Back to Journals » Journal of Inflammation Research » Volume 16
Electroacupuncture Pretreatment Attenuates Learning Memory Impairment Induced by Repeated Propofol Exposure and Modulates Hippocampal Synaptic Plasticity in Rats
Authors Fan S , Wang X, Gao N, Wei S
Received 24 July 2023
Accepted for publication 27 September 2023
Published 16 October 2023 Volume 2023:16 Pages 4559—4573
DOI https://doi.org/10.2147/JIR.S427925
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Shunqin Fan, Xijun Wang, Ning Gao, Songli Wei
Department of Anesthesiology, International Zhuang Medical Hospital Affiliated to Guangxi University of Traditional Chinese Medicine, Nanning, People’s Republic of China
Correspondence: Xijun Wang, Department of Anesthesiology, International Zhuang Medical Hospital affiliated to Guangxi University of Traditional Chinese Medicine, No. 8 Qiu Yue Road, Liang Qing District, Nanning, Guangxi, People’s Republic of China, Email [email protected]
Background: Recurrent propofol anesthesia in the peak of neurodevelopment may lead to learning-memory decline. This study aimed to examine the efficacy of electroacupuncture pretreatment in ameliorating the aforementioned learning memory deficits and to explore its underlying mechanisms in a rat model of repeated propofol exposure.
Methods: 10-day-old Sprague Dawley rats were randomly assigned to five groups: the control, fat emulsion, propofol, electroacupuncture pretreatment and electroacupuncture pretreatment combined with propofol groups. The electroacupuncture pretreatment involved three consecutive daily sessions, while propofol was received intraperitoneally once daily for five days. Following the modeling period, the rats’ learning-memory performance was assessed using the New Novel Arm Y-maze, New Object Recognition, and Morris Water Maze. The Nissl staining method was used to observe the development of hippocampal neurons, while Golgi staining was employed to observe hippocampal synaptic development.
Results: The electroacupuncture pretreatment significantly attenuated the learning and memory impairment induced by recurring propofol exposure in rats. Additionally, it facilitated the development of hippocampal neurons and synaptic plasticity in the hippocampus. Immunofluorescence and Western Blot analyses were conducted to detect the expression of proteins related to apoptosis, learning memory, and synaptic plasticity. In the propofol group, the pro-apoptotic factors Caspase-3 and Bax was up-regulated, while the anti-apoptotic factor Bcl-2 was down-regulated, as compared to the blank group. Additionally, the phosphorylated cAMP-response element binding protein (pCREB), brain-derived neurotrophic factor (BDNF), synaptophysin, and growth associated protein-43 (GAP-43) was significantly decreased. In contrast, the electroacupuncture pretreatment combined with propofol group exhibited decreased the Caspase-3 and Bax and increased the Bcl-2, as compared to the propofol group, meanwhile, the pCREB, BDNF, Synaptophysin and GAP-43 was increased.
Conclusion: Our findings indicate that electroacupuncture pretreatment can alleviate the learning and memory impairment induced by recurring propofol exposure in rats. This is achieved by enhancing hippocampal synaptic plasticity, activating the pCREB/BDNF pathway and inhibiting neuronal apoptosis.
Keywords: electroacupuncture pretreatment, propofol, learning memory, synaptic plasticity
Introduction
Millions of infants, toddlers, and preschoolers undergo general anesthesia annually for various surgical procedures in China, and propofol is the most frequently used drug for this purpose. Although propofol has been deemed safe for the adult brain in current clinical practice, it is unclear whether it is safe for the developing brain. Multiple animal studies have reported that high doses of propofol or repeated exposures to the drug can cause long-term learning and memory impairment in animals.1–4 The toxic effects of propofol are predominantly evidenced by the apoptosis of hippocampal neurons, degradation of synaptic structures, and neuroinflammatory responses, with the developing brain displaying a heightened susceptibility to such effects.5,6 The developing brain exhibits diminished compensatory responses to injurious stimuli and heightened susceptibility to anesthesia-induced neurotoxicity.7,8 In humans, the developing brain denotes the period from the third trimester of pregnancy until three years of age, whereas in rats, the peak neuronal development occurs around one to two weeks post-birth.9,10 Although propofol’s use in infant and child anesthesia has been extensive with low incidence of adverse reactions, safety concerns surrounding its use warrants substantial attention. Consequently, the cellular mechanisms and signaling pathways mediating propofol-induced learning memory impairment remain unclear. Consequently, various protective measures to mitigate such impairment have emerged in recent year.11–13
The formation and maintenance of learning memory significantly relies on the hippocampal synaptic plasticity.14 Propofol-induced inhibition of hippocampal neural circuits decreases hippocampal synaptic plasticity and disrupts synaptic signaling, resulting in impaired learning and memory.15 The study observed that16 high doses of propofol resulted in reduced total dendritic count and length along with neuronal apoptosis. As a crucial component of synaptic plasticity, dendrites significantly impact neural signaling and impairments in dendritic development and restricted nerve signal transmission can significantly affect synaptic plasticity.17 Current research is focused on addressing the key issue of restoring synaptic plasticity and mitigating learning memory impairment that may arise from repeated propofol exposure.
