Back to Journals » Journal of Inflammation Research » Volume 15
Elevated ALCAM Expression Associated with Endotypes and Postoperative Recurrence in Chronic Rhinosinusitis with Nasal Polyps
Authors Zhang H, Xie S, Fan R, Wang F, Xie Z, Jiang W
Received 22 November 2021
Accepted for publication 27 January 2022
Published 15 February 2022 Volume 2022:15 Pages 1063—1077
DOI https://doi.org/10.2147/JIR.S350609
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Hua Zhang,1– 3,* Shaobing Xie,1– 3,* Ruohao Fan,1– 3 Fengjun Wang,1– 3 Zhihai Xie,1– 3 Weihong Jiang1– 3
1Department of Otolaryngology Head and Neck Surgery, Xiangya Hospital of Central South University, Changsha, Hunan, 410008, People’s Republic of China; 2Hunan Province Key Laboratory of Otolaryngology Critical Diseases, Changsha, Hunan, 410008, People’s Republic of China; 3National Clinical Research Center for Geriatric Disorders, Xiangya Hospital of Central South University, Changsha, Hunan, 410008, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Weihong Jiang, Department of Otolaryngology Head and Neck Surgery, Xiangya Hospital of Central South University, Changsha, Hunan, 410008, People’s Republic of China, Email [email protected]
Background: Chronic rhinosinusitis with polyps (CRSwNP) is characterized by high heterogeneity and postoperative recurrence rate. This study aimed to explore the clinical significance of activated leukocyte cell adhesion molecule (ALCAM) in endotyping CRSwNP and predicting its recurrence.
Methods: We recruited 120 CRSwNP patients including 70 non-eosinophilic CRSwNP (neCRSwNP) and 50 eosinophilic CRSwNP (eCRSwNP) patients, and 40 healthy controls (HCs). Serum and tissue samples were collected. Serum ALCAM levels were detected by enzyme-linked immunosorbent assay (ELISA), and tissue ALCAM expression was assessed by reverse transcription-polymerase chain reaction (RT-PCR), Western blotting (WB) and immunohistochemistry (IHC). The predictive values of ALCAM expression for CRSwNP endotypes and postoperative recurrence were assessed.
Results: The serum levels of ALCAM were significantly increased in CRSwNP patients in comparison with HCs and were correlated with the peripheral eosinophil count, tissue eosinophil counts, and percentage. Multivariate analysis and receiver operating characteristic (ROC) curve highlighted that serum ALCAM levels were associated with CRSwNP endotypes. Tissue ALCAM expression was significantly enhanced in CRSwNP patients, especially in eCRSwNP patients. At the end of the study, 110 patients completed the follow-up schedule, 78 patients were categorized into the non-recurrent group, and the other 32 patients were included in the recurrent group. The serum ALCAM levels were elevated in the recurrent group compared with the non-recurrent group, and ALCAM expression in the tissue was significantly elevated. The ROC curve exhibited a high predictive ability of serum ALCAM in predicting postoperative recurrence. Logistic regression and Kaplan–Meier curves demonstrated that serum ALCAM was an independent risk factor for postoperative recurrence.
Conclusion: This is the first report suggesting that ALCAM expression was upregulated and associated with mucosal eosinophil infiltration and CRSwNP recurrence. Serum ALCAM could be a promising biomarker for distinguishing endotypes and predicting postoperative recurrence in CRwNP patients.
Keywords: activated leukocyte cell adhesion molecule, chronic rhinosinusitis with nasal polyps, endotypes, recurrence, biomarker
Introduction
Chronic rhinosinusitis (CRS) is a highly prevalent disease characterized by chronic inflammation of the nasal cavity and sinuses, and it has impacted approximately 10% of the Chinese population.1,2 CRS is a multifactorial disease and is frequently grouped into two phenotypes based on the presence or absence of nasal polyps: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP).3,4 Accordingly, CRSwNP is a highly heterogeneous disorder with massive infiltration of inflammatory cells and mediators in the tissue. Eosinophilic-driven Th2 inflammation is predominant in its underlying pathomechanism.5,6 CRSwNP is further categorized into noneosinophilic (neCRSwNP) and eosinophilic (eCRSwNP) types based on the eosinophil percentage in poly tissues.7,8 These subtypes of CRSwNP exhibit distinct pathophysiological properties, clinical manifestations, prognosis and postoperative recurrence rates.9,10 Given that, preoperatively discriminating CRSwNP endotypes and predicting postoperative recurrence are pivotal to achieving personalized treatments and adjusting follow-up protocols. Therefore, it is urgently necessary to explore objective indicators or biomarkers for distinguishing CRSwNP endotypes and predicting postoperative recurrence to develop treatment strategies and treatment outcomes.
