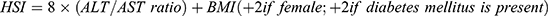Back to Journals » International Journal of Women's Health » Volume 15
Elevated Hepatic Steatosis Index is Associated with the Development of Adverse Maternal, but Not Adverse Neonatal, Outcomes: A Retrospective Cohort Study
Authors Chai TY , Byth K , George J, Pasupathy D, Cheung NW
Received 5 December 2022
Accepted for publication 15 February 2023
Published 13 April 2023 Volume 2023:15 Pages 589—598
DOI https://doi.org/10.2147/IJWH.S399085
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Everett Magann
Thora Y Chai,1– 3 Karen Byth,2,4 Jacob George,2,5,6 Dharmintra Pasupathy,3 N Wah Cheung1– 3
1Department of Diabetes and Endocrinology, Westmead Hospital, Westmead, Australia; 2Faculty of Medicine and Health, The University of Sydney, Sydney, NSW, Australia; 3Reproduction and Perinatal Centre, The University of Sydney, Sydney, NSW, Australia; 4Western Sydney Local Health District Research Education Network, Westmead, NSW, Australia; 5Storr Liver Centre, Westmead Millennium Institute for Medical Research, Westmead, NSW, Australia; 6Department of Gastroenterology and Hepatology, Westmead Hospital, Westmead, NSW, Australia
Correspondence: Thora Y Chai, Email [email protected]
Objective: To determine whether an elevated hepatic steatosis index (HSI), a non-invasive test for possible metabolic dysfunction-associated fatty liver disease (MAFLD), is associated with the development of adverse pregnancy outcomes.
Material and Methods: A retrospective cohort study was conducted on adult women with singleton pregnancies who delivered at two tertiary hospitals from August 2014 to December 2017. Aspartate aminotransaminase (AST) and alanine aminotransaminase (ALT) levels obtained 12 months pre-gravid, or during pregnancy but prior to screening for gestational diabetes mellitus (GDM), were extracted and linked with oral glucose tolerance test results. The HSI was calculated using the following equation: 8 × (ALT/AST ratio) + BMI (+2 if female; +2 if diabetes mellitus present) and considered elevated if > 36. Multiple logistic regression analysis was used to quantify the association between elevated HSI and each composite adverse pregnancy outcome after adjusting for independent maternal risk factors.
Results: Over 40-months, 11929 women were eligible and of these, 1885 had liver enzymes collected. Women with an elevated HSI (> 36) were more likely multiparous and overweight/obese compared to those women with a non–elevated HSI (≤ 36). Elevated HSI was significantly associated with a composite of adverse maternal outcomes (adjusted odds ratio (aOR) 1.55 95% CI 1.11– 2.17, p=0.01), although a non-significant increased risk of a composite of adverse neonatal outcomes occurred after multivariable adjustment (aOR 1.17, 95% CI 0.94– 1.45, p=0.17).
Conclusion: Over and above known maternal risk factors, women with elevated HSI were more likely to develop adverse maternal, but not adverse neonatal outcomes.