Electroacupuncture has been applied to childhood illnesses with a long-term prognosis, such as Autism Spectrum Disorder and Anorexia Nervosa.18,19 Furthermore, studies have found that electroacupuncture can treat chronic urinary retention caused by lower motor neuron damage following pediatric lumbosacral surgery.20 Electroacupuncture, which combines traditional Chinese acupuncture with modern electrical stimulation, offers the advantages of being a simple, safe, and easily operable technique.21 By modulating the frequency, intensity, and waveform of stimulation, electroacupuncture effectively regulates the internal environment and suppresses central inflammation, thus demonstrating potential efficacy in models of brain injury.22,23 Previous reports suggest that stimulating the “Baihui” point (GV20) through electroacupuncture can increase dopamine levels in the cerebral cortex and hippocampus of SD rats. Moreover, such stimulation can also regulate neuronal plasticity, potentially reducing the degree of post-ischemic brain atrophy.24 Stimulation of the “Baihui” and “Dazhui” (GV14) points through electroacupuncture can effectively regulate the Bcl-2/Bax ratio, leading to upregulation of anti-apoptotic protein levels. As a result, electroacupuncture can prevent neuronal apoptosis.25,26 Furthermore, electroacupuncture was found to enhance the expression of CREB protein and facilitate the repair of synaptic plasticity in the hippocampus of rats modeling post-traumatic stress disorder.27 CREB proteins play a crucial role in the regulation of synaptic plasticity and memory formation.28 Rats exhibiting cognitive and memory impairment demonstrated a significant reduction in hippocampal expression of pCREB.29 The cerebral protective effects of pCREB are carried out through the regulation of various genes, including BDNF.30
BDNF plays a crucial role in preserving cellular survival and modulating synaptic function.31,32 Studies suggest that enhancing hippocampal expression of BDNF is strongly correlated with improved learning and memory.33 The modulation of synaptic plasticity is a significant function of the pCREB/BDNF signaling pathway. Our hypothesis posits whether pretreatment with electroacupuncture can mitigate propofol-induced learning and memory impairment in rats by repairing synaptic plasticity and suppressing neuronal apoptosis via this pathway.
Materials and Methods
Animals
The Ethics Committee of Guangxi University of Traditional Chinese Medicine approved this experimental study (DW20230313-036). We obtained the 7-day-old Sprague Dawley rats (14–16g) from Changsha Tianqin Biotechnology Co. Ltd., China, for our experiments. We purchased a total of 8 cages, with 12 rats per cage. After screening, the rats were randomly divided into 5 groups, with a male-to-female ratio of 2:1 in each group. In addition, we mated 6 female rats at 8 weeks of age with male rats at a ratio of 1:2 to produce offspring. After reaching 7 days of age, the offspring were randomly divided into 5 groups at a male-to-female ratio of 2:1, with each group consisting of 3 rats. In the end, a total of 105 rats were included in the experiment (n=21/group). The surplus animals were properly disposed of, and none of the animals included in the experiment died. We placed 7-day-old Sprague Dawley rats with their dams in a specialized animal facility that maintained a 12-hour light/dark cycle and a room temperature of 20–22°C. They were adaptively fed for 3 days in this facility. The rats were provided ad libitum access to drinking water and feed. Following post-anesthesia resuscitation, we placed the rats in a separate incubator and returned them to their dams after they autonomously regained full body movements.
Experimental Groups and Interventions
Both the electroacupuncture pretreatment group and the electroacupuncture pretreatment compound propofol group received electroacupuncture for three consecutive days, once daily. We administered electroacupuncture pretreatment using an SDZ-II electronic needle therapy instrument (Hua-Tuo brand, Suzhou Medical Supplies Factory Co., Ltd.). We targeted the “Baihui” and “Dazhui” points for electroacupuncture pretreatment. The “Baihui” point is located at the midpoint of the line connecting the anterior edges of the two ears and lies in the middle of the parietal bone. The “Dazhui” point is situated between the seventh cervical vertebra and the first thoracic vertebra in the middle of the back. We inserted the needle subcutaneously to a depth of 2–3mm at a 45° angle to the skin. For effective acupoint stimulation, we used stimulation parameters of 1 mA intensity, 2/15 Hz sparse and dense wave, applied for 30 min. Additionally, we observed a slight tremor of the skin tissue around the stimulation point. Both the propofol and the electroacupuncture pretreatment combination propofol groups received intraperitoneal injections of 100 mg/kg propofol daily for five consecutive days after the electroacupuncture pretreatment period. The anesthesia was induced with an initial injection of 50 mg/kg propofol, followed by another 50 mg/kg when the righting reflex was restored. Our preliminary study identified that this anesthesia method produces sedation and hypnosis lasting up to 4–5 hours while considering the respiratory and cardiovascular suppression effects of propofol. Following anesthesia, the rats were positioned in a pre-oxygenated incubator atop a thermostatic heating blanket. The fat emulsion group received 0.1 mL peritoneal injections of fat emulsion daily for five successive days, whereas the blank group received 0.1 mL saline injections following the same protocol (Figure 1).
Y Maze
The Y maze was conducted using the BW-MYM103 equipment (bio-will co., Ltd, China), which is comprised of three 120° angled arms, each arm measured 30cm in length, 8cm in width, and 12cm in height. The novel arm, starting arm, and other arms were arranged sequentially within this structure. The experiment consisted of two distinct phases. During the first phase, the novel arm was obstructed by a partition, and the rats were permitted to explore the starting arm and remaining arms for a period of three minutes. After a two-hour interval, the second phase commenced by removing the partition of the novel arm, enabling the rats to freely explore all three arms for an additional three minutes. The process involved recording the time and path of each rat’s entry into the novel arm to evaluate their spatial recognition memory. Following each experiment, the labyrinth underwent a thorough wipe-down with 70% alcohol and was allowed to evaporate completely prior to subsequent testing. Randomly select 6 rats from each group (n=6) with a male-to-female ratio of 1:1 for this experiment.
New Object Recognition
The second day after the completion of the Y-maze, we conducted a new object recognition experiment using the BW-OF302 apparatus (bio-will co., Ltd, China), which included a black box (50cm x 50cm x 40cm) and two object blocks. This experiment consisted of two phases. During the initial phase, two square object blocks (each measuring 4cm x 4cm x 4cm) were placed at the top-left and top-right corners of the box, each situated 6 cm from the adjacent sides of the box. The rats were permitted to explore freely for five minutes. In the subsequent stage, after a two-hour interval, the square object block located in the top-left corner was replaced with a spherical object block (5cm in diameter), while maintaining its position. During the 5-minute exploration, we recorded the number of times that the rats sniffed and touched the new object block, with a designated criterion being either nasal tip or upper limb touch. The recognition index (RI), calculated as the time spent exploring the new object divided by the sum of the time spent exploring both the new and old objects, served as an indicator of the rats’ ability to learn and remember. The rats (n=6) that completed the Y-maze will continue with this experiment.