Activated leukocyte cell adhesion molecule (ALCAM; CD166) is a glycoprotein belonging to the immunoglobulin superfamily, and it is expressed on a variety of cell types.11,12 Previous studies have demonstrated that ALCAM is implicated in diverse pathophysiological processes, including tumor progression and metastasis and T cell activation and proliferation, and it is involved in malignant tumors, autoimmune diseases, and inflammatory diseases.13–15 In recent years, the role of ALCAM in the immune response has attracted attention; elevated ALCAM expression was observed in the tissue and serum of patients with asthma and allergic rhinitis, suggesting that ALCAM may be involved in airway inflammation.16–18 Thus, the present study explored the role of ALCAM in CRSwNP pathogenesis and evaluated its value in discriminating CRSwNP endotypes and predicting postoperative recurrence.
Materials and Methods
Participants and Settings
We prospectively recruited 120 CRSwNP patients who underwent nasal endoscopic surgery in our tertiary clinic between June 2018 and August 2018. CRSwNP was diagnosed based on disease history, physical examination, nasal endoscopy, and sinus computed tomography (CT) findings according to the guidelines of the European Position Paper on Rhinosinusitis and Nasal Polyps 2012.19 Accompanied with allergic rhinitis and asthma were confirmed by physicians based on disease history, skin tests and/or specific IgE tests, and pulmonary function. The inclusion criteria were listed as follows: 1) age over 18 years; 2) treatment with nasal endoscopic surgery; and 3) volunteering to participate in this study and providing nasal polyp samples; patients with CRSwNP were excluded if they had other nasal or sinus diseases, such as fungal sinusitis, allergic fungal rhinosinusitis, cystic fibrosis, or malignancy; autoimmune diseases or eosinophilic diseases; or a history of immunotherapy, antibiotics, nasal or systemic corticosteroids, or anti-allergic drugs within four weeks. We also excluded patients aged < 18 years or >70 years. Forty patients undergoing septoplasty without other nasal diseases were recruited as the control group in the present study as previously described, and middle turbinate mucosa was collected as control tissue.20 Control subjects were excluded if they were on immunotherapy, had severe heart and kidney dysfunction, or had inflammatory or autoimmune conditions. This study was approved by the Medical Ethics Committee of Xiangya Hospital of Central South University (permission no. 201801402). All subjects provided informed consent before they were enrolled.
Endotyping CRSwNP
Nasal polyps were obtained from CRSwNP patients during the operation, immersed in 10% formalin and embedded in paraffin wax. The embedded tissues were sectioned into 5-μm thickness sections and stained with hematoxylin and eosin (H&E). Two independent pathologists who were blinded to the clinical data counted the numbers and percentages of eosinophils in 10 randomly selected high-power fields (HPF). The diagnosis of eCRSwNP and neCRSwNP was conducted as previously described: eCRSwNP was defined when the percentage of tissue eosinophils was > 10% of the total inflammatory cells; otherwise neCRSwNP was diagnosed.20,21
Postoperative Follow-Up and Evaluation
Postoperative medical treatment was performed according to the standard recommendations, including nasal saline irrigation, intranasal budesonide, and oral clarithromycin for three months after surgery, and patients with eCRSwNP were suggested to receive intranasal budesonide for more than six months.22–24 A postoperative follow-up schedule was performed for more than two years: weekly for one month, monthly for six months, every three months in the first year, and subsequently, every six months. Recurrence was considered when CRSwNP symptoms were present despite the rescue regimen of antibiotics and oral steroids, and endoscopic evidence was obtained during outpatient clinic follow-up as previously described.22,25,26
Serum ALCAM Level Detection
Non-anticoagulant peripheral venous blood samples were collected from CRSwNP patients and HCs preoperatively and stored at room temperature for 1–2 hours. The coagulated blood samples were centrifuged, and the supernatants were collected and stored at −80°C for subsequent experiments. All serum samples were thawed and centrifuged before use, and the sources of the samples were blinded to the researchers who evaluated the clinical data to minimize the risk of measurement bias. The serum ALCAM levels were quantified by a commercial ELISA kit (CUSABIO, Wuhan, China) according to the manufacturer’s instructions. All samples were diluted at 1:20 and conducted in duplicate to improve the assay precision.
