Back to Journals » Nature and Science of Sleep » Volume 13
Electrophysiological Evaluation in Identifying Unique Sleep Features Among Anti-LGI1 Encephalitis Patients During Active and Recovery Phase
Authors Liu X , Yang L, Han Y , Xu J, Pang Z , Du Y , Feng Y , Lin Y
Received 28 December 2020
Accepted for publication 31 March 2021
Published 3 May 2021 Volume 2021:13 Pages 527—536
DOI https://doi.org/10.2147/NSS.S299467
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ahmed BaHammam
Xiaoyun Liu,1,* Liling Yang,1,* Yuxiang Han,1 Jingjing Xu,2 Zaiying Pang,1 Yifeng Du,1 Yabo Feng,1,* Youting Lin1,*
1Department of Neurology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan City, Shandong Province, 250021, People’s Republic of China; 2Department of Geriatrics, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan City, Shandong Province, 250021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yabo Feng; Youting Lin
Department of Neurology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, No. 324, Jingwu Road, Huaiyin Zone, Jinan City, Shandong Province, 250021, People’s Republic of China
Tel/Fax +86-531‐68776354
Email [email protected]; [email protected]
Objective: The purpose of this study was to illustrate the electrophysiological features of sleep disturbances in patients with anti-leucine-rich glioma-inactivated protein 1 (anti-LGI1) encephalitis in both active and recovery stages.
Methods: Retrospectively filed video electroencephalogram (VEEG) and polysomnography (PSG) data in 24 patients with anti-LGI1 encephalitis were analyzed in comparison with that in 20 individuals without sleep disorders as control group.
Results: Sleep efficiency (SE) and total sleep time involving REM and NREM sleep were significantly decreased in patients with anti-LGI1 encephalitis during the active stage compared to that during the recovery stage and in the control group. Imbalanced sleep structure was found, demonstrated by elevated N1, decreased N3 and REM components, as well as abnormal N2 structure characterized with significantly lower spindle duration and density during the active stage. These findings were independent of the presence of nocturnal episodic events or sleep hyperkinetic movements (HMs). HMs were present in 11/23 patients throughout NREM and REM sleep (nonspecific in sleep stages) during the active stage. During the recovery stage, SE and sleep structures were dramatically improved, including the percentage of N3 and REM sleep, spindle duration and density. Ten of 11 patients with HMs were followed up. HMs were totally remitted in 3 patients and still persistent in 1, while evolved into REM sleep behavior disorder (RBD) in 4 with comorbid periodic limb movement syndrome (PLMS) in 3/4, and only PLMS in 2.
Conclusion: Sleep disturbances were remarkable and intrinsic features in active anti-LGI1 encephalitis, marked by overall disruptions of both NREM and REM sleep, as well as the presence of HMs, which tend to evolve into RBD or PLMS during the recovery stage. Long-term follow-up with PSG is needed, especially for those patients with severe sleep disturbances during the active phase.
Keywords: anti-leucine-rich glioma-inactivated protein 1 encephalitis, video electroencephalogram, polysomnography, sleep disturbances, REM sleep behavior disorder, periodic limb movement in sleep
Plain Language Summary
Polysomnography (PSG) studies in patients with anti-leucine-rich glioma-inactivated protein 1 (anti-LGI1) encephalitis are rare in the literature, though sleep disorder is a common presentation. We have systematically conducted video electroencephalogram (VEEG) and PSG monitoring in patients with anti-LGI1 encephalitis during active and recovery stages, in attempt to delineate the electrophysiological features associated with anti-LGI1 encephalitis. Severe sleep disturbances were discovered during the active stage, marked by overall disruption of both NREM and REM structures, as well as the presence of sleep hyperkinetic movements (HMs), all of which responded well with immunotherapy. HMs tend to evolve into RBD or PLMS during the recovery stage and deserve a long-term follow-up.