Morris Water Maze
The second day after the completion of the NOR test. The Morris Water Maze test was conducted using the BW-MMWM101 equipment (bio-will co., Ltd, China), which comprised a circular pool (120cm in inner diameter), a platform (10cm), and a video capture system. The pool was sectioned into four quadrants, and the test was performed in a noise-free environment, while maintaining the water temperature at 23°C. To prevent the rats from visually detecting the platform in the pool, a specific dosage of artificial edible melanin was mixed into the water. Additionally, the circumference of the water maze was enclosed with curtains, which were utilized to regulate the room’s illumination and to sidestep the interference of glare from the external light source affecting the video capture. To prevent the rats from visually detecting the platform in the pool, a specific dosage of artificial edible melanin was mixed into the water. Additionally, the circumference of the water maze was enclosed with curtains, which were utilized to regulate the room’s illumination and to sidestep the interference of glare from the external light source affecting the video capture. During days 2–5, the platform was situated 0.6 cm below the waterline, and the rats were consecutively placed into the four quadrants and permitted to explore for 90 seconds. The time it took for the rat to first locate the platform was defined as the escape latency period. If the rat was unable to locate the platform within 90 seconds, it was directed to and allowed to remain on the platform for 30 seconds. On the sixth day, rats were sequentially placed into the pool from the farthest quadrant, where the original platform was situated, three times consecutively. Each rat was given a 90-second exploration period during which the time it took for the rat to cross the area where the platform was originally located was recorded. If the rat did not cross the area within the allotted time, 90 seconds was recorded. The ANY-maze video tracking analysis system (version: 4.99, Stoelting, USA, Inc.) was employed to record the average number of platform crossings, the average time spent in the target quadrant, and the average distance traveled, among other aspects. These recorded measurements were used to evaluate the rats’ learning and memory abilities. The rats (n=6) that completed the Y-maze and New Object Recognition will continue with this experiment.
Golgi Staining
Following anesthesia, the rats’ brains were expeditiously extracted by decapitation on ice. The brains’ surface blood was rinsed with distilled water and subsequently fixed with Golgi staining fixative for a duration exceeding 48 hours. The rat brain tissue was sliced 2–3mm thick and rinsed multiple times with physiological saline. The tissue was placed in a 45mL round bottom EP tube and submerged in Golgi staining solution. The tube was kept in a cool and ventilated area, sheltered from light, for 14 days. Afterward, the tissue was washed thrice with distilled water. It was then soaked overnight in 80% glacial acetic acid, softened, and washed again with distilled water. The tissue was finally immersed in 30% sucrose and fixed. The tissues were sliced into 100 um sections utilizing an oscillating slicer, affixed on gelatin slides, and air dried in a cool, ventilated environment overnight. The dried tissue slides were treated with concentrated ammonia water for 15 minutes, and subsequently washed with distilled water for 1 minute. The slides were then treated with acid hardening fixing solution for 15 minutes, washed with distilled water for 3 minutes, and left to dry. Finally, the sections were sealed with glycerin gelatin. Dendrites were observed under a 1000x microscope, utilizing at least 3 neurons for each group. Dendrites were analyzed with Sholl analysis, and their density and number of dendritic crossings were calculated and analyzed. Randomly select 3 rats (n=3) from each group for this experiment.
TUNEL Staining
The TUNEL staining method was utilized to analyze the apoptosis of hippocampal neurons in each group of rats at the culmination of modeling. While under anesthesia, the rats underwent perfusion with 100 mL of saline, followed by another round of perfusion with 100 mL of 4% paraformaldehyde when clear effluent was obtained. Subsequently, the brains were promptly extracted and stored in 4% paraformaldehyde. After 48 hours, the brains were dehydrated, embedded, and sliced into 200 um coronal sections utilizing a microtome. These sections underwent dewaxing, rehydration, proteinase K repair, membrane breakage, incubation with reaction solution, DAPI restaining, sealing, and photographic analysis as per staining guidelines. Three fields of view were captured for each group, with the averaged values being used to assess the hippocampal apoptosis in each group. Randomly select 3 rats (n=3) from each group for this experiment.
Nissl Staining
The chemical composition of Nissl bodies, which is the central site of protein synthesis in neurons, comprises a nucleic acid protein that contains iron. Upon neuronal stimulation, the cytosolic Nissl bodies are notably reduced or can even vanish. The sections were fractionated in 95% ethanol for a minimum of 5 hours at room temperature, followed by treatment with 75% ethanol and saline and subsequent staining with 0.1% cresyl violet for 2–3 minutes. The sections were then sequentially treated with distilled water, ethanol, and xylene, before being sealed with neutral resin. The hippocampal Nissl bodies were examined under a microscope, with at least 3 fields of view being taken for each group and the average value being utilized in the analysis of the survival of hippocampal Nissl bodies. Randomly select 3 rats (n=3) from each group for this experiment.
Immunofluorescence Staining
Paraffin sections were subjected to dewaxing, hydration, and PBS washing to facilitate antigen repair. This was followed by an overnight incubation at 4°C with the primary antibodies rabbit anti-pCREB (1:1000, GB114322, Servicebio, China), BDNF (1:150, GB11559, Servicebio, China), Caspase-3 (1:150, GB11009-1. Servicebio, China), and Bcl-2 (1:200, GB114322, Servicebio, China). The next day, the secondary antibodies were incubated for an hour at 37°C, and the nuclei were restained with DAPI. After undergoing anti-fluorescence quenching treatment, the samples were observed under an inverted microscope. For each group, three fields of view were chosen, and ImageJ software was employed for analysis. The cumulative fluorescence intensity mean value was considered the protein expression. Randomly select 3 rats (n=3) from each group for this experiment.