RNA Extraction and qRT-PCR Analysis
Tissue specimens were harvested during surgery and immediately snap-frozen in liquid nitrogen. Tissue total RNA was extracted with Ncmzol reagent (New Cell & Molecular Biotech, Suzhou, China) and quantified by a Nanodrop 2000c (Thermo Fisher Scientific, Pittsburg, USA). RNA was reverse transcribed into cDNA using a SureScript First-strand cDNA synthesis kit (US EVERBRIGHT, Suzhou, China). Primers for ALCAM and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed and synthesized (Ruibo Biotechnology, Guangzhou, China). qRT-PCR was performed using 100ng of cDNA and SYBR Green qPCR Supermix (US EVERBRIGHT, Suzhou, China) following the manufacturer’s protocols. The mRNA expression was calculated using the comparative threshold cycle (2−ΔΔCT) method. The primers used are shown in Table S1.
Western Blotting
Collected tissue specimens were completely lysed in cell lysis buffer, followed by centrifugation and the supernatants collection. The protein concentrations were detected of by the BCA protein kit (New Cell & Molecular Biotech, Suzhou, China) according to the instructions. Separated the extracted protein by gel electrophoresis (Beyotime Biotech, Shanghai, China) and blotted onto PVDF membranes (0.45 mm). The PVDF membrane were sealed with 5% non-fat milk for 1 h, then incubated with the primary antibodies anti-ALCAM and anti-GAPDH (Affinity Biosciences, Changzhou, China) overnight at 4 °C. Then, the membranes were incubated with corresponding HRP-conjugated (1:5000) secondary antibody for 2 h at room temperature. The blot bands were examined by Molecular Imager Chemidoc XRS System (UVP, Ltd., USA), and the band densities were quantified with a computerized densitometer.
IHC Staining of ALCAM
Collected tissue specimens were fixed, embedded, sectioned, and stained, and IHC analysis was conducted by utilizing a streptavidin-biotin complex (SABC) kit (Weiao Biological Technology) as previously described.27,28 The sections were incubated overnight at 4 °C with primary antibody against ALCAM (Affinity Biosciences, Changzhou, China), and then incubated with an anti-rabbit secondary antibody for 30 minutes, and 3′3-diaminobenzidine (DAB) was used for visualization. The pathological changes in nasal tissues were assessed by two senior pathologists in a double-blinded manner. Two representative images were selected in each group and displayed at 200x and 400x magnification.
Statistical Analysis
All data were recorded as the mean ± standard deviation. For normally distributed variables, one-way analysis of variance (ANOVA) or Student’s t-test was performed; otherwise, Kruskal–Wallis H-test or Mann–Whitney U-test was applied. Spearman correlation tests were utilized to assess the associations between ALCAM expression and the clinical variables. Logistic regression analysis and receiver operating characteristic (ROC) curves were performed to determine the ability of serum ALCAM to distinguish CRSwNP endotypes and predicting postoperative recurrence. Furthermore, based on the cutoff value, relapse-free survival curves of postoperative recurrence were constructed utilizing the Kaplan-Meier method. All statistical analyses were performed using SPSS statistics software version 19.0 (IBM, Chicago, IL, USA). For all tests, P < 0.05 was regarded as statistically significant.
Results
Baseline Data of All Subjects
The demographic and clinical data of all participants are shown in Table 1. Among 120 CRSwNP patients, 70 patients were categorized into the neCRSwNP group, and 50 patients were classified into the eCRSwNP group. The typical H&E staining of neCRSwNP, eCRSwNP, and HC are exhibited in Figure S1. Compared to the HC and neCRSwNP groups, patients in the eCRSwNP group exhibited a higher rate of accompanying allergic rhinitis and asthma, and higher levels of peripheral eosinophil count and percentage (P < 0.05). The tissue eosinophil count and percentage and disease duration in the eCRSwNP group were increased compared with those in the neCRSwNP group (P < 0.05).
