Back to Journals » Journal of Pain Research » Volume 16
Electroacupuncture Exerts Chondroprotective Effect in Knee Osteoarthritis of Rabbits Through the Mitophagy Pathway
Authors Xing L , Chen X, Guo C, Zhu W , Hu T , Ma W, Du M, Xu Y , Guo C
Received 7 April 2023
Accepted for publication 3 August 2023
Published 21 August 2023 Volume 2023:16 Pages 2871—2882
DOI https://doi.org/10.2147/JPR.S416242
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Longfei Xing,1,* Xilin Chen,2,3,* Changqing Guo,1 Wenting Zhu,4 Tingyao Hu,1 Weiwei Ma,1 Mei Du,1 Yue Xu,1 Changqing Guo5
1School of Acupuncture-Moxibustion and Tuina, Beijing University of Chinese Medicine, Beijing, People’s of Republic of China; 2Department of Acupuncture and Rehabilitation, The Fifth College of Clinical Medicine, Guangzhou University of Traditional Chinese Medicine, Guangzhou, People’s Republic of China; 3Department of Acupuncture and Rehabilitation, Guangdong Second Hospital of Traditional Chinese Medicine, Guangzhou, People’s Republic of China; 4The Third Affiliated Hospital of Beijing University of Chinese Medicine, Beijing, 100029, People’s Republic of China; 5Department of Medical Technology, Shijiazhuang Medical College, Shijiazhuang, Hebei Province, People’s of Republic of China
*These authors contributed equally to this work
Correspondence: Changqing Guo, Shijiazhuang Medical College, Shijiazhuang, Hebei Province, 050599, People’s of Republic of China, Tel +86-13081033381, Fax +86-82517626, Email [email protected]
Purpose: Mitochondrial dysfunction of chondrocytes has become an area of focus in Knee Osteoarthritis (KOA) in recent years. Activation of mitophagy could promote the survival of chondrocytes and alleviate cartilage degeneration. The aim of this study was to explore whether mitophagy was involved in the cartilage protection of KOA rabbits after electroacupuncture (EA) intervention.
Methods: The rabbits were divided into 3 groups, Control group, KOA group, EA group, with 6 rabbits in each group. KOA model rabbits were established by modified Videman’s extended immobilization method for 6 weeks and randomly divided into KOA group and EA group. The rabbits in EA group were treated every other day for 3 weeks. The degree of cartilage degeneration was detected by Safranine O-Fast Green staining and immunofluorescence. The morphological changes of chondrocytes mitochondria were detected by transmission electron microscope. ATP concentration in cartilage was measured by ATP Assay Kit. The changes of Pink1-Parkin signal pathway were detected by immunofluorescence, Western blot, and Real-time PCR.
Results: The morphology showed that EA could reduce the degeneration of KOA cartilage and increase the distribution of collagen II. We also found that EA could activate mitophagy in KOA rabbit chondrocytes to remove damaged mitochondria and restore mitochondrial homeostasis, which was manifested as increasing the expression of LC3 II/I, promoting the colocalization of TOM20 and LC3B, reducing the accumulation of mitochondrial markers outer mitochondrial membrane 20 (TOM20) and inner mitochondrial membrane 23 (TIM23), and increasing ATP production in chondrocytes. This regulation might be achieved by upregulating the Pink1-Parkin signal pathway.
Conclusion: EA may play a role in protecting KOA cartilage by activating mitophagy mediated through Pink1-Parkin pathway.