Introduction
Sleep disorder has been recognized as a common presentation ranging from 45.3% to 73% in autoimmune encephalitis,1,2 with higher prevalence in voltage-gated potassium channel complex (VGKC) autoimmunity (93%). However, sleep disorder was reported in only 65%~86% patients with anti-leucine-rich glioma-inactivated protein 1 (anti-LGI1) encephalitis, a subtype of VGKC, with lower occurrence than that in VGKC.2–4 Therefore, sleep disturbance has been probably overlooked in anti-LGI1 encephalitis.
In VGKC autoimmune encephalitis, the commonly reported disturbances in sleep and associated electrophysiological features include lowered sleep efficiency (SE), loss of slow wave sleep (SWS), rapid eye movement (REM) sleep3 and occurrence of REM sleep behavior disorder (RBD).3,5 Immunologically, VGKC autoimmune encephalitis has two main subtypes: LGI1 and contactin-associated protein–2 (CASPR2), targeting LGI and CASPR2 membrane receptors respectively and thus associated with distinctive clinical features. CASPR2 is closely linked with Morvan syndrome and sleep manifestations whereas LGI1 is closely associated with limbic encephalitis, although their spectrum could overlap to some extent.5–7 To date, only several case series exist regarding polysomnography (PSG) studies on anti-LGI1 encephalitis mainly demonstrating a fragmenting sleep architecture, alteration in spindle activity, abnormal SWS and REM, dream enactment and RBD.2,8 Thus, further systematic investigations are needed so as to elucidate details about the electrophysiological characteristics, and their underlying mechanisms and potential biomarkers in anti-LGI1 encephalitis.
We have defined hyperkinetic movements (HMs) as excessive movements including simple actions such as diffused and migrating myoclonic or twisting movements, and complex movements mimicking daily life activities, which predominantly occur at the beginning of light sleep and can nearly last throughout the sleep state in severe cases.9 Although HMs can also be detected during the resting awake state, the close association of HMs with sleep has led to the hypothesis that HMs could be a sort of sleep disturbance. For further confirming such a hypothesis, studies on the evolution of the semiology of HMs are needed.
We have systematically conducted video electroencephalogram (VEEG) and PSG monitoring in patients with anti-LGI1 encephalitis in both active and recovery stages from January 2015, in attempt to delineate the electrophysiological features associated with anti-LGI1 encephalitis. In this study, we analyzed the retrospectively filed VEEG and PSG data in patients with anti-LGI1 encephalitis including active and recovery stages in an attempt to demonstrate the features of both NREM and REM sleep structures in association with clinical pictures including responses to immunotherapy.
Methods
All the data were from the Department of Neurology, Shandong Provincial Hospital Affiliated to Shandong First Medical University from January 2015 to December 2019. The study was approved by the ethics committee of the Shandong Provincial Hospital affiliated to Shandong First Medical University, and conducted according to the Declaration of Helsinki principles. Written informed consent was provided by all participants.
Overall, 24 consecutive patients with anti-LGI1 encephalitis were enrolled as encephalitis group, all of whom had at least one overnight VEEG monitoring session. Thirteen patients were studied during both active and recovery stages, 6 were monitored only during active stage and 5 only during recovery stage. Three and 13 patients had undergone PSG during active phase and recovery phase respectively. All patients were treated with corticosteroid, of whom, 12 plus intravenous immunoglobulin and 3 plus intravenous immunoglobulin and rituximab. Twenty individuals without sleep disorders were included as control group undergoing overnight VEEG monitoring with normal EEG under the same conditions. The monitoring procedure is shown in Figure 1.
 |
Figure 1 Flow chart of the study. |
All patients fulfilled the diagnostic criteria of anti-LGI1 encephalitis proposed by Graus et al.10 Long-term follow-up was performed by telephone approximately once every 3 months and at the clinic every 6 months.