 |
Table 1 The Demographic and Clinical Features of Participants |
Serum ALCAM Level in CRSwNP and Its Association with CRSwNP Endotypes
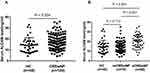
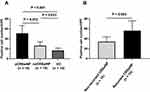
The ALCAM concentrations were elevated in the serum of CRSwNP patients in comparison with the HCs, and the ALCAM levels were markedly increased in the eCRSwNP group compared with those in the neCRSwNP (Figure 1, P < 0.05). The Spearman correlation results in Table 2 showed that elevated serum ALCAM levels were correlated with the peripheral eosinophil count (r = 0.186, P = 0.042), tissue eosinophil count (r = 0.654, P < 0.001) and percentage (r = 0.680, P < 0.001). The binary logistic regression analysis results demonstrated that the peripheral eosinophil percentage and serum ALCAM level were associated with CRSwNP endotypes (Table 3). The ROC curves shown in Figure 2 suggested that serum ALCAM (area under the curve (AUC) = 0.747) had a stronger ability to predict CRSwNP endotypes than the peripheral eosinophil percentage (AUC = 0.681).
 |
Table 2 Relationship Between Serum ALCAM and Clinical Variables in CRSwNP Patients |
 |
Table 3 Binary Logistic Regression Analysis of Factors Associated with eCRSwNP |
Evaluation of the Predictive Value of Serum ALCAM for CRSwNP Recurrence
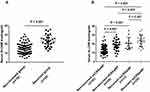
A total of 110 patients completed the postoperative follow-up schedule; 32 patients experienced polyp recurrence and were categorized into the recurrent group, and the other 78 patients were included in the non-recurrent group. The demographics and clinical characteristics in the two groups were listed in Table 4. The rate of accompanying allergic rhinitis, tissue eosinophil count and percentage, and the percentage of eCRSwNP were higher in the recurrent group than in the non-recurrent group (P < 0.05). The serum ALCAM levels were increased in the recurrent group compared with the non-recurrent group, and the serum ALCAM concentrations were significantly elevated in the recurrent eCRSwNP group compared with the non-recurrent eCRSwNP and non-recurrent neCRSwNP groups (Figure 3, P < 0.05). The binary logistic regression analysis results in Table 5 showed that serum ALCAM levels were associated with the recurrence of CRSwNP. The ROC curves demonstrated that serum ALCAM presented a high predictive value for CRSwNP recurrence (Figure 4A, AUC = 0.823). Patients were classified into the low ALCAM level group (< 24.1 ng/mL) and the high ALCAM level group (> 24.1 ng/mL) according to the cutoff value of serum ALCAM cutoff value obtained from the ROC curve, Kaplan-Meier curves showed that these two groups exhibited significantly different rates of postoperative recurrence (Figure 4B, P < 0.05).
 |
Table 4 Demographic Characteristics Between Primary and Recurrent CRSwNP Patients |
 |
Table 5 Binary Logistic Regression Analysis of Factors Associated with the Recurrence of CRSwNP |
ALCAM Expression in Nasal Tissues
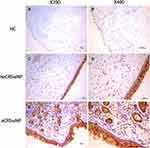
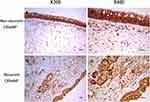
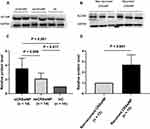
As shown in Figure 5, the ALCAM mRNA levels were significantly increased in the CRSwNP group than the HC group (P = 0.047), especially in the eCRSwNP group (P = 0.003). No significant difference was observed between the non-recurrent CRSwNP group and the recurrent CRSwNP group (P = 0.162). Figures 6 and 7 showed the representative IHC images, ALCAM staining was mainly located in the nasal epithelium and gland areas, and the numbers of ALCAM protein positive cells were significantly greater in CRSwNP patients in comparison with HCs, especially in eCRSwNP patients (Figure 8A). Compared with non-recurrent CRSwNP, ALCAM expression was markedly enhanced in nasal epithelial cells, submucosal cells, and glandular cells in the tissue of recurrent CRSwNP (Figure 8B). Moreover, Western blotting (WB) results presented that the protein expression levels of ALCAM were enhanced in eCRSwNP patients than neCRSwNP patients and HCs, and this difference was also observed between non-recurrent CRSwNP and recurrent CRSwNP groups (Figure 9).