Keywords: liver steatosis, pregnancy outcomes, liver function tests, maternal health, neonatal health
Introduction
Metabolic dysfunction-associated fatty liver disease (MAFLD), formerly non-alcoholic fatty liver disease (NAFLD), is the most common chronic liver disorder worldwide, with strong associations to obesity and type 2 diabetes.1,2 Over the last decade, the largest rise in MAFLD incidence occurred amongst young adults (<40 years).3 The prevalence of MAFLD in pregnancy has also tripled, increasing from 10.5 per 100000 to 28.9 per 100000 pregnancies.4
Of concern, MAFLD has been associated with adverse pregnancy outcomes.4–6 A Swedish study found women with MAFLD had higher risks of gestational diabetes mellitus (GDM), pre-eclampsia, neonate prematurity and low birth weight.5 A United States study additionally ascertained postpartum hemorrhage more frequently occurred in women with MAFLD compared to those without.4 As both these studies were retrospective audits, the diagnosis of MAFLD was determined from the International Classification of Diseases (ICD) codes, raising the possibility that MAFLD was likely underdiagnosed. More recently, an Australian meta-analysis determined that MAFLD (diagnosed on imaging and/or histology) was associated with a 3-fold higher risk of GDM and pre-eclampsia, and a 2-fold higher risk of pre-term birth and large-for-gestational age (LGA) neonates.6
Although liver biopsy remains the gold standard for diagnosing MAFLD, most of its diagnosis can be achieved through non-invasive measures.1,2 Serum liver enzymes, particularly aspartate aminotransferase (AST) and alanine aminotransferase (ALT), have been used to form clinical indices of hepatic steatosis,7,8 such as the Hepatic Steatosis Index (HSI).9 The HSI is a non-invasive test, in which a score >36 has been associated with the presence of hepatic steatosis.9 Developed in 2009 by Lee et al, HSI uses the parameters of body mass index (BMI), ALT/AST ratio and the presence of diabetes or female gender.9 A recent study found that elevated HSI values were associated with the development of GDM and LGA,10 although these were the only pregnancy outcomes analysed in a predominantly Chinese cohort of women. To our knowledge, no study assessing HSI and its association with the development of adverse pregnancy outcomes in a multiethnic population has previously been performed. Our aim is to determine whether an elevated HSI (>36) is associated with the development of a composite of adverse maternal or neonatal outcomes in a multiethnic cohort of women.
Materials and Methods
Study Design
A retrospective cohort study was conducted on adult women (≥18 years old) who delivered singleton pregnancies at two tertiary referral hospitals in Sydney, Australia, from August 2014 to December 2017. If a woman delivered more than once during the 40-month study period, only her earliest pregnancy was included. Ethics approval was gained from the Western Sydney Local Health District Human Research Ethics Committee (WSLHD HREC 2019/ETH01935). As de-identified patient data was used, written informed patient consent was waived for this study. Our study also complies with the Declaration of Helsinki.
Serum Liver Enzymes (ALT and AST)
Liver enzyme levels for ALT and AST obtained 12 months pre-gravid, or during pregnancy but prior to screening for GDM at 24–28 weeks gestation, were extracted from the Institute of Clinical Pathology and Medical Research (ICPMR) database. ALT and AST were measured with the Siemens Dimension Vista® 1500 System analyzer (Siemens Healthcare Diagnostics Inc, Newark DE, USA). The low and high normal reference intervals for AST (5–30U/L) and ALT (5–35U/L) were derived from the ICPMR database, which conforms with the Australasian Association of Clinical Biochemists Harmonisation of Adult Reference Intervals in Australia and New Zealand.11
Hepatic Steatosis Index (HSI)
The HSI was calculated using the following equation:
For our study, pre-pregnancy BMI was used. A HSI score >36 (AUC 0.82, 95% confidence intervals (CI) 0.81–0.83) was used as the cut-off to define hepatic steatosis or possible MAFLD.9
Data Collection
Information regarding maternal demographics (age, ethnicity, smoking, alcohol consumption, medical history, medication use), anthropometric data (height, pre-pregnancy weight, pre-pregnancy BMI) and obstetric history (gravidity, parity, gestational hypertension, pre-eclampsia, eclampsia, cesarean delivery, induction of labor) were extracted from the ObstetriX database. Pre-pregnancy BMI was further categorised in our study depending on ethnicity.12,13 For East Asian and South Asian ethnicities, underweight was defined as BMI <18.5kg/m2; healthy weight was defined as BMI between 18.5 and 23.0kg/m2; overweight was defined as BMI between 23.1 and 27.5kg/m2 and obese was defined as BMI >27.5kg/m2.12 For European and Other ethnicities, underweight was defined as BMI <18.5kg/m2; healthy weight was defined as BMI between 18.5 and 25.0kg/m2; overweight was defined as BMI between 25.1 and 30.0kg/m2 and obese was defined as BMI >30.0kg/m2.13
Women with multiple pregnancies, documented chronic liver disease (ie hepatitis B/C, autoimmune liver disease, hemochromatosis, Wilson’s disease, primary biliary cholangitis, primary sclerosing cholangitis) or moderate-to-excessive alcohol intake (>2 standard drinks per day) were excluded from our study. ObstetriX (Cerner Sverige AB, Sweden) is an electronic patient record system which is used in many government-managed hospitals in New South Wales, Australia. Data are entered contemporaneously by healthcare staff in either inpatient or outpatient settings during the entire pregnancy cycle.
