598-017-13989-7
4. Meurette O, Mehlen P. Notch signaling in the tumor microenvironment. Cancer Cell. 2018;34(4):536–548. doi:10.1016/j.ccell.2018.07.009
5. van Es JH, van Gijn ME, Riccio O, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435(7044):959–963. doi:10.1038/nature03659
6. Yong T, Sun A, Henry MD, Meyers S, Davis JN. Down regulation of CSL activity inhibits cell proliferation in prostate and breast cancer cells. J Cell Biochem. 2011;112(9):2340–2351. doi:10.1002/jcb.23157
7. Li JY, Huang WX, Zhou X, Chen J, Li Z. Numb inhibits epithelial-mesenchymal transition via RBP-Jκ-dependent Notch1/PTEN/FAK signaling pathway in tongue cancer. BMC Cancer. 2019;19(1):391. doi:10.1186/s12885-019-5605-5
8. Chow MT, Luster AD. Chemokines in cancer. Cancer Immunol Res. 2014;2(12):1125–1131. doi:10.1158/2326-6066.CIR-14-0160
9. Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17(9):559–572. doi:10.1038/nri.2017.49
10. Puchert M, Obst J, Koch C, Zieger K, Engele J. CXCL11 promotes tumor progression by the biased use of the chemokine receptors CXCR3 and CXCR7. Cytokine. 2020;125:154809. doi:10.1016/j.cyto.2019.154809
11. Gao Q, Wang S, Chen X, et al. Cancer-cell-secreted CXCL11 promoted CD8(+) T cells infiltration through docetaxel-induced-release of HMGB1 in NSCLC. J Immunother Cancer. 2019;7(1):42. doi:10.1186/s40425-019-0511-6
12. Tokunaga R, Zhang W, Naseem M, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - A target for novel cancer therapy. Cancer Treat Rev. 2018;63:40–47. doi:10.1016/j.ctrv.2017.11.007
13. Chu D, Zheng J, Wang W, et al. Notch2 expression is decreased in colorectal cancer and related to tumor differentiation status. Ann Surg Oncol. 2009;16(12):3259–3266. doi:10.1245/s10434-009-0655-6
14. Aster JC, Pear WS, Blacklow SC. The varied roles of notch in cancer. Annu Rev Pathol. 2017;12:245–275. doi:10.1146/annurev-pathol-052016-100127
15. Vinson KE, George DC, Fender AW, et al. The Notch pathway in colorectal cancer. Int J Cancer. 2016;138(8):1835–1842. doi:10.1002/ijc.29800
16. VanDussen KL, Carulli AJ, Keeley TM, et al. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139(3):488–497. doi:10.1242/dev.070763
17. Braune EB, Tsoi YL, Phoon YP, et al. Loss of CSL unlocks a hypoxic response and enhanced tumor growth potential in breast cancer cells. Stem Cell Rep. 2016;6(5):643–651. doi:10.1016/j.stemcr.2016.03.004
18. Chen X, Thiaville MM, Chen L, et al. Defining NOTCH3 target genes in ovarian cancer. Cancer Res. 2012;72(9):2294–2303. doi:10.1158/0008-5472.CAN-11-2181
19. Hu XB, Feng F, Wang YC, et al. Blockade of notch signaling in tumor-bearing mice may lead to tumor regression, progression, or metastasis, depending on tumor cell types. Neoplasia. 2009;11(1):32–38. doi:10.1593/neo.81008
20. Liu B, Lin X, Yang X, et al. Downregulation of RND3/RhoE in glioblastoma patients promotes tumorigenesis through augmentation of notch transcriptional complex activity. Cancer Med. 2015;4(9):1404–1416. doi:10.1002/cam4.484
21. Lv Q, Shen R, Wang J. RBPJ inhibition impairs the growth of lung cancer. Tumour Biol. 2015;36(5):3751–3756. doi:10.1007/s13277-014-3015-5
22. Avila JL, Kissil JL. Notch signaling in pancreatic cancer: oncogene or tumor suppressor? Trends Mol Med. 2013;19(5):320–327. doi:10.1016/j.molmed.2013.03.003
23. Nagao H, Setoguchi T, Kitamoto S, et al. RBPJ is a novel target for rhabdomyosarcoma therapy. PLoS One. 2012;7(7):e39268. doi:10.1371/journal.pone.0039268
24. Thierry GR, Gentek R, Bajenoff M. Remodeling of reactive lymph nodes: dynamics of stromal cells and underlying chemokine signaling. Immunol Rev. 2019;289(1):42–61. doi:10.1111/imr.12750
25. Li X, Wang M, Gong T, et al. A S100A14-CCL2/CXCL5 signaling axis drives breast cancer metastasis. Theranostics. 2020;10(13):5687–5703. doi:10.7150/thno.42087
26. Nazari A, Ahmadi Z, Hassanshahi G, et al. Effective treatments for bladder cancer affecting CXCL9/CXCL10/CXCL11/CXCR3 axis: a review. Oman Med J. 2020;35(2):e103. doi:10.5001/omj.2020.21
27. Gao YJ, Liu L, Li S, et al. Down-regulation of CXCL11 inhibits colorectal cancer cell growth and epithelial-mesenchymal transition. Onco Targets Ther. 2018;11:7333–7343. doi:10.2147/OTT.S167872
28. Benhadjeba S, Edjekouane L, Sauvé K, Carmona E, Tremblay A. Feedback control of the CXCR7/CXCL11 chemokine axis by estrogen receptor α in ovarian cancer. Mol Oncol. 2018;12(10):1689–1705. doi:10.1002/1878-0261.12362
29. Liubomirski Y, Lerrer S, Meshel T, et al. Notch-mediated tumor-stroma-inflammation networks promote invasive properties and CXCL8 expression in triple-negative breast cancer. Front Immunol. 2019;10:804. doi:10.3389/fimmu.2019.00804
30. Azizidoost S, Asnafi AA, Saki N. Signaling-chemokine axis network in brain as a sanctuary site for metastasis. J Cell Physiol. 2019;234(4):3376–3382. doi:10.1002/jcp.27305
31. Ibrahim SA, Gadalla R, El-Ghonaimy EA, et al. Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Mol Cancer. 2017;16(1):57. doi:10.1186/s12943-017-0621-z
32. Chen H, Edwards R, Tucci S, et al. Chemokine 25-induced signaling suppresses colon cancer invasion and metastasis. J Clin Invest. 2012;122(9):3184–3196. doi:10.1172/JCI62110
33. Procopio MG, Laszlo C, Al Labban D, et al. Combined CSL and p53 downregulation promotes cancer-associated fibroblast activation. Nat Cell Biol. 2015;17(9):1193–1204.
34. Wang WJ, Yao Y, Jiang LL, et al. Knockdown of lymphoid enhancer factor 1 inhibits colon cancer progression in vitro and in vivo. PLoS One. 2013;8(10):e76596. doi:10.1371/journal.pone.0076596
35. Maciaczyk D, Picard D, Zhao L, et al. CBF1 is clinically prognostic and serves as a target to block cellular invasion and chemoresistance of EMT-like glioblastoma cells. Br J Cancer. 2017;117(1):102–112. doi:10.1038/bjc.2017.157
36. Al Labban D, Jo SH, Ostano P, et al. Notch-effector CSL promotes squamous cell carcinoma by repressing histone demethylase KDM6B. J Clin Invest. 2018;128(6):2581–2599. doi:10.1172/JCI96915
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.