Western Blot
The rats in each group were decapitated promptly while under anesthesia, and the hippocampal and cortical tissues were removed on ice to extract total proteins. The proteins in the gel underwent electrophoresis, followed by transfer to PVDF membranes and closure using skimmed milk for 2 hours. Subsequently, rabbit anti-CREB (1:1000, GB111052, Servicebio, China), rabbit anti-pCREB (1:450, GB114684, Servicebio, China), rabbit anti-BDNF (1:1000, GB11559, Servicebio, China), rabbit anti-Bcl-2 (1:1000, GB113375, Servicebio, China), rabbit anti-Bax (1:1000, GB11690, Servicebio, China), rabbit anti-Caspase-3 (1:1000, GB11767C, Servicebio, China), rabbit anti-GAP-43 (1:2000, GB12095, Servicebio, China), rabbit anti-Synaptophysin (1:10,000, GB11553, Servicebio, China), and rabbit anti-β-actin (1:1000, GB11001, Servicebio, China) primary antibody solutions were incubated overnight at 4°C. On the second day, the membranes were washed with TBST and incubated with fluorescent goat anti-rabbit IgG (1:10,000, SA5-35571, Thermo) at 4°C for an hour. After utilizing a membrane sweeper, the grayscale values of the bands were analyzed using ImageJ software. The rats used in the experiment were selected from the offspring born through mating, with 3 rats per group (n=3).
Statistical Analysis
The generated results are presented in the form of mean ± standard deviation (SD). SPSS software (version 27.0.1) was utilized to conduct all statistical analyses. One-way analysis of variance (ANOVA) was applied to compare differences in normally distributed data, and, subsequently LSD multiple comparison tests were performed. Two-way repeated-measures ANOVA applied in the MWM escape latency data analysis. In cases where the data is non-normally distributed, Kruskal–Wallis analysis was employed first, followed by Bonferroni two-by-two comparison. All data need to undergo homogeneity of variance testing. If the data meet the homogeneity of variance assumption, post-hoc multiple comparisons will be conducted using the LSD method. If the data do not meet the homogeneity of variance assumption, post-hoc multiple comparisons will be conducted using the Dunnett-T3 method. The threshold for assuming statistically significant difference is p value < 0.05.
Results
Electroacupuncture Pretreatment Attenuates Learning Memory Impairment Induced by Repeated Propofol Exposure in Rats
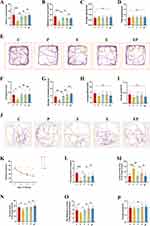
Rats’ short-term spatial learning memory ability was evaluated using the new novel arm Y-maze. Rats in the P group exhibited significantly less time (P < 0.001, η2=0.475) and distance (P < 0.001, η2=0.493) during exploration in the new novel arm compared to the other groups. Conversely, rats in the EP group displayed significantly more time (P < 0.001) and distance (P = 0.009) while exploring the new novel arm compared to the P group (Figure 2A and B). Motor speed (P > 0.05, η2=0.014) and total exploration distance (P > 0.05, η2=0.024) did not exhibit significant differences between the groups (Figure 2C and D). These findings suggest that repeated propofol exposure has an adverse effect on rats’ spatial learning memory abilities, but that electroacupuncture pretreatment can potentially mitigate the neurotoxic impact of prolonged propofol exposure. The new object recognition experiment was also used to appraise the cognitive memory ability of the rats. Our outcomes suggest that the Recognition Index (P < 0.05, η2=0.272) and the number of sniffing touches (P < 0.05, η2=0.552) were significantly lower in the P group when compared to the other groups. In contrast, the Recognition Index (P < 0.05) and the number of sniffing touches (P < 0.001) were significantly higher in the EP group when compared to the P group (Figure 2F and G). No significant difference was observed in motor speed (P > 0.05, η2=0.118) and total exploration path length (P > 0.05, η2=0.150) between groups (Figure 2H and I). To investigate the spatial learning memory ability of rats further, we employed the Morris water maze experiment (Figure 2J). As revealed by the two-way repeated measures ANOVA, Our observations indicated a gradual decrease in escape latency for all groups. However, the P group displayed a significantly higher escape latency than the other groups, which suggests an impairment in the learning memory of the P group. Conversely, escape latency was significantly lower in the EP group than in the P group, and no significant differences were observed with the other groups, (Figure 2K). Twenty-four hours after the final Morris water maze (MWM) training, we performed an MWM spatial exploration experiment by removing the platform. Our findings revealed that the number of times the rats crossed the platform (P < 0.05, η2=0.397), the total time (P < 0.05, η2=0.340) and distance (P < 0.05, η2=0.383) explored in the target quadrant in the P group were significantly lower than other groups. The time taken to cross the platform for the first time was significantly higher in the EP group. Relative to the P group, the EP group showed a significant increase in the number of platform crossings (P < 0.05), total time (P < 0.05), and total distance (P < 0.05) explored in the target quadrant, and a significant decrease in the time taken to cross the platform for the first time (P < 0.001, η2=0.467) (Figure 2L–O). We observed no significant difference in locomotor speed (P > 0.05, η2=0.153) among all groups of rats (Figure 2P). In light of the above results, it can be inferred that repeated propofol exposure in developing rats caused learning memory impairment. However, our data indicates that electroacupuncture pretreatment effectively mitigated this toxic effect.