The diagnosis of GDM was determined using the International Association of Diabetes in Pregnancy Study Groups (IADPSG) 2010 criteria,14 which recommends universal testing with a standard one-step, 2-hour 75g oral glucose tolerance test (OGTT) at 24–28 weeks gestation. GDM was diagnosed if any one of the three OGTT blood glucose levels (BGLs) exceeded the following thresholds: fasting BGL ≥5.1 mmol/L, 1-hour BGL ≥10.0 mmol/L and 2-hour BGL ≥8.5 mmol/L.14 OGTT BGLs (fasting, 1-hr and 2-hr) were obtained from ObstetriX.
Neonatal demographics (gestational age (weeks), gender, birthweight) and neonatal complications – including pre-term birth (delivery at <37 weeks gestation), neonatal hypoglycemia (defined as neonate capillary BGL <2.6mmol/L) and admissions into neonatal high dependency units or HDU (either neonatal special care nursery unit or neonatal intensive care unit) – were extracted from ObstetriX. Neonate birthweight percentiles were calculated from an online weight centile calculator (Grow Bulk Centile Calculator, version 6.7.3_13) customised for maternal height, weight, ethnicity and parity as well as neonatal birthweight, gender and gestational age (days).15 Small-for-gestational age (SGA) was defined as neonatal birthweight below the 10th percentile and LGA was defined as neonatal birthweight above the 90th percentile.
Outcome Measures
A composite of adverse maternal outcomes was defined as having one or more of GDM or any maternal hypertensive complication (gestational hypertension, pre-eclampsia or eclampsia). A composite of adverse neonatal outcomes was defined as the presence of one or more of pre-term birth, SGA or LGA neonates, neonatal hypoglycemia, or neonatal HDU admissions.
Statistical Analysis
A dataset was created in Microsoft Excel (Microsoft Office 365 version 16.0, Redmond, WA, USA: Microsoft Corp) and data linkage was performed using the study participants identification number, serum liver enzyme levels and OGTT BGLs (fasting, 1-hour, 2-hour). Data were analyzed using IBM SPSS Statistics version 27 (IBM SPSS Statistics, Version 27.0. Armonk, NY, USA: IBM Corp). Frequencies and percentages were used to summarize categorical variables and the median and interquartile range (IQR) were used for continuous variables. Chi-squared or Fisher’s exact test were used to test for associations between categorical variables. Mann–Whitney U-tests were used to test for differences in the distribution of continuous variables between groups. Two-tailed tests with a 5% level of significance were used throughout.
HSI levels could only be calculated for pregnant women who had liver enzymes collected. Odds ratios (OR) with 95% CI were used to quantify the strength of association between elevated HSI (>36) and each dichotomous outcome variable. Multiple logistic regression analysis with backward stepwise variable selection was used to identify independent maternal risk factors associated with each outcome. Candidate variables for selection in each model were those maternal risk factors (excluding HSI status and whether liver enzymes were taken during pregnancy) associated with the outcome at p-value <0.20. For each dichotomous outcome variable, the OR with 95% CI for HSI status (Elevated >36 vs Non-Elevated ≤36) adjusted for independent maternal risk factors was then calculated.
Results
Over 40-months, a total of 13330 women delivered and 11929 women with singleton pregnancies were considered eligible (Figure 1). Only 1885 women had liver enzymes collected (Figure 1), of whom 1407 (74.6%) had liver enzymes collected during pregnancy, but prior to GDM screening, and 478 (25.4%) had liver enzymes collected up to 12 months pre-gravid.
 |
Figure 1 Flow diagram illustrating selection of study participants from August 2014 to December 2017. |
Women who had serum liver enzymes collected pre-gravid or during pregnancy were more likely multiparous, of Caucasian ethnicity, had a higher pre-pregnancy BMI, underwent higher rates of induced labor or cesarean delivery and experienced increased adverse maternal outcomes than those women who did not have serum liver enzymes collected (Supplementary Table 1). Neonates of these women were more likely born premature, admitted into neonatal HDU and experienced neonatal hypoglycemia compared to women without liver enzymes collected (Supplementary Table 1).