Keywords: osteoarthritis, electroacupuncture, mitophagy, mitochondria, Pink1-Parkin
Introduction
In the past 10 years, the number of patients with disability due to Osteoarthritis (OA) has increased by 114.5%, among which Knee Osteoarthritis (KOA) is the most common one, with its morbidity increasing year by year, causing a huge economic burden to the world.1 It is characterized by pathologic changes in whole joint histologies, including progressive destruction of articular cartilage, synovial inflammation, and fibrosis, osteophyte formation and subchondral bone alterations.2 Articular cartilage degeneration is a typical pathological change of KOA. Biochemical and mechanical factors interact with each other, resulting in changes in mechanical properties such as elastic modulus and Poisson’s ratio of cartilage, and various pathological changes such as extracellular matrix synthesis, catabolic imbalance, and chondrocytes energy metabolism disorders. Previous studies have suggested that articular cartilage has no blood vessels and lacks blood supply, so chondrocytes are in a low-oxygen environment, anaerobic glycolysis is the main way to produce energy, and mitochondria-mediated aerobic respiration plays an insignificant role in the energy metabolism of articular cartilage.3 However, recent studies have shown that the surface of cartilage is in a relative aerobic environment, the oxygen partial pressure is about 7%, and the mitochondria-dependent tricarboxylic acid cycle is still relatively active. About 25% of ATP in chondrocytes comes from the oxidative phosphorylation of mitochondria.4 As the most important organelle involved in cell energy metabolism, mitochondria dysfunction is closely related to extracellular matrix metabolism, cell apoptosis, and senescence.5 In recent years, researchers have recognized that6 mitochondrial damage is closely related to the pathogenesis of KOA, and the accumulation of damaged mitochondria is an important factor in the progression of cartilage degradation, as well as a potential target for KOA treatment.7
In the progress of KOA, the damaged mitochondria will decrease the production of ATP, which in turn affects the synthesis of type II collagen in the extracellular matrix.8 In the same time, the outer membrane of damaged mitochondria will depolarize, and produce a large number of reactive oxygen species, which can damage the macromolecules such as DNA, lipids, and proteins in the cell, and damage the normal mitochondria, forming a vicious cycle to accelerate cartilage degeneration.9 Activation of mitophagy is the key to removing damaged mitochondria, breaking this vicious cycle, and reducing oxidative stress and inflammation levels in chondrocytes. PTEN-induced kinase 1 (Pink1)-Parkin is a key pathway for regulating mitophagy.10 Pink1 is fixed on the outer membrane of depolarized mitochondria and recruits Parkin to ubiquitinate the mitochondrial membrane, thereby binding to autophagy factor LC3 to initiate the degradation program of mitophagy.11 Studies have confirmed that activation of Pink1 and Parkin could effectively activate mitophagy of chondrocytes and improve the survival rate of chondrocytes.12,13 Therefore, mitochondrial autophagy is an important therapeutic target for KOA.
Currently, there is no single treatment that can completely cure KOA. 2019 American College of Rheumatology/Arthritis Foundation Guideline recommended acupuncture (including hand acupuncture, electroacupuncture, etc.) as a non-drug treatment for KOA, believing that acupuncture can effectively reduce pain in patients with KOA.14 2020 Chinese Guidelines for TCM Diagnosis and Treatment of Knee Arthritis also stated that acupuncture can be applied to patients with KOA at all stages.15 Previous studies have shown that EA can down-regulate the expression of knee inflammatory cytokines, reduce chondrocytes oxidative injury, and down-regulate the expression of MMP-13 to reduce cartilage degeneration, improve muscle atrophy, correct knee biomechanical load, and improve cartilage mechanical properties, thereby exerting protective effects on cartilage, reducing knee pain, and improving knee mobility.16–19 Recent studies have shown that EA can regulate mitochondrial function of KOA chondrocytes and reduce chondrocyte apoptosis.20 It has been accepted that EA can regulate mitophagy in cerebrovascular diseases.21 Whether EA could effectively regulate mitophagy and exert chondroprotective effect in cartilage remains to be further explored.
Based on this, the KOA rabbit model was established through the modified Videman’s extended immobilization method, and the hypothesis was explored, including whether EA intervention could exert chondroprotective effect by activating mitophagy and removing damaged mitochondria in chondrocytes, and what is the role of PINK1-Parkin pathway in cartilage in this process.