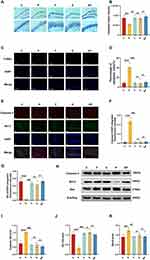
Electroacupuncture Pretreatment Attenuates Neuronal Apoptosis Induced by Repeated Propofol Exposure
The decrease in Nissl bodies served as a reflection of neuronal damage. Our Nissl staining results indicated a significant reduction in the integrated optical density of the P group relative to all other groups (P < 0.05, η2=0.773); Our findings depicted a significant increase in the integrated optical density of the EP group when compared to the P group (P < 0.001), without any significant difference from the other groups (P > 0.05) (Figure 3A and B). These results suggest that repeated propofol exposure can impede the development of hippocampal neurons in developing rats. However, our data indicates that electroacupuncture can counteract this toxic effect induced by repeated propofol exposure. TUNEL staining was employed to identify neuronal apoptosis. Our results exhibited a marked increase in the percentage of apoptotic cells in the P group compared to other groups (P < 0.001, η2=0.897). Moreover, we observed a significant decline in the percentage of apoptotic cells in the EP group when compared to the P group (P < 0.001), with no significant variations across the other groups (P > 0.05) (Figure 3C and D). According to our TUNEL staining results, repeated propofol exposure stimulated the apoptosis of hippocampal neurons in developing rats, while electroacupuncture pretreatment successfully counteracted the neurotoxic effects induced by repeated propofol exposure. Our immunofluorescence findings revealed a significant increase in Caspase-3 expression (P < 0.001, η2=0.980) and a significant decrease in Bcl-2 expression (P < 0.001, η2=0.922) in the P group when compared to all other groups; We observed a significant decrease in Caspase-3 expression (P < 0.001) and a significant increase in Bcl-2 expression (P < 0.001) in the EP group when compared to the P group (Figure 3E), with no significant difference from the other groups (P > 0.05) in both expressions (Figure 3F and G). Our Western Blot analysis revealed a marked increase in Caspase-3 (P < 0.001, η2=0.814) and Bax expressions (P < 0.05, η2=0.599) and a significant decrease in Bcl-2 expression (P < 0.001, η2=0.940) in the P group when compared to all other groups (Figure 3H). Conversely, Caspase-3 (P < 0.001) and Bax expressions decreased (P < 0.05) and Bcl-2 expression increased (P < 0.001) in the EP group compared to the P group (Figure 3I and J), with no significant difference from the other groups in all three expressions (P > 0.05) (Figure K). Our Immunofluorescence and Western Blot analyses revealed that repeated propofol exposure upregulated the expression of pro-apoptotic factors and downregulated the expression of anti-apoptotic factors, whereas electroacupuncture pretreatment successfully mitigated the neuronal apoptotic effect triggered by repeated propofol exposure.
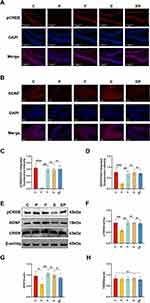
pCREB/BDNF Signaling Pathway Changes After Electroacupuncture Pretreatment and Repeated Propofol Exposure
pCREB and BDNF are critical factors in the creation and protection of learning memory and the facilitation of synaptic plasticity formation and maintenance. Our Immunofluorescence findings revealed a significant decrease in pCREB (P < 0.001, η2=0.842) and BDNF (P < 0.001, η2=0.789) expression in the P group when compared to all other groups, with a significant upregulation of pCREB (Figure 4A) and BDNF (Figure 4B) expression in the EP group when compared to the P group (P < 0.001). The expression of both pCREB and BDNF was not significantly different from all other groups (P > 0.05) in the EP group (Figure 4C and D). Correspondingly, our Western Blot analysis demonstrated that pCREB (P < 0.05, η2=0.709) and BDNF (P < 0.05, η2=0.570) expression was significantly lower in the P group (Figure 4E), while in the EP group, pCREB and BDNF expression was significantly upregulated compared to the P group (P < 0.05) (Figure 4F). However, pCREB and BDNF expression in the EP group was not significantly different from all other groups (Figure 4G and H). Our findings indicate that repeated exposure to propofol leads to a reduction in the expression of proteins related to learning and memory. However, electroacupuncture pretreatment can successfully reverse the altered expression of the pCREB/BDNF signaling pathway that results from repeated propofol exposure.
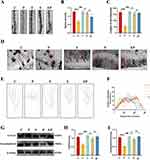
Electroacupuncture Pretreatment Attenuates Synaptic Plasticity Damage Induced by Repeated Propofol Exposure
Through our Golgi staining analysis of hippocampal synapses in developing rats, we observed a significant reduction in dendritic spine density (P < 0.001, η2=0.915) and total dendritic length (P < 0.001, η2=0.964) in the P group compared to all other groups (Figure 5A and B). Conversely, in the EP group, dendritic spine density and total dendritic length (P < 0.001) were increased when compared to the P group, and showed no significant difference from all other groups (P > 0.05) (Figure 5C and D). Notably, there was no significant difference between groups regarding the number of dendrites (P > 0.05) (Figure 5E and F). Our Western Blot analysis of proteins related to synaptic development indicated that GAP-43 (P < 0.001, η2=0.851) and Synaptophysin (P < 0.001, η2=0.838) expression were reduced in the P group compared to all other groups (Figure 5G). In contrast, GAP-43 (P < 0.001) and Synaptophysin (P < 0.001) expression were significantly higher in the EP group than in the P group (Figure 5H), and was not significantly different from all other groups (P > 0.05) (Figure 5I). Taken together, our findings indicate that repeated exposure to propofol led to notable synaptic damage in the hippocampus of developing rats, primarily evidenced by reduced dendritic spine density and dendritic length. In contrast, electroacupuncture pretreatment efficiently mitigated the synaptic damage resulting from repeated propofol exposure and successfully restored synaptic plasticity in the hippocampus.