Table 1 summarizes the study cohort by HSI status. Of the 1885 women who had liver enzymes collected, 1260 (66.8%) had an Elevated HSI >36 and 372 women (19.7%) had GDM. Supplementary Tables 2 and 3 summarizes the characteristics of our cohort who had liver enzymes collected pre-gravid or during pregnancy respectively, as according to HSI status.
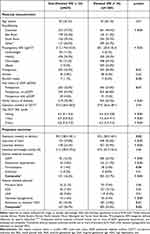 |
Table 1 Characteristics of 1885 Women by HSI Status Who Had Delivered from Aug 2014 to Dec 2017 |
Women in the Elevated HSI group were more likely to be of Caucasian ethnicity, overweight or obese, multiparous, had higher OGTT BGL results, underwent higher rates of induced labor or cesarean delivery and experienced increased rates of GDM, gestational hypertension or pre-eclampsia compared to women in the Non-Elevated HSI group (Table 1). Neonates of Elevated HSI women were more likely to experience hypoglycemia and HDU admissions than neonates born to women with Non-Elevated HSI on univariate analysis (Table 1).
Table 2 presents unadjusted and adjusted OR (aOR) with 95% CI for the development of adverse maternal outcomes in those with Elevated HSI (>36) versus Non-Elevated HSI (≤36). After adjusting for the independent maternal risk factors, elevated HSI was associated with the development of GDM, gestational hypertension and the overall composite of adverse maternal outcomes (Table 2).
 |
Table 2 Association Between the Presence of Adverse Maternal Outcomes and HSI Status (Elevated >36 Vs Non-Elevated ≤36) |
Table 3 presents unadjusted and aOR with 95% CI for the development of adverse neonatal outcomes in those with Elevated HSI (>36) versus Non-Elevated HSI (≤36). After adjusting for the independent maternal risk factors, elevated HSI was not significantly associated with the development of adverse neonatal outcomes.
 |
Table 3 Association Between the Presence of Adverse Neonatal Outcomes and HSI Status (Elevated >36 Vs Non-Elevated ≤36) |
Discussion
By extending the investigation of HSI and its association with adverse pregnancy outcomes into a multiethnic cohort of women, we identified that elevated HSI (>36) was associated with the development of adverse maternal, but not adverse neonatal outcomes. Prior studies have examined the association of liver enzyme levels and its development of adverse pregnancy outcomes, such as GDM and pre-eclampsia.16–20 By itself, liver enzyme levels, such as ALT, are poor predictors of MAFLD.21 As such, non-invasive indices of hepatic steatosis may be a simple measure (over imaging) used to assess for probable MAFLD. HSI is easier to calculate than other measures of hepatic steatosis, such as the Fatty Liver Index,22 as it involves clinical parameters (other than liver enzyme levels) that are routinely collected during pregnancy.
Our study builds on the findings of prior studies assessing the consequences of MAFLD in pregnancy, especially its association with adverse maternal complications.4–6,23–28 The concurrent presence of MAFLD in pregnancy may further worsen the insulin-resistant state of pregnancy, thereby increasing the risk of GDM.24,25 We identified that women with an elevated HSI had a 1.5-fold increased odds of developing GDM, although this association was attenuated after multivariable adjustment, particularly with BMI. Our adjusted odds were lower than those identified in Song et al study for GDM, which were between 2–3-fold.10 However, pre-pregnancy BMI was not included in their multivariable analysis.10
Our study also identified a 3-fold increased odds of developing gestational hypertension, although more severe maternal hypertensive complications (pre-eclampsia and eclampsia) were not found to be significant after multivariable adjustment. A similar 2–3-fold higher risk of gestational hypertension and pre-eclampsia occurred in women with MAFLD compared to those without in previous studies.4–6,27,28 The pathological mechanisms behind MAFLD inducing pregnancy-associated hypertension may be associated with insulin resistance and its inappropriate activation of the renin–angiotensin system.4,27,28 As MAFLD, obesity, GDM and pregnancy-associated hypertension are all known cardiometabolic risk factors,28–30 pregnancy (or even conception planning) is an opportune time to screen reproductive-aged women with any of these risk factors for future cardiovascular disease and organise long-term follow-up if required.