Materials and Methods
Instruments and Reagents
Instruments needed are disposable acupuncture needles (0.2x13mm, Huanqiu, Suzhou, China), and HANS acupoint nerve stimulator (LH202H, Huawei Industry Development, Beijing, China). ATP assay kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The following primary antibodies were used in this study: anti-TOM20, anti-TIM23, anti-Pink1, anti-Parkin (Proteintech, Chicago, IL, USA), anti-LC3 (MBL, Tokyo, Japan), anti-GAPDH (Abcam, Boston, Mass, USA), horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Servicebio, Wuhan, China), horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Servicebio, Wuhan, China), Alexa Fluor 594 labeled goat anti-rabbit IgG (ZSGB-BIO, Beijing, China), Alexa Fluor 488 labeled goat anti-mouse IgG (ZSGB-BIO, Beijing, China), Prestained Protein Marker II (10–200kDa) (Servicebio, Wuhan, China).
Animals and Study Design
Male New Zealand rabbits (n=18, weighing 2.0–2.5kg and aged 6 months old), were purchased from Fulongtengfei Experimental Animal Research Institute (Beijing, China, Certificate Number: SYXK 2018–0041). The rabbits were housed under a specific condition, namely, 20–25°C, 50~60% humidity, and a 12 h light/12 h dark circadian cycle, with free access to regular chow diet. After being habituated to the environment for 1 week, rabbits were randomly divided into three groups (n=6): Control group (control), KOA group (KOA), and KOA + Electroacupuncture group (EA). The modified Videman method was used to immobilize the left hind limb of rabbits in an upright position for 6 weeks to induce KOA in KOA-related groups.22 After 6 weeks of model preparation, the knee joint mold was removed. And then the EA group were treated for 3 weeks. During this period, the other two groups did not receive any treatment except for normal grasping and binding fixation. After the intervention, rabbits were sacrificed by air embolism in the state of anesthesia and indicators were detected.
KOA Model
After 1 week of adaptive feeding, the modified Videman method was performed for KOA modeling. After a 12-h fast and water deprivation, rabbits were anesthetized with 3% pentobarbital sodium (30mg/kg). The rabbits were fixed to the operating table, and the left hindlimbs were shaved. With their left hindlimbs in a completely straight position, the groin to toe area was fixed with a resin bandage. Two orthopedic casting tapes were applied to reinforce the fixation, and finally an anti-bite bandage was applied. During fixation, blood supply of the left hind limbs was determined by the toe shape and color, and the fixed mold was adjusted as needed. The mold was removed after 6 weeks of fixation in KOA-related groups. The control group did not perform any operation. The Lequesne MG scale was used to evaluate the KOA model, and the score was greater than or equal to 4 points, which determined that the KOA model was successfully prepared.23
EA Intervention
Acupoints Xuehai (SP10), Liangqiu (ST34), Neixiyan (EX-LE4), and Waixiyan (EX-LE5) were selected for EA intervention according to Experimental Acupuncture and Moximography24 combined with the method of Comparative Biology. After disinfection, the rabbits were treated with disposable acupuncture needles into the acupoints with a depth of 5mm, and then connected to HANS Acupoint Nerve stimulator for EA intervention for 15min on SP10 and ST34, EX-LE4, and EX-LE5 (parameters: density wave, 2/100Hz, 3mA), once every other day for 3 weeks.
Safranine O-Fast Green Stanning
The chondro-subchondral bone complex was fixed with 4% paraformaldehyde (Servicebio, Wuhan, China) for 72h, and then decalcified with EDTA decalcification solution. The decalcification solution was changed every 4 days for 8 weeks until the syringe needle could easily pierce into the tissue. The tissues were then dehydrated, transparent, waxed, and embedded in paraffin. Paraffin sections with a thickness of 4μm were prepared from sagittal view with a paraffin microtome (Leica, Wetzlar, Hessen, Germany). Cartilage sections were stained with Modified Safranine O-Fast Green FCF Cartilage Stain Kit (Solarbio, Wuhan, China). Two researchers evaluated the stained sections according to the Mankin scoring system25 without knowing the groups.