Discussion
In the present prospective study, we first detected the soluble form and membrane-bound form of ALCAM in serum and tissue samples by ELISA, qRT-PCR and IHC, respectively, and we found that both forms of ALCAM were markedly increased in CRSwNP, especially in eCRSwNP and recurrent CRSwNP. Moreover, we revealed that serum ALCAM levels were correlated with the degree of mucosal eosinophil infiltration and were closely associated with eCRSwNP and an increased risk of postoperative recurrence. Taken together, these results indicated that ALCAM expression was associated with the development of eCRSwNP and CRSwNP recidivation, and serum ALCAM could be a novel biomarker to distinguish endotypes and predict postoperative recurrence in CRSwNP patients.
ALCAM is a type I transmembrane molecule expressed on several immune and non-immune cells, especially dendritic cells (DCs), and it plays a major role in mediating the immune response.29–31 Growing evidence has demonstrated that ALCAM is overexpressed in malignant tumors, inflammatory diseases, and autoimmune diseases, and the soluble form of ALCAM has increasingly utilized as a biomarker in the diagnosis, disease progression, and prognosis in various clinical disorders.14,32,33 A recent publication found that urinary soluble ALCAM levels were increased in systemic lupus erythematosus patients and that elevated ALCAM concentrations closely associated with disease activity and the occurrence of lupus nephritis.34 Kim et al13 reported that ALCAM expression was enhanced in the serum and lung tissue of allergic asthma models and the sputum and serum of asthma patients, and they speculated that ALCAM might be a potential target. In the present study, we revealed that the ALCAM level was increased in both serum and tissue samples of CRSwNP patients and differentially expressed in eCRSwNP and neCRSwNP. The statistical analysis results demonstrated that serum ALCAM exhibited promising value in predicting CRSwNP endotypes. Therefore, we speculated that ALCAM played a crucial role in eosinophilic inflammation in CRSwNP and contributed to the development of eCRSwNP, but the underlying mechanism remained uncertain. The Th2 cytokines mediated immune response is predominates in the pathogenesis of CRSwNP, and tissue eosinophil infiltration is closely related to the pathological feature of eCRSwNP.8,10,35 ALCAM is an essential molecule for stabilizing the immunological synapses (ALCAM-CD6) between DCs and T cells and promoting DC-mediated T cell polarization toward Th2.12,15 When the nasal mucosa is exposed to irritants, allergens and bacteria, the allergens are captured by DCs, and the expression of ALCAM is enhanced. Elevated levels of ALCAM promote DC activation and Th2 immune response, and then aggravate tissue eosinophilic inflammation.4,36,37 Furthermore, the overexpression of ALCAM in nasal polyps could infiltrate into peripheral blood as a soluble form and increase circulating levels of ALCAM, and the elevated serum ALCMA, in turn, can promote the recruitment of eosinophils into local tissue (Figure 10).
Currently, functional endoscopic sinus surgery remains the mainstream choice when the first line of medical treatment is ineffective in CRSwNP patients.38–40 Although there is a significant improvement in symptoms and quality of life, a large number of patients respond poorly and suffer a high risk of recurrence, especially in eCRSwNP cases.37,41,42 Therefore, predicting the recurrence of CRSwNP is challenging and urgently needed in clinical rhinology. Although several methods and indicators have been reported to be associated with postoperative recurrence, including computed tomography25 and peripheral eosinophilic and basophilic trends,40 there is no objective biomarker with high sensitivity and specificity currently available in clinical practice. Here, we observed that ALCAM levels were significantly enhanced in the serum and tissue of the recurrent CRSwNP group, particularly in recurrent eCRSwNP patients. Additionally, the statistical analysis results showed that serum ALCAM was closely associated with postoperative recurrence. Prior publications have identified that the degree of Th2 inflammation and eosinophil infiltration in tissue were closely correlated with postoperative recurrence in CRSwNP.10,43,44 In our study, we found that the elevated serum ALCAM levels were positively correlated with blood and tissue eosinophilia, and the rate of eCRSwNP was higher in the recurrent group, which suggested that ALCAM might promote eosinophilia and be involved in recurrence. Recent publications have revealed that soluble ALCAM is an inflammatory biomarker and it is associated with eosinophilic inflammation in asthma and allergic rhinitis.16,18 High concentrations of ALCAM in peripheral blood promote DC-dependent Th2 inflammatory responses and enhance tissue eosinophilia, resulting in a poor prognosis and a high risk of recidivation (Figure 10). All the above results implied that serum ALCAM seemed to be a powerful biomarker for preoperatively predicting recurrence in CRSwNP patients.