However, our study did not identify elevated HSI as being an independent predictor of adverse neonatal outcomes after multivariable adjustment. Unlike Song et al,10 we did not find a significant association between elevated HSI and the development of LGA neonates, despite experiencing similar LGA rates (around 8–9%). Prior studies have found that MAFLD during pregnancy was associated with an increased risk of adverse neonatal outcomes, although results remain conflicting. MAFLD has been associated with a higher risk of having both SGA5 or LGA neonates,4,6,23 whilst pre-term birth4,5 and extreme prematurity (<32 weeks)5 have also been reported. Differences are likely secondary to the total sample size of the studies, varying predominant ethnicities of the populations examined and confounding variables able to be captured and assessed.
Our study is not without limitations. The use of HSI has not yet been validated in pregnant populations, although it has previously been investigated in a pregnant cohort.10 Our study has also included serum liver enzymes collected both pre-gravid and during the first two trimesters of pregnancy. Most studies have focused on liver enzymes collected either pre-gravid16,17 or during pregnancy.18–20 The inclusion of pre-gravid AST and ALT in our study was important as prior studies have determined that abnormal values were associated with the development of adverse maternal outcomes, such as GDM or pre-eclampsia.16,17 Whilst a majority of studies analysed liver enzyme levels during pregnancy and determined associations with adverse pregnancy outcomes,18–20 liver enzyme levels can change during the course of pregnancy. Whilst AST levels did not significantly change between matched pregnant and non-pregnant women, ALT levels were slightly elevated (although remained within reference limits) in second-trimester pregnant women.31 A later study by Gohel et al found that ALT and AST levels did not significantly deviate until the third trimester of pregnancy, where slight increases in levels were identified.32 Hence, ALT and AST values collected during the third trimester of pregnancy were excluded from our study. ALT and AST values can also differ between specific ethnicities, with more values noted in the upper limits of set reference ranges amongst Black American and Hispanic populations.33 Both these population groups are not as prevalent in Australia and hence we did not feel it was necessary to account for ethnic disparities in AST or ALT values in our study.
The presence of selection bias is likely in our study cohort, as not only were the pregnant women who had serum liver enzymes collected overweight or obese, but also suffered more complicated pregnancies when compared to those women who did not have serum liver enzymes collected. Furthermore, the retrospective nature of this study raises the possibility that we may not have completely excluded all participants with chronic liver disease, or with moderate-to-excessive alcohol intake, even though clinical information were mostly inputted by healthcare staff into ObstetriX. Our study could also be further strengthened by correlating elevated HSI with imaging results suggestive of hepatic steatosis, but this information was not available on the ObstetriX database. Nevertheless, major strengths of our study include being one of the largest such study to date, with 1885 women from multiethnic backgrounds, compared to the next largest which had 1082 Chinese women.10 We were also able to obtain the majority of the OGTT BGLs and other confounding variables through ObstetriX.
Conclusions
Our study indicates that over and above known maternal risk factors, elevated HSI >36, suggestive of possible MAFLD, was significantly associated with the development of GDM and pregnancy-associated hypertension but not adverse neonatal outcomes. Given the increasing prevalence of MAFLD amongst young adults and its cardiometabolic consequences in later life, routine measurement of liver enzymes may be useful in young reproductive-aged women who are obese, either in early pregnancy or during pregnancy-planning, to help with the calculation of HSI. However, longitudinal cohort studies and intervention studies are needed to help validate the utility and effectiveness of such a measure.
Abbreviations
ALT, alanine aminotransferase; aOR, adjusted odds ratio; AST, aspartate aminotransferase; BGL, blood glucose level; BMI, body mass index; CI, confidence intervals; GDM, gestational diabetes mellitus; HDU, high dependency unit; HSI, Hepatic Steatosis Index; IADPSG, International Association of Diabetes in Pregnancy Study Groups; ICD, International Classification of Diseases; LGA, large-for-gestational neonates; MAFLD, metabolic dysfunction–associated fatty liver disease; NAFLD, non-alcoholic fatty liver disease; OGTT, oral glucose tolerance test; OR, odds ratio; SGA, small-for-gestational neonates.