ATP Assay
An appropriate amount of fresh cartilage tissue was weighed, and 9 times of the volume of cold double distilled water was added to the ratio of weight (g): volume (mL) = 1:9. Then the homogenate was homogenized in an ice water bath to prepare 10% homogenate and determine the protein concentration. ATP concentration in cartilage tissue (μmol/g) was measured according to the instruction manual of ATP Assay Kit. The researchers were blind to the grouping of specimens.
Transmission Electron Microscopy
A volume of 1mm×1mm×1mm cartilage was removed from the sacrificed rabbit knee with a sharp knife and then fixed in pre-cooled electron microscopy fixation solution at 4°C (Servicebio, Wuhan, China) for 2–4h. After dehydration, infiltration, embedding, and sectioning, the cartilage was double stained with uranium and observed by transmission electron microscope (HITACHI, Tokyo, Japan).
Western Blot
Fresh cartilage was dissected from the left knee of the sacrificed rabbit with a rongeur, ground with liquid nitrogen in a mortar and collected into a centrifuge tube. Ten times the volume of RIPA lysate (Servicebio, Wuhan, China) and one-tenth volume of PMSF (Servicebio, Wuhan, China) were added to the centrifuge tube to extract total protein. Total protein was quantified using BCA kit (Solarbio, Wuhan, China). Before the start of electrophoresis, 1.5μL and 3μL ladders were added to two different lanes. Electrophoresis was carried out with 12% SDS-polyacrylamide gel (SDS-PAGE) at 80V for 2h, and then transferred to 0.22μm PVDF membranes (Millipore, Boston, Mass, USA) for 30 min at 100V. Subsequently, the PVDF membrane was placed in rapid blocking solution (Solarbio, Wuhan, China) for 20 min at room temperature. The membranes were then incubated overnight with primary antibodies against LC3 (1:1000), TOM20 (1:2000), TIM23 (1:5000), Pink1 (1:1000), Parkin (1:2000), and GAPDH (1:10,000) on a shaker at 4°C. The second day, the membranes were incubated with horseradish peroxidase (HRP)-labeled secondary antibody for 1h at room temperature, and finally the color was developed with the hypersensitive ECL chemiluminescence kit. The exposure system software is Image Lab(TM) touch software, the software version is 2.4.0.03, and the imager model is ChemiDoc(TM) MP. The exposure application is Chemiluminescent Blot. In chemiluminescence processes, optimal auto-exposure mode is considered first. If blot cannot be displayed by optimal auto-exposure mode, manual exposure mode is selected. Image J (Rockville, USA) software was used to analyze the gray values of protein bands.
Real-Time Quantitative Polymerase Chain Reaction (RT-PCR) Analysis
Cartilage samples were obtained using the same method as Western Blot. RNA extraction solution (Servicebio, Wuhan, China) was used to homogenize and extract total RNA. RNA was reverse transcribed into cDNA according to the RNA Reverse Transcription Kit instructions. Real-time PCR was performed using qPCR SYBR Green Master Mix (Servicebio, Wuhan, China) for cDNA amplification. Specific PCR primers for GAPDH, Pink1, and Parkin were constructed based on published sequences. The PCR reaction conditions were first set to 95°C for 5 min and then entered the following cycles: 95°C for 30s, 60°C for 30s, 72°C for 30s, a total of 45 cycles. The relative expression levels of target genes were calculated by 2-ΔΔCT method and normalized to the mRNA expression levels of GAPDH. The primers upstream and downstream are shown in Table 1. The researchers were blind to the grouping of specimens.