Discussion
To assess the effects of repeated propofol exposure, we administered a daily dose of 100 mg/kg propofol via intraperitoneal injection. To simulate the effects of prolonged surgical anesthesia during clinical anesthesia or prolonged sedation in the intensive care unit, we administered 50 mg/kg propofol via a single intraperitoneal injection. When the rats showed partial recovery from the righting reflex, we administered another intraperitoneal injection of 50 mg/kg propofol. This administration method was chosen based on the recognized pharmacological effects of propofol, including cardiovascular and respiratory depression. Our findings confirm that repeated propofol exposure can lead to learning and memory deficits in developing rats, consistent with FDA warnings. Importantly, our study also presents evidence suggesting that electroacupuncture pretreatment can effectively mitigate such deficits resulting from repeated propofol exposure. To evaluate the learning and memory ability of rats subjected to repeated propofol exposure under electroacupuncture pretreatment, we conducted behavioral experiments using the new novel arm Y-maze, new object recognition, and Morris water maze. Our study involved 10-d-old SD rats, and we established a model of repeated propofol exposure with concurrent electroacupuncture pretreatment. Our data revealed a significant impairment of learning and memory ability in rats following repeated propofol exposure. However, rats exposed to repeated propofol after electroacupuncture pretreatment exhibited learning and memory ability that was comparable to that of normal controls. These results suggest that electroacupuncture pretreatment may confer cerebral protection, which is generally consistent with previous literature.34–36
In our study, we found that repeated propofol exposure in developing rats caused a significant decrease in hippocampal Nissl bodies, an increase in the number of TUNEL-positive cells, elevated expression of Caspase-3 and Bax, and decreased expression of Bcl-2. However, electroacupuncture pretreatment reversed these effects of repeated propofol exposure and attenuated neuronal apoptosis.
Here is increasing evidence supporting the notion that synaptic plasticity plays a prominent role in the establishment and upkeep of learning and memory during development.37,38 Synaptic plasticity consists of structural and functional changes. Repeated exposure to propofol can potentially impact both facets of synaptic plasticity, although the precise mechanism remains unclear. Our research focused on examining synaptic structure and related proteins. Our findings demonstrate that dendritic spine density and dendritic length were notably reduced in the hippocampus of rats repeatedly exposed to propofol, resulting in a severe impairment of synaptic plasticity. Following electroacupuncture pretreatment, the dendritic spine density and length remained similar to those of the blank control group. This signified the effectiveness of electroacupuncture pretreatment in mitigating the effects of propofol on synaptic plasticity. Conversely, repeated exposure to propofol led to a significant reduction in the expression of BDNF and pCREB protein. However, electroacupuncture pretreatment successfully restored the expression of both pCREB and BDNF to the levels observed in the control group. Electroacupuncture pretreatment proved effective in reducing the impact of propofol on synaptic plasticity, as evidenced by the preservation of dendritic spine density and length compared to the control group. Conversely, repeated exposure to propofol caused a remarkable decline in the expression of BDNF and pCREB protein, though electroacupuncture pretreatment successfully restored the levels of both proteins to those observed in the control group.39 pCREB is a key protein in synaptic plasticity,40 and also promotes the formation and maintenance of long-term potentiation (LTP).41 The mechanism of LTP plays a major role in the establishment of synaptic plasticity and is simultaneously an integral mechanism for the formation of learning and memory.42 Heightened expression of pCREB and BDNF in the hippocampus of rats has been shown to correlate with enhanced cognitive function and memory retention.43 Several positive regulators in the body facilitate synaptic plasticity and LTP, thereby consolidating memory. BDNF and pCREB are two such regulators that offer positive feedback and promote the formation and retention of learning and memory.44 The detection of pCREB, total CREB and BDNF expression was performed using both immunofluorescence and WB techniques. Our findings indicated that repeated exposure to propofol resulted in a substantial decrease in pCREB and BDNF expression, while the expression of total CREB remained unchanged. In contrast, the combined application of electroacupuncture pretreatment and repeated exposure to propofol yielded significantly higher levels of pCREB and BDNF expression compared to the repeated propofol exposure group. The expression of total CREB was unaffected by these conditions. The expression of synaptic-associated proteins, namely GAP43 and Synaptophysin, was quantified in our study. Our results revealed that repeated exposure to propofol led to a marked reduction in the expression of both GAP43 and Synaptophysin. However, electroacupuncture pretreatment prevented the suppression of these proteins’ expression by propofol exposure, thereby highlighting its protective role against synaptic damage inflicted by propofol exposure.
Due to financial and technical constraints on brain electrophysiology, this study did not explore functional changes in LTP/LTD and its related ion channels. To clarify the role of pCREB/BDNF signaling in electroacupuncture pretreatment for mitigating learning memory deficits caused by repeated propofol exposure, subsequent experiments will employ pathway-specific agonists or inhibitors to assess relevant indicators. As a non-pharmacological approach, electroacupuncture is a safe intervention for pediatric patients whose liver and kidney functions are not yet fully developed. However, the optimal parameter settings for electroacupuncture remain unknown, hence controlled experiments are necessary to determine them.
Our study investigated the effect of electroacupuncture pretreatment in reducing learning memory impairment caused by repeated propofol exposure and the underlying mechanisms involved. We found that electroacupuncture pretreatment (1) ameliorated learning memory deficits associated with repeated propofol exposure in developing rats; (2) modulated synaptic plasticity, facilitated neuronal function, and promoted the formation and retention of learning memory; and (3) relied, at least partly, on the activation of the pCREB/BDNF signaling pathway. Although further validation of our findings is required, our study provides insights into the potential therapeutic applications of electroacupuncture in treating propofol-induced learning deficits in a developing brain.
Ethical Approval
All animal experiments were conducted in compliance with National Institutes of Health guidelines and were approved by the Ethics Committee of Guangxi University of Traditional Chinese Medicine (DW20230313-036).
Acknowledgments
During the preparation of this work the authors used ChatGPT in order to make the sentences more concise, coherent and readable.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors have read and approved the final submitted manuscript.