Data Sharing Statement
The datasets and resources generated and analyzed during our study are available from the corresponding author, TYC, on reasonable request.
Ethics Approval and Informed Consent
The study protocol was approved by the Western Sydney Local Health District Human Research Ethics Committee (HREC 2019/ETH01935), including a waiver for written patient consent, as de-identified data was used. Our study also complies with the Declaration of Helsinki.
Acknowledgments
The authors would like to thank the staff at NSW Health Pathology West, formerly Institute of Clinical Pathology of Medical Research, for their help with extracting and de-identifying the liver enzyme levels.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The author(s) disclose receipt of the following financial support: T.Y.C is supported by the Jerry Koutts Scholarship 2020, provided by the Institute of Clinical Pathology & Medical Research (ICPMR), Westmead Hospital and the Western Sydney Local Health District Research Education Network Grant 2021, provided by the Western Sydney Local Health District Research Education Network, Westmead Hospital. J.G. is supported by the Robert W. Storr Bequest to the Sydney Medical Foundation, University of Sydney and received research grants from the National Health and Medical Research Council of Australia (NHMRC) Program (APP1053206, APP1149976) and Project (APP1107178 and APP1108422). N.W.C received a research grant from the Diabetes Australia Research Program 2021 (Y21GCHEW).
Disclosure
The authors do not have any potential conflicts of interest with respect to the research, authorship, and/or publication of this article to declare.
References
1. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi:10.1016/j.jhep.2020.03.039
2. Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014. doi:10.1053/j.gastro.2019.11.312
3. Allen AM, Therneau TM, Larson JJ, Coward A, Somers VK, Kamath PS. Non-alcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology. 2018;67(5):1726–1736. doi:10.1002/hep.29546
4. Sarkar M, Grab J, Dodge JL, et al. Non-alcoholic fatty liver disease in pregnancy is associated with adverse maternal and perinatal outcomes. J Hepatol. 2020;73(3):516–522. doi:10.1016/j.jhep.2020.03.049
5. Hagström H, Höijer J, Ludvigsson JF, et al. Adverse outcomes of pregnancy in women with non-alcoholic fatty liver disease. Liver Int. 2016;36(2):268–274. doi:10.1111/liv.12902
6. El Jamaly H, Eslick GD, Weltman M. Systematic review with meta-analysis: non-alcoholic fatty liver disease and the association with pregnancy outcomes. Clin Mol Hepatol. 2022;28(1):52–66. doi:10.3350/cmh.2021.0205
7. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American association for the study of liver diseases, American college of gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–2023. doi:10.1002/hep.25762
8. Tsai E, Lee TP. Diagnosis and evaluation of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis, including non-invasive biomarkers and transient elastography. Clin Liver Dis. 2018;22(1):73–92. doi:10.1016/j.cld.2017.08.004
9. Lee JH, Kim D, Kim HJ, et al. Hepatic steatosis index: a simple screening tool reflecting non-alcoholic fatty liver disease. Dig Liver Dis. 2010;42(7):503–508. doi:10.1016/j.dld.2009.08.002
10. Song S, Duo Y, Zhang Y, et al. The predictive ability of hepatic steatosis index for gestational diabetes mellitus and large-for-gestational age infant compared with other non-invasive indices among Chinese pregnancies: a preliminary double-center cohort study. Diabetes Metab Syndr Obes. 2021;14:4791–4800. doi:10.2147/DMSO.S335364
11. Koerbin G, Tate JR. Harmonising adult reference intervals in Australia and New Zealand – the continuing story. Clin Biochem Rev. 2016;37(3):121–129.