NIHON KOHDEN E1200 EEG was applied for VEEG and PSG monitoring. We recorded electrooculogram and chin electromyogram in addition to the standard 21 electroencephalogram channels according to the International 10–20 System. Recording parameters included the sampling rate at 200 Hz, the low filter at 1.6 Hz and the high filter at 70 Hz. During PSG monitoring electrooculogram and chin electromyogram were routinely recorded. We did not use routine 6 EEG channels recording during PSG considering the channels were too few to distinguish epileptic events from abnormal sleep behaviors. For PSG recordings, extremity electromyogram, naso-oral airflow, thoracic and abdominal effort, and oxygen saturation by pulse oximeter were applied in addition to the above VEEG montages to reveal sleep-related events. Macrostructure of sleep and associated events were analyzed using Polysmith software according to AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications and AASM interpretation manual 2.1,11 and the following macrostructure variables were determined: total sleep time (TST), time and percentage in individual NREM sleep stages (N1, N2, N3) and REM sleep, sleep efficiency (SE), and arousal index (AI). Sleep spindle characteristics were manually identified from the longest periods of N2 stage sleep, including spindle duration, spindle frequency and spindle density. Sleep onset was identified by disintegration of the posterior dominant rhythm (PDR), and sleep stages were recognized by the sleep physiological markers (such as vertex sharp-wave, K complex, spindle, and non-reading rapid eye movement with atonia) for patients who had persistent slow EEG activities during wakefulness. Sleep parameters were independently analyzed by two experienced clinical electrophysiologists, and ambiguous points were discussed to ultimately achieve agreement.
All statistical analyses were performed using R, version 3.5.3. P <0.05 was considered to indicate a statistically significant difference.
Results
Clinical Characteristics
Twenty-four patients (21males) with anti-LGI1 encephalitis were enrolled in this study. The median age at disease onset was 53.5 years old (range 28~70), and the median duration from onset to admission was 116 days (range 7~540). The common clinical symptoms included cognitive decline (95.8%), seizures (95.8%), sleep disorders (70.8%), autonomic symptoms (58.3%), faciobrachial dystonic seizures (FBDS) (45.8%), and abnormal behavior (delirium, confabulation, hallucination, apathy, personality change and self-mutilation) (33.3%). MRI anomalies were found in 22 of 24 patients (91.7%) with T2-FLAIR or T1 hyperintensity. Details are shown in Table 1.
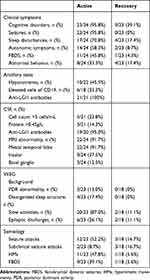 |
Table 1 Clinical Characteristics of Anti-LGI1 Encephalitis |
One patient was lost to follow-up, and 23 patients were followed for a median of 94 (17~268) weeks. Memory impairments were reported in 9 patients, personality changes in 4 patients, sleep disorders in 4 patients, hyperhidrosis in 2 patients, and FBDS in 1 patient. EEG seizures were discovered in 3/18 patients, but no clinical seizures were reported in all patients during the recovery period.
Sleep quality declined by a score >5 in 8 out of 21 patients who completed the Pittsburgh Sleep Quality Index (PSQI) during the recovery period at a median of 94 (17~268) weeks although sleep disorder was not a common complaint. Three patients experienced relapses. One patient relapsed at the 28th week (1 month after withdrawal of 15 mg prednisone) presenting with monthly FBDS which remained until the last visit at the 90th week even after further immunotherapy was administered. One relapsed at the 60th week after thoracic vertebra fracture, presenting with generalized seizure attacks, insomnia, HMs, and visual hallucination. One relapsed at the 30th week, two weeks after withdrawal of 10 mg prednisone because of an accident involving third-degree burns over 50% of total surface area, and presented with delirium, hallucination and HMs (under immunotherapy at the last follow-up of this study).