Funding
This work was supported by Innovation Project of Guangxi Graduate Education of GXUCM (#YCSY2023059), the initiation of talent introduction and scientific research fund project for Guangxi International Zhuang Medicine Hospital (#GZ2021RC009), Research Fund Project of Guangxi Traditional Chinese Medicine Administration (#GXZYZ20210352), and Innovation Team of High-level Talent Cultivation from Guangxi University of Chinese Medicine (#2022B007). No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tesic V, Joksimovic SM, Quillinan N, et al. Neuroactive steroids alphaxalone and CDNC24 are effective hypnotics and potentiators of GABAA currents, but are not neurotoxic to the developing rat brain. Br J Anaesth. 2020;124(5):603–613. doi:10.1016/j.bja.2020.01.013
2. Yang Y, Yi J, Pan M, Hu B, Duan H. Edaravone alleviated propofol-induced neural injury in developing rats by BDNF/TrkB pathway. J Cell Mol Med. 2021;25(11):4974–4987. doi:10.1111/jcmm.16422
3. Zeng Z, Yao J, Zhong J, et al. The role of the lncRNA-LRCF in propofol-induced oligodendrocyte damage in neonatal mouse. Neurochem Res. 2021;46(4):778–791. doi:10.1007/s11064-020-03205-w
4. Zhou H, Xie Z, Brambrink AM, Yang G. Behavioural impairments after exposure of neonatal mice to propofol are accompanied by reductions in neuronal activity in cortical circuitry. Br J Anaesth. 2021;126(6):1141–1156. doi:10.1016/j.bja.2021.01.017
5. Shen X, Dong Y, Xu Z, et al. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013;118(3):502–515. doi:10.1097/ALN.0b013e3182834d77
6. Stratmann G, Sall JW, May LD, et al. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110(4):834–848. doi:10.1097/ALN.0b013e31819c463d
7. Apai C, Shah R, Tran K, Pandya shah S. Anesthesia and the developing brain: a review of sevoflurane-induced neurotoxicity in pediatric populations. Clin Ther. 2021;43(4):762–778. doi:10.1016/j.clinthera.2021.01.024
8. Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23(3):876–882. doi:10.1523/JNEUROSCI.23-03-00876.2003
9. Brambrink AM, Evers AS, Avidan MS, et al. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012;116(2):372–384. doi:10.1097/ALN.0b013e318242b2cd
10. Klintsova AY, Hamilton GF, Boschen KE. Long-term consequences of developmental alcohol exposure on brain structure and function: therapeutic benefits of physical activity. Brain Sci. 2012;3(1):1–38. doi:10.3390/brainsci3010001
11. Liu F, Liu S, Patterson TA, et al. Protective effects of xenon on propofol-induced neurotoxicity in human neural stem cell-derived models. Mol Neurobiol. 2020;57(1):200–207. doi:10.1007/s12035-019-01769-5
12. Liu F, Rainosek SW, Sadovova N, et al. Protective effect of acetyl-L-carnitine on propofol-induced toxicity in embryonic neural stem cells. Neurotoxicology. 2014;42:49–57. doi:10.1016/j.neuro.2014.03.011
13. Liu F, Liu S, Patterson TA. Effects of xenon-based anesthetic exposure on the expression levels of Polysialic Acid Neural Cell Adhesion Molecule (PSA-NCAM) on human neural stem cell-derived neurons. Mol Neurobiol. 2020;57(1):217–225. doi:10.1007/s12035-019-01771-x
14. Torii T, Sato A, Nakahara Y, Iwahashi M, Itoh Y, Iramina K. Frequency-dependent effects of repetitive transcranial magnetic stimulation on the human brain. Neuroreport. 2012;23(18):1065–1070. doi:10.1097/WNR.0b013e32835afaf0
15. She YJ, Xu HP, Gao Y, Wang Q, Zheng J, Ruan X. Calpain-TRPC6 signaling pathway contributes to propofol-induced developmental neurotoxicity in rats. Neurotoxicology. 2023;95:56–65. doi:10.1016/j.neuro.2023.01.004
16. Engelhardt T, MacDonald J, Galley HF, Webster NR. Webster NR Selective phosphodiesterase 5 inhibition does not reduce propofol sedation requirements but affects speed of recovery and plasma cyclic guanosine 3’,5’-monophosphate concentrations in healthy volunteers. Anesth Analg. 2005;101(4):1050–1053. doi:10.1213/01.ane.0000168264.41341.7d
17. Briner A, Nikonenko I, De Roo M, Dayer A, Muller D, Vutskits L. Developmental Stage-dependent persistent impact of propofol anesthesia on dendritic spines in the rat medial prefrontal cortex. Anesthesiology. 2011;115(2):282–293. doi:10.1097/ALN.0b013e318221fbbd
18. Lee B, Ko MM, Lee SH, Chang GT. Acupuncture for the treatment of childhood anorexia: a systematic review and meta-analysis. Complement Ther Med. 2022;71(102893):102893. doi:10.1016/j.ctim.2022.102893