12. World Health Organization. Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi:10.1016/S0140-6736(03)15268-3
13. World Health Organization. Expert Consultation. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452.
14. Metzger BE, Gabbe SG, Persson B, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. doi:10.2337/dc10-0719
15. Perinatal Institute. Gestation Network: centile Calculator; 2013. Available from: www.gestation.net/index.htm.
16. Park JY, Kim WJ, Chung YH, et al. Association between pregravid liver enzyme levels and gestational diabetes in twin pregnancies: a secondary analysis of national cohort study. Sci Rep. 2021;11(1):18695. doi:10.1038/s41598-021-98180-9
17. Cho GJ, Kim HY, Park JH, et al. Pre-pregnancy liver enzyme levels and risk of pre-eclampsia in a subsequent pregnancy: a population-based cohort study. Liver Int. 2018;38(5):949–954. doi:10.1111/liv.13617
18. Chen X, Chen H, Zhang Y, et al. Maternal liver dysfunction in early pregnancy predisposes to gestational diabetes mellitus independent of preconception overweight: a prospective cohort study. BJOG. 2022;129(10):1695–1703. doi:10.1111/1471-0528.17117
19. Lee SM, Park JS, Han YJ, et al. Elevated alanine aminotransferase in early pregnancy and subsequent development of gestational diabetes and pre-eclampsia. J Korean Med Sci. 2020;35(26):e198. doi:10.3346/jkms.2020.35.e198
20. Yarrington CD, Cantonwine DE, Seely EW, McElrath TF, Zera CA. The association of alanine aminotransferase in early pregnancy with gestational diabetes. Metab Syndr Relat Disord. 2016;14(5):254–258. doi:10.1089/met.2015.0106
21. Ma X, Liu S, Zhang J, et al. Proportion of NAFLD patients with normal ALT value in overall NAFLD patients: a systematic review and meta-analysis. BMC Gastroenterol. 2020;20(1):10. doi:10.1186/s12876-020-1165-z
22. Bedogni G, Bellentani S, Miglioli L, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi:10.1186/1471-230X-6-33
23. Lee SM, Jung YM, Choi ES, et al. Metabolic dysfunction-associated fatty liver disease and subsequent development of adverse pregnancy outcomes. Clin Gastroenterol Hepatol. 2021;20:2542–2550.e8. doi:10.1016/j.cgh.2021.11.007
24. De Souza LR, Berger H, Retnakaran R, et al. Non-alcoholic fatty liver disease in early pregnancy predicts dysglycemia in mid-pregnancy: prospective study. Am J Gastroenterol. 2016;111(5):665–670. doi:10.1038/ajg.2016.43
25. Lee SM, Kwak SH, Koo JN, et al. Non-alcoholic fatty liver disease in the first trimester and subsequent development of gestational diabetes mellitus. Diabetologia. 2019;62(2):238–248. doi:10.1007/s00125-018-4779-8
26. Chai TYL, Rajaratnam RM, Deng D, George J, Pasupathy D, Cheung NW. The prevalence of gestational diabetes mellitus in women diagnosed with non-alcoholic fatty liver disease during pregnancy: a systematic review and meta-analysis. J Diabetes Complications. 2021;35(9):107991. doi:10.1016/j.jdiacomp.2021.107991
27. Herath RP, Siriwardana SR, Ekanayake CD, Abeysekara V, Kodithuwakku SUA, Herath HP. Non-alcoholic fatty liver disease and pregnancy complications among Sri Lankan women: a cross sectional analytical study. PLoS One. 2019;14(4):e0215326. doi:10.1371/journal.pone.0215326
28. Jung YM, Lee SM, Hong S, et al. The risk of pregnancy-associated hypertension in women with nonalcoholic fatty liver disease. Liver Int. 2020;40(10):2417–2426. doi:10.1111/liv.14563
29. Zhou MS, Schulman IH, Zeng Q. Link between the renin-angiotensin system and insulin resistance: implications for cardiovascular disease. Vasc Med. 2012;17(5):330–341. doi:10.1177/1358863X12450094
30. Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021;143(18):e902–e16. doi:10.1161/CIR.0000000000000961
31. Bacq Y, Zarka O, Bréchot JF, et al. Liver function tests in normal pregnancy: a prospective study of 103 pregnant women and 103 matched controls. Hepatology. 1996;23(5):1030–1034. doi:10.1002/hep.510230514
32. Gohel MG, Joshi AG, Anand JS, Makadia JS, Kamariya CP. Evaluation of changes in liver function test in first, second and third trimester of normal pregnancy. Int J Reprod Contracept Obstet Gynecol. 2013;2(4):616–620. doi:10.5455/2320-1770.ijrcog20131225
33. Johnston DE. Special considerations in determining liver function tests. Am Fam Physician. 1999;59(8):2223–2230.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.