Routine EEG Characteristics
EEG recordings were conducted in 23 patients (daytime EEG 4, overnight EEG 19) during the active phase, lasting an average of 12.7 hours (range 4.5~23); in 18 patients during the recovery phase at a median of 23.5 (12~198) weeks, lasting an average of 13.1 hours (range 8.5~16.5).
During the active phase, EEG recordings showed an abnormal posterior dominant rhythm (PDR) in 3/23 patients. Persistent slow activities were found in 7 patients, and paroxysmal slow activities were found in 13 patients. Focal interictal discharges were detected in 6 patients, and periodic discharges were recorded in 2 patients. Overall episodic events including epileptic events and FBDS were recorded in 17 patients. FBDS were recorded in 9 patients, with a frequency ranging from 0.2 to 14 times per hour, involving bilateral limbs in 6/9 patients. Seventeen types of seizures were recorded in 12 patients, with a frequency ranging from 0.2 to 11.6 times per hour. During the recovery stage, paroxysmal slow activities were observed in 2 patients, epileptic discharges in 2 patients, and EEG seizures in 3 out of 18 patients.
Sleep Electrophysiology
In the 23 patients during the active phase, sleep staging could not be made in 2 patients due to the absence of physiological markers (such as PDR, K complex and spindles), and in 1 patient due to persistent delirium status resulting in sleep absence in two overnight recordings. HMs were observed in 11/23 during both awake and sleeping states, activated immediately by light sleep without NREM/REM specificity except for one patient who presented HMs during awaking and REM sleep but not during NREM sleep. Among the 11 patients with HMs, 8 patients were absent of REM sleep and all the other 3 patients with REM sleep displayed REM sleep behavior disorder (RBD). VEEG recordings were completed in 10 of the 11 patients during recovery stage. HMs were totally remitted in 3 patients and still persistent in 1, while evolved into REM sleep behavior disorder (RBD) in 4 with comorbid periodic limb movement syndrome (PLMS) in 3/4, and only PLMS in 2. Those patients without HMs during the active phase had no REM without atonia (RWA) or PLMS in either the active or recovery stages.
Of the 3 patients undergoing PSG during the active phase, 1 had obstructive sleep apnea hypopnea syndrome (OSAHS). Of the 13 patients undergoing PSG during the recovery phase, 8 have moderate to severe OSAHS and 1 had mild OSAHS.
Quantitative analysis of sleep structures during the active stage was conducted in 17 patients having identifiable sleep stages during overnight monitoring. Based on their overnight sleep parameters, fragmented and imbalanced sleep structure was a distinctive feature in the active cohort compared with the controls (Figure 2). SE was significantly decreased (p value 0.0001). TST, NREM and REM duration were significantly shortened (p values 0.0006, 0.0148, and <0.001 respectively). Increased AI (p value 0.0007) and imbalanced sleep structures, with an increased percentage of N1 and decreased percentage of N3 and REM (p values <0.001, 0.0005 and 0.0002, respectively) were observed. In addition, the longest N2 stage sleep, spindle duration and spindle density were significantly lower than those of the controls (p values 0.007, <0.001, and 0.001, respectively) (Table S1). Sleep parameters had no significant differences between subgroups with or without episodic events, nor did those between subgroups with or without HMs (Figure 3, Tables S2 and S3). During the recovery phase, SE (p value 0.0339) and sleep structure including duration and percentage of N3 (p values 0.0139, 0.0192, respectively), REM sleep (p values 0.0073, 0.007, respectively) and spindle duration and density (p values <0.001, 0.0012, respectively) were dramatically improved compared with those during the active phase. The recovery group had lower TST and NREM than controls (Table S1) but sleep architecture, constitution and spindle parameters were not significantly different between the two groups.
Discussion
To our knowledge, this is the first study conducting quantitative controlled analysis of sleep structures in order to identify detailed features of sleep disturbances in both active and recovery stages of anti-LGI1 encephalitis. Sleep-activated HMs were common presentations during the active stage, and the main EEG features could be summarized as follows: decreased sleep efficiency, fragmented sleep structure, and highly disorganized sleep background with loss of spindles, SWS, and REM sleep. Sleep disturbance responded well to immunotherapy, however, should be closely monitored during long-term follow-up.