19. Cheuk DK, Wong V. Acupuncture for autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2011;9:CD007849.
20. Yang M, Gao S, Yao H. Effects of electroacupuncture on pediatric chronic urinary retention: a case-series study. Front Pediatr. 2023;11(1194651). doi:10.3389/fped.2023.1194651
21. Tang Y, Wang T, Yang L. Acupuncture for post-operative cognitive dysfunction: a systematic review and meta-analysis of randomized controlled trials. Acupunct Med. 2020;39(5):423–431. doi:10.1177/0964528420961393
22. Han YG, Qin X, Zhang T, et al. Electroacupuncture prevents cognitive impairment induced by lipopolysaccharide via inhibition of oxidative stress and neuroinflammation. Neurosci Lett. 2018;683:190–195. doi:10.1016/j.neulet.2018.06.003
23. Liu PR, Zhou Y, Zhang Y, Diao S. Electroacupuncture alleviates surgery-induced cognitive dysfunction by increasing α7-nAChR expression and inhibiting inflammatory pathway in aged rats. Neurosci Lett. 2017;659:1–6. doi:10.1016/j.neulet.2017.08.043
24. Chuang C-M, Hsieh C-L, Li T-C, Lin J-G. Acupuncture stimulation at Baihui acupoint reduced cerebral infarct and increased dopamine levels in chronic cerebral hypoperfusion and ischemia-reperfusion injured Sprague-Dawley rats. Am J Chin Med. 2007;35(5):779–791. doi:10.1142/S0192415X07005260
25. Chen A, Lin Z, Lan L, et al. Electroacupuncture at the Quchi and Zusanli acupoints exerts neuroprotective role in cerebral ischemia-reperfusion injured rats via activation of the PI3K/Akt pathway. Int J Mol Med. 2021;30(4):791–796.
26. Xue X, You Y, Tao J, et al. Electro-acupuncture at points of Zusanli and Quchi exerts anti-apoptotic effect through the modulation of PI3K/Akt signaling pathway. Neurosci Lett. 2014;558:14–19. doi:10.1016/j.neulet.2013.10.029
27. Li M, Li K, Ding N, Xie YQ, Niu K, Zhang H. Effect of electroacupuncture on expression of CREB and its ability to bind to synaptic proteins in amygdala and hippocampus of rats with post-traumatic stress disorder. Zhen Ci Yan Jiu. 2020;45(7):517–523. doi:10.13702/j.1000-0607.190709
28. Mertz C, Krarup S, Jensen CD, et al. Aspects of cAMP signaling in epileptogenesis and seizures and its potential as drug target. Neurochem Res. 2020;45(6):1247–1255. doi:10.1007/s11064-019-02853-x
29. Lee H, Son Y, Lee M. Sodium butyrate prevents radiation-induced cognitive impairment by restoring pCREB/BDNF expression. Neural Regen Res. 2019;14(9):1530–1535. doi:10.4103/1673-5374.255974
30. Sugiura S, Kitagawa K, Omura-Matsuoka E, et al. CRE-mediated gene transcription in the peri-infarct area after focal cerebral ischemia in mice. J Neurosci Res. 2004;75(3):401–407. doi:10.1002/jnr.10881
31. Gómez-Palacio-Schjetnan A, Escobar ML. Neurotrophins and synaptic plasticity. Curr Top Behav Neurosci. 2013;15:117–136.
32. Gray JD, Milner TA, McEwen BS. Dynamic plasticity: the role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience. 2013;239:214–227. doi:10.1016/j.neuroscience.2012.08.034
33. El Hayek L, Khalifeh M, Zibara V, et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal Brain-Derived Neurotrophic Factor (BDNF). J Neurosci. 2019;39(13):2369–2382. doi:10.1523/JNEUROSCI.1661-18.2019
34. Wang HL, Liu FL, Li RQ, et al. Electroacupuncture improves learning and memory functions in a rat cerebral ischemia/reperfusion injury model through PI3K/Akt signaling pathway activation. Neural Regen Res. 2021;16(6):1011–1016. doi:10.4103/1673-5374.300454
35. Xie L, Liu Y, Zhang N, et al. Electroacupuncture improves M2 microglia polarization and glia anti-inflammation of hippocampus in Alzheimer’s disease. Front Neurosci. 2021;15:689629.
36. Shao SM, Park KH, Yuan Y, et al. Electroacupuncture attenuates learning and memory impairment via PI3K/Akt pathway in an amyloid β25-35-induced Alzheimer’s disease mouse model. Evid Based Complement Alternat Med. 2022;2022:3849441. doi:10.1155/2022/3849441
37. Yu C-J, Wang M, Li R-Y. TREM2 and microglia contribute to the synaptic plasticity: from physiology to pathology. Mol Neurobiol. 2023;60(2):512–523. doi:10.1007/s12035-022-03100-1
38. Huang YS, Mendez R, Fernandez M, Richter JD. CPEB and translational control by cytoplasmic polyadenylation: impact on synaptic plasticity, learning, and memory. Mol Psychiatry. 2023;2023:1–9.
39. Kim S, Kim MS, Park K, et al. Hippocampus-dependent cognitive enhancement induced by systemic gintonin administration. J Ginseng Res. 2016;40(1):55–61. doi:10.1016/j.jgr.2015.05.001
40. Zhang XQ, Mu JW, Wang HB, et al. Increased protein expression levels of pCREB, BDNF and SDF-1/CXCR4 in the hippocampus may be associated with enhanced neurogenesis induced by environmental enrichment. Mol Med Rep. 2016;14(3):2231–2237. doi:10.3892/mmr.2016.5470
41. Paramanik V, Thakur MK. Role of CREB signaling in aging brain. Arch Ital Biol. 2013;151(1):33–42. doi:10.4449/aib.v151i1.1461
42. Sánchez-Rodríguez I, Temprano-Carazo S, Jeremic D. Recognition memory induces natural LTP-like hippocampal synaptic excitation and inhibition. Int J Mol Sci. 2022;23(18):10806. doi:10.3390/ijms231810806
43. Nam SM, Choi JH, Yoo DY, et al. Effects of curcumin (Curcuma longa) on learning and spatial memory as well as cell proliferation and neuroblast differentiation in adult and aged mice by upregulating brain-derived neurotrophic factor and CREB signaling. J Med Food. 2014;17(6):641–649. doi:10.1089/jmf.2013.2965
44. Hernandez PJ, Abel T. The role of protein synthesis in memory consolidation: progress amid decades of debate. Neurobiol Learn Mem. 2008;89(3):293–311. doi:10.1016/j.nlm.2007.09.010
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.