Some weaknesses should be mentioned in our study. First, this study was limited to a small sample size in a single medical center among patients with the same ethnicity in Changsha, which may increase the risk of selection bias and influence its generalization. Second, there is a lack of international consensus on the diagnostic criteria for eCRSwNP and recurrent CRSwNP. Last, the period of follow-up was relatively short, which may weaken the conclusions. Further nationwide studies with a larger sample size and unified diagnostic criteria are needed to confirm and investigate the role of ALCMA in CRSwNP.
In conclusion, we confirmed an association between ALCAM and CRSwNP. We demonstrated that ALCAM was upregulated and associated with mucosal eosinophilia and CRSwNP recurrence. Our findings also suggested that serum ALCAM could be an effective biomarker to distinguishendotypes and predict postoperative recurrence in CRSwNP patients and provide a novel intervention target to improve precision treatment.
Abbreviations
CRSwNP, chronic rhinosinusitis with polyps; ALCAM, activated leukocyte cell adhesion molecule; HCs, healthy controls; ELISA, enzyme-linked immunosorbent assay; qRT-PCR, quantitative real-time polymerase chain reaction; IHC, immunohistochemistry; ROC, receiver operating characteristic; CRS, chronic rhinosinusitis; CRSsNP, chronic rhinosinusitis without nasal polyps; H&E, hematoxylin and eosin; HPF, high-power fields; SABC, streptavidin-biotin complex; DAB, diaminobenzidine; AUC, area under the curve; ANOVA, one-way analysis of variance.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the recommendations of the Declaration of Helsinki. The Human Ethical Committee of Xiangya Hospital of Central South University approved this study; all participants provided informed consent.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81770985, No. 81800917, and No. 81873695) and the Natural Science Foundation of Hunan Province (No.2020JJ4910).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest in preparing this article.
References
1. Bachert C, Marple B, Schlosser RJ, et al. Adult chronic rhinosinusitis. Nat Rev Dis Primers. 2020;6(1):86. doi:10.1038/s41572-020-00218-1
2. Xu X, Reitsma S, Wang Y, Fokkens WJ. Highlights in the advances of chronic rhinosinusitis. Allergy. 2021;76(11):3349–3358. doi:10.1111/all.14892
3. Hopkins C. Chronic rhinosinusitis with nasal polyps. N Engl J Med. 2019;381(1):55–63. doi:10.1056/NEJMcp1800215
4. Kariyawasam HH. Chronic rhinosinusitis with nasal polyps: insights into mechanisms of disease from emerging biological therapies. Expert Rev Clin Immunol. 2019;15(1):59–71. doi:10.1080/1744666X.2019.1541738
5. Cho SH, Hamilos DL, Han DH, Laidlaw TM. Phenotypes of chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2020;8(5):1505–1511. doi:10.1016/j.jaip.2019.12.021
6. McCormick JP, Thompson HM, Cho DY, Woodworth BA, Grayson JW. Phenotypes in chronic rhinosinusitis. Curr Allergy Asthma Rep. 2020;20(7):20. doi:10.1007/s11882-020-00916-6
7. Delemarre T, Bochner BS, Simon HU, Bachert C. Rethinking neutrophils and eosinophils in chronic rhinosinusitis. J Allergy Clin Immunol. 2021;148(2):327–335. doi:10.1016/j.jaci.2021.03.024
8. Bochner BS, Stevens WW. Biology and function of eosinophils in chronic rhinosinusitis with or without nasal polyps. Allergy Asthma Immunol Res. 2021;13(1):8–22. doi:10.4168/aair.2021.13.1.8
9. Lou H, Zhang N, Bachert C, Zhang L. Highlights of eosinophilic chronic rhinosinusitis with nasal polyps in definition, prognosis, and advancement. Int Forum Allergy Rhinol. 2018;8(11):1218–1225. doi:10.1002/alr.22214