This quantitative controlled analysis of sleep structures based on EEG features revealed a highly disorganized macrostructure of sleep during the active stage of anti-LGI1 encephalitis. Compared to the control group, patients during active stage had severe loss of spindles, SWS and REM sleep, suggesting that the evolution of sequential sleep stages was disturbed and reaching deeper stages became more challenging.3,12,13 We previously reported that there are abundant nocturnal episodic events during active phase of anti-LGI1 encephalitis, thus it is plausible to attribute the disorganized sleep structure to sleep interruption by these motor events. However, as described in this study, patients without motor events still encounter pronounced disruption of sleep structure during the active phase, suggesting that sleep instability in the anti-LGI1 group was independent of the presence of motor events. In other words, the sleep disturbances could be an intrinsic consequence of encephalitis targeting sleep-associated structures.
There are several types of descriptions regarding the frequent nocturnal motor overactivity in patients with anti-LGI1 encephalitis, such as restless sleep, RBD and HMs. Our study showed that motor overactivity could be observed during wakefulness besides during sleep, so we have referred these motor overactivity as HMs, which has been thoroughly discussed in a previous study.9 According to our observations, with the disease evolving into recovery stage, HMs remitted in most cases but they tended to transform into RBD and/or PLMS. We postulated that HMs might be part of the sleep disturbance spectrum sharing some similar underlying mechanisms with RBD/PLMS. Nevertheless, HMs fulfilled the diagnostic criteria for neither PLMS nor RBD during the active phase (as shown in Figures 4 and 5). In detail, the features of HMs include nearly sustained and nonstereotyped muscle movements with a predominance in upper limbs without difference between NREM and REM sleep, which are distinctive from the core features of PLMS (priority, leg-predominance and partial inhibition in REM). In addition, some features of HMs can be used to distinguish HMs from typical RBD. These features include lack of dream recall, enactment and violent actions, and most importantly, bursts of EMG potentials starting from NREM. Thus, RWA in these patients seemed more likely to be an extension of HMs from awaking/NREM to REM sleep but not a REM-specific phenomenon like RBD. RWA was observed in only 3 patients with HMs and occurred much less frequently during the active than recovery stage, which might be due to severely disrupted sleep structure with such a limited REM sleep time in the former group. In fact, the remaining eight patients with HMs had no REM sleep, so prolonged monitoring might be necessary to characterize motor events in REM sleep.
Status dissociates (SD), also called agrypnia excitata, characterized by indistinctive awake/NREM/REM state boundaries, loss of conventional features of NREM sleep (spindles and SWS), unstable REM sleep and motor overactivation, has been observed in patients with prion diseases, delirium tremens, neurodegeneration and VGKC encephalitis.14–17 The results of this study indicate that sleep disturbances associated with anti-LGI1 encephalitis are congruent to the definition of SD. That disruption of sleep components could also be detected in the subgroup without HMs, indicating that the overall disintegration and disarrangement of sleep structure is an essential nature of SD independent of the presence of HMs which though might be a marker of severity and were prone to evolve into RBD/PLMS.
The mechanism underlying SD has been unclear. Some authors presumed that the occurrence of SD is related with dysfunction of thalamolimbic system releasing the hypothalamus and brainstem reticular formation from cortico-limbic inhibitory control.15,17 Apart from aforementioned diseases with diffuse lesions, SD has been reported in a patient after subtotal removal of a silent cavernoma in the ponto-mesencephalic tegmentum,18 indicating that brainstem could play a key role in SD. The fact that anti-LGI1 encephalitis can involve limbic system, motor cortex, striatum thalamus and brainstem as mainly immunological targets is consistent with these hypotheses.9,19 This is also supported by findings in mouse that LGI1 is widely expressed in neurons and some axonal terminals throughout the CNS including the limbic cortex, hypothalamus, locus coeruleus, raphe nuclei and thalamus.7 Thus, we postulate that the disruption of sleep structure in anti-LGI1 encephalitis might be due to an imbalance of neuronal inhibition and excitation in the cortex-basal ganglia/thalamus-brain stem network leading to dysfunction in the critical regions associated with the generation and consolidation of sleep.
Our case–control study further confirmed the finding in some case series that sleep events could dramatically respond to immunotherapy as demonstrated by both complaints and PSG evaluation.3,5 However, the potential long-term consequences of anti-LGI1 encephalitis on sleep deserved attention since a study of more than 2 years follow-up showed that persistent insomnia existed in 21% patients.4 In this study, PSG monitoring demonstrated RBD, PLMS and shortened TST during the recovery stage, also indicating a long-term sleep disturbance. In our series, OSAHS was rather common in patients having no previous history of snoring or daytime somnolence. We speculate that the increase body mass index caused by glucocorticoids may contribute to the increased risk of OSAHS.
Our study showed that relapses occurred at the 28~60th week after the initiation of immunotherapy, probably related to some active stress events or non-standard immunotherapy. In some cases, it is a challenge to make a diagnosis of relapse because the manifestations indicating relapse such as cognitive decline, FBDS, sleep disturbances and EEG anomalies during the recovery stage are easily mistaken as residual ones from the active stage. For instance, relapses had been suspected based on the presentation of EEG seizures in one patient but later was excluded after screening for antibodies in serum and CSF. In contrast, HMs, combined with the disorganized sleep structures and the disruption of NREM sleep biomarkers such as spindles and SWS, might be more convincing in indicating relapses. However, studies with larger samples and longer follow-up periods are needed to confirm such a hypothesis.
A main pitfall in this study was the small number of sample and the limited PSG montages especially during the active stage, mainly due to patients’ intolerance to PSG or EEG monitoring before immunotherapy. Wireless wearable devices could help increase the feasibility of obtaining those data in the future. Another drawback was that the PSG monitoring time-points in relation to disease process were inconsistent among all patients during recovery stage. Strictly designed longitudinal studies with precise time points could probably provide a more reliable profile of these abnormal sleep-related events during the recovery stage.
In conclusion, sleep disturbance could be a remarkable and intrinsic feature for active anti-LGI1 encephalitis, marked by sleep fragmentation, ambiguous or total loss of sleep physiological rhythms, disrupted organization of both NREM and REM sleep, and the presence of HMs. HMs are likely to evolve into RBD and PLMS during the recovery stage, thus long-term follow-up with PSG is needed, especially for those patients with severe sleep disturbances during the active phase.
Abbreviations
AI, arousal index; Anti-LGI1, anti-leucine-rich glioma-inactivated protein 1; CASPR2, contactin-associated protein-2; CSF, cerebrospinal fluid; FBDS, faciobrachial dystonic seizures; HMs, hyperkinetic movements; OSAHS, obstructive sleep apnea hypopnea syndrome; PDR, posterior dominant rhythm; PLMS, periodic limb movement in sleep; PSG, polysomnography; PSQI, Pittsburgh Sleep Quality Index; RBD, REM sleep behavior disorder; REM, rapid eye movement; RWA, REM without atonia; SD, status dissociates; SE, sleep efficiency; SWS, slow wave sleep; TST, total sleep time; VEEG, video electroencephalogram; VGKC, voltage-gated potassium channel complex.
Acknowledgments
Xiaoyun Liu and Liling Yang should be regarded as co-first authors. Yabo Feng and Youting Lin should be regarded as co-corresponding authors. The authors are grateful to the patients for their participations in our study. This paper was completed by the efforts of all authors. The authors are grateful to Dr Xu Shangchen for editing language.