10. Ho J, Hamizan AW, Alvarado R, Rimmer J, Sewell WA, Harvey RJ. Systemic predictors of eosinophilic chronic rhinosinusitis. Am J Rhinol Allergy. 2018;32(4):252–257. doi:10.1177/1945892418779451
11. Sulaj A, Kopf S, Gröne E, et al. ALCAM a novel biomarker in patients with type 2 diabetes mellitus complicated with diabetic nephropathy. J Diabetes Complicat. 2017;31(6):1058–1065. doi:10.1016/j.jdiacomp.2017.01.002
12. Ferragut F, Vachetta VS, Troncoso MF, Rabinovich GA, Elola MT. ALCAM/CD166: a pleiotropic mediator of cell adhesion, stemness and cancer progression. Cytokine Growth Factor Rev. 2021;61:27–37. doi:10.1016/j.cytogfr.2021.07.001
13. Kim YS, Kim MN, Lee KE, et al. Activated leucocyte cell adhesion molecule (ALCAM/CD166) regulates T cell responses in a murine model of food allergy. Clin Exp Immunol. 2018;192(2):151–164. doi:10.1111/cei.13104
14. Oh MS, Hong JY, Kim MN, et al. Activated leukocyte cell adhesion molecule modulates Th2 immune response in atopic dermatitis. Allergy Asthma Immunol Res. 2019;11(5):677–690. doi:10.4168/aair.2019.11.5.677
15. von Lersner A, Droesen L, Zijlstra A. Modulation of cell adhesion and migration through regulation of the immunoglobulin superfamily member ALCAM/CD166. Clin Exp Metastasis. 2019;36(2):87–95. doi:10.1007/s10585-019-09957-2
16. Semitekolou M, Xanthou G. Activated leukocyte cell adhesion molecule: a novel regulator of allergic inflammation in the airways. Am J Respir Crit Care Med. 2018;197(8):973–975. doi:10.1164/rccm.201801-0196ED
17. Kim MN, Hong JY, Shim DH, et al. Activated leukocyte cell adhesion molecule stimulates the T-cell response in allergic asthma. Am J Respir Crit Care Med. 2018;197(8):994–1008. doi:10.1164/rccm.201703-0532OC
18. Xie S, Zhang H, Wang F, et al. Activated leukocyte cell adhesion molecule as a biomarker for disease severity and efficacy of sublingual immunotherapy in allergic rhinitis. Int Immunopharmacol. 2020;88:106975. doi:10.1016/j.intimp.2020.106975
19. Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50(1):1–12. doi:10.4193/Rhino12.000
20. Zhong B, Yuan T, Du J, et al. The role of preoperative blood eosinophil counts in distinguishing chronic rhinosinusitis with nasal polyps phenotypes. Int Forum Allergy Rhinol. 2021;11(1):16–23. doi:10.1002/alr.22636
21. Xie S, Zhang H, Liu Y, et al. The role of serum metabolomics in distinguishing chronic rhinosinusitis with nasal polyp phenotypes. Front Mol Neurosci. 2020;7:593976. doi:10.3389/fmolb.2020.593976
22. Qi S, Yan B, Liu C, Wang C, Zhang L. Predictive significance of Charcot-Leyden Crystal mRNA levels in nasal brushing for nasal polyp recurrence. Rhinology. 2020;58(2):166–174. doi:10.4193/Rhin19.296
23. Brescia G, Barion U, Zanotti C, Giacomelli L, Martini A, Marioni G. The prognostic role of serum eosinophil and basophil levels in sinonasal polyposis. Int Forum Allergy Rhinol. 2017;7(3):261–267. doi:10.1002/alr.21885
24. Lu PC, Lee TJ, Huang CC, Chang PH, Chen YW, Fu CH. Serum eosinophil cationic protein: a prognostic factor for early postoperative recurrence of nasal polyps. Int Forum Allergy Rhinol. 2021;11(4):766–772. doi:10.1002/alr.22664
25. Meng Y, Zhang L, Lou H, Wang C. Predictive value of computed tomography in the recurrence of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2019;9(11):1236–1243. doi:10.1002/alr.22355
26. Mueller SK, Wendler O, Nocera A, et al. Escalation in mucus cystatin 2, pappalysin-A, and periostin levels over time predict need for recurrent surgery in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2019;9(10):1212–1219. doi:10.1002/alr.22407
27. Ma Y, Zheng C, Shi L. The role of YKL40 in the pathogenesis of CRS with nasal polyps. Eur Arch Oto-Rhino-Laryngol. 2018;275(2):431–438. doi:10.1007/s00405-017-4859-2