Funding
Funding for this work has been provided by Natural Science Foundation of Shandong Province (No. ZR2020MH152).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Deng S, Qiu K, Liu H, Wu X, Lei Q, Lu W. Clinical characteristics and short-term prognosis of autoimmune encephalitis: a single-center cohort study in Changsha, China. Front Neurol. 2019;10:539. doi:10.3389/fneur.2019.00539
2. Blattner MS, de Bruin GS, Bucelli RC, Day GS. Sleep disturbances are common in patients with autoimmune encephalitis. J Neurol. 2019;266:1007–1015. doi:10.1007/s00415-019-09230-2
3. Cornelius JR, Pittock SJ, McKeon A, et al. Sleep manifestations of voltage-gated potassium channel complex autoimmunity. Arch Neurol. 2011;68:733–738. doi:10.1001/archneurol.2011.106
4. van Sonderen A, Thijs RD, Coenders EC, et al. Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology. 2016;87:1–8.
5. Iranzo A, Graus F, Clover L, et al. Rapid eye movement sleep behavior disorder and potassium channel antibody-associated limbic encephalitis. Ann Neurol. 2006;59:178–181. doi:10.1002/ana.20693
6. Silber MH. Autoimmune sleep disorders. Handb Clin Neurol. 2016;133:317–326.
7. Irani SR, Pettingill P, Kleopa KA, et al. Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol. 2012;72:241–255. doi:10.1002/ana.23577
8. Serdaroğlu E, Tezer Fİ, Saygi S. Autoimmune epilepsy and/or limbic encephalitis can lead to changes in sleep spindles. Noro Psikiyatr Ars. 2018;55:320–324. doi:10.5152/npa.2017.19442
9. Liu X, Han Y, Yang L, et al. The exploration of the spectrum of motor manifestations of anti-LGI1 encephalitis beyond FBDS. Seizure. 2020;76:22–27. doi:10.1016/j.seizure.2019.12.023
10. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. doi:10.1016/S1474-4422(15)00401-9
11. American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events summary of updates in version 2.1; 2014. Available from: https://aasm.org/clinical-resources/scoring-manual/.
12. Barone DA, Krieger AC. Sleep disturbances in voltage-gated potassium channel antibody syndrome. Sleep Med. 2016;21:171–173. doi:10.1016/j.sleep.2015.11.012
13. Lin N, Hao H, Guan H, et al. Sleep disorders in leucine-rich glioma-inactivated protein 1 and contactin protein-like 2 antibody-associated diseases. Front Neurol. 2020;11:696. doi:10.3389/fneur.2020.00696
14. Lugaresi E, Provini F, Cortelli P. Agrypnia excitata. Sleep Med. 2011;12(Suppl 2):S3–10. doi:10.1016/j.sleep.2011.10.004
15. Provini F. Agrypnia excitata. Curr Neurol Neurosci Rep. 2013;13:341. doi:10.1007/s11910-013-0341-8
16. Hazin R, Abuzetun JY, Giglio P, Khan F. Agrypnia excitata: current concepts and future prospects in management. J Neuropsychiatry Clin Neurosci. 2009;21:126–131. doi:10.1176/jnp.2009.21.2.126
17. Antelmi E, Ferri R, Iranzo A, et al. From state dissociation to status dissociatus. Sleep Med Rev. 2016;28:5–17. doi:10.1016/j.smrv.2015.07.003
18. Provini F, Vetrugno R, Pastorelli F, et al. Status dissociatus after surgery for tegmental ponto-mesencephalic cavernoma: a state-dependent disorder of motor control during sleep. Mov Disord. 2004;19:719–723. doi:10.1002/mds.20027
19. Navarro V, Kas A, Apartis E, et al. Motor cortex and hippocampus are the two main cortical targets in LGI1-antibody encephalitis. Brain. 2016;139:1079–1093. doi:10.1093/brain/aww012
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