28. Wang XZ, Wan Z, Xue WJ, Zheng J, Li Y, Ding CG. B-cell activating factor predicts acute rejection risk in kidney transplant recipients: a 6-month follow-up study. Front Immunol. 2019;10:1046. doi:10.3389/fimmu.2019.01046
29. Yang Y, Ma Y, Gao H, et al. A novel HDGF-ALCAM axis promotes the metastasis of Ewing sarcoma via regulating the GTPases signaling pathway. Oncogene. 2021;40(4):731–745. doi:10.1038/s41388-020-01485-8
30. Münsterberg J, Loreth D, Brylka L, et al. ALCAM contributes to brain metastasis formation in non-small-cell lung cancer through interaction with the vascular endothelium. Neuro-Oncology. 2020;22(7):955–966. doi:10.1093/neuonc/noaa028
31. Zarghami N, Soto MS, Perez-Balderas F, et al. A novel molecular magnetic resonance imaging agent targeting activated leukocyte cell adhesion molecule as demonstrated in mouse brain metastasis models. J Cereb Blood Flow Metab. 2021;41(7):1592–1607. doi:10.1177/0271678X20968943
32. Darvishi B, Boroumandieh S, Majidzadeh AK, Salehi M, Jafari F, Farahmand L. The role of activated leukocyte cell adhesion molecule (ALCAM) in cancer progression, invasion, metastasis and recurrence: a novel cancer stem cell marker and tumor-specific prognostic marker. Exp Mol Pathol. 2020;115:104443. doi:10.1016/j.yexmp.2020.104443
33. Michel L, Grasmuck C, Charabati M, et al. Activated leukocyte cell adhesion molecule regulates B lymphocyte migration across central nervous system barriers. Sci Transl Med. 2019;11(518). doi:10.1126/scitranslmed.aaw0475
34. Ding H, Lin C, Cai J, et al. Urinary activated leukocyte cell adhesion molecule as a novel biomarker of lupus nephritis histology. Arthritis Res Ther. 2020;22(1):122. doi:10.1186/s13075-020-02209-9
35. Yılmaz İ. Type 2 chronic rhinosinusitis with nasal polyps: from phenotype to endotype. J Allergy Clin Immunol Pract. 2021;9(1):600–601. doi:10.1016/j.jaip.2020.11.009
36. Heffler E, Malvezzi L, Boita M, et al. Immunological mechanisms underlying chronic rhinosinusitis with nasal polyps. Expert Rev Clin Immunol. 2018;14(9):731–737. doi:10.1080/1744666X.2018.1512407
37. Toro MDC, Antonio MA, Alves Dos Reis MG, de Assumpcao MS, Sakano E. Achieving the best method to classify Eosinophilic Chronic Rhinosinusitis: a systematic review. Rhinology. 2021;59(4):330–339. doi:10.4193/Rhin20.512
38. Sella GCP, Tamashiro E, Sella JA, et al. Asthma Is the Dominant Factor for Recurrence in Chronic Rhinosinusitis. J Allergy Clin Immunol Pract. 2020;8(1):302–309. doi:10.1016/j.jaip.2019.08.007
39. Du K, Zheng M, Zhao Y, et al. A nomogram combing peripheral parameters for estimation of CRSwNP recurrence. Am J Rhinol Allergy. 2021;35:578–586.
40. Brescia G, Contro G, Giacomelli L, Barion U, Frigo AC, Marioni G. Blood eosinophilic and basophilic trends in recurring and non-recurring eosinophilic rhinosinusitis with nasal polyps. Am J Rhinol Allergy. 2021;35(3):296–301. doi:10.1177/1945892420953960
41. Hong SN, Kim YS, Cha H, et al. Endotype-related recurrence pattern of chronic rhinosinusitis in revision functional endoscopic sinus surgery. Auris Nasus Larynx. 2021. doi:10.1016/j.anl.2021.07.010
42. Hopkins C, Lund V. Does time from previous surgery predict subsequent treatment failure in chronic rhinosinusitis with nasal polyps? Rhinology. 2021;59(3):277–283. doi:10.4193/Rhin21.017
43. Bayar Muluk N, Cingi C, Scadding GK, Scadding G. Chronic rhinosinusitis-could phenotyping or endotyping aid therapy? Am J Rhinol Allergy. 2019;33(1):83–93. doi:10.1177/1945892418807590
44. Avdeeva K, Fokkens W. Precision medicine in chronic rhinosinusitis with nasal polyps. Curr Allergy Asthma Rep. 2018;18(4):25. doi:10.1007/s11882-018-0776-8
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.