 |
Table 1 Primers Used in This Study |
Immunofluorescence
After dewaxing and dehydration, the cartilage paraffin sections were subjected to antigen repair by pepsin digestion solution (Servicebio, Wuhan, China) at 37°C for 30min. After treatment with tissue autofluorescence quencher (Servicebio, Wuhan, China) for 30min and blocking with 10% concentrated sheep serum (Boster, Wuhan, China) for 1h, antibody was incubated. For immunofluorescence staining, primary antibodies against collagen typeII (1:100) were incubated overnight at 4°C, and for double immunofluorescence staining, they were incubated with primary antibodies against TOM20 (1:100) and LC3 (1:100), Pink1 (1:100), and Parkin (1:100) overnight at 4°C. Alexa Fluor labeled secondary antibody (1:400) was incubated at 37°C for 1h. The samples were sealed with Antifade Mounting Medium with DAPI (ZSGB-Bio, Beijing, China), observed with fluorescence microscope (Olympus, Tokyo, Japan), and analyzed by Image J. The researchers were blind to the grouping of specimens.
Statistical Analysis
The experimental data were analyzed using SPSS 21.0 software (version 20.0, SPSS, Inc., Chicago, IL, USA). When data conform to normal distribution and variance is homogeneous, one-way analysis of variance (ANOVA) and LSD’s test were applied for determining the statistical significance of multiple group comparisons, and data are expressed as mean± standard deviation (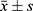 ). When data do not conform to a normal distribution and variance is not homogeneous, a nonparametric test (Mann–Whitney) is used, and data are expressed as median (interquartile range)/M (QR). A value of P < 0.05 was considered statistically significant. Higher significance levels were established at P < 0.01.
). When data do not conform to a normal distribution and variance is not homogeneous, a nonparametric test (Mann–Whitney) is used, and data are expressed as median (interquartile range)/M (QR). A value of P < 0.05 was considered statistically significant. Higher significance levels were established at P < 0.01.
Results
EA Reduced the Lequesne MG Score
As shown in Figure 1, the results of Lequesne MG scores on the left knee joint before and after intervention in each group of rabbits were statistically analyzed by nonparametric tests because the data did not conform to the normal distribution. After 6 weeks of immobilization in the left hind limb extension using the modified Videman method, the rabbits developed impaired mobility of the affected limb, atrophy of the thigh muscles, and palpable fibrocords at the tendon attachment. Compared with the control group, Lequesne MG scores between the KOA group and the EA group were significantly increased (P < 0.01), and there were no significant differences between the KOA group and the EA group (P > 0.05). It shows that the KOA rabbit molds were successful, and the baseline between the KOA group and the EA group is consistent, which is comparable.
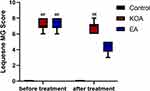 |
Figure 1 Effect of EA on Lequesne MG score on the knee joint of the left hind limb of Knee Osteoarthritis(KOA) rabbits. Values were median (interquartile range)/M (QR); N=6. ##P < 0.01 vs Control. |
After the intervention, compared with the control group, Lequesne MG score of the KOA group increased significantly (P < 0.01). Compared with the KOA group, there was no significant difference in Lequesne MG score in the EA group (P > 0.05), but there was a significant downward trend, indicating that EA could reduce knee pain and swelling and improve knee dysfunction in KOA model rabbits to a certain extent.
EA Alleviated Cartilage Degeneration in KOA Rabbits
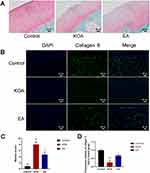
The degree of cartilage degeneration was evaluated by observing the staining of Safranine O-Fast Green and collagen II. Compared with the control group, the cartilage thickness in the KOA group was thinner, the cartilage surface was defective, the number of chondrocytes was necrotic and decreased, and the superficial cartilage showed a lack of Safranine O staining, indicating the degradation of proteoglycans (Figure 2A). Compared with the KOA group, the cartilage defect in the EA group was not obvious, and the absence of Safranine O staining was mild, indicating that proteoglycan degradation was reduced after treatment. Compared with the control group, the scores of the KOA group were significantly increased (P < 0.01), and the scores of the EA group were significantly decreased (P < 0.01; Figure 2C).
Collagen II immunofluorescence showed that compared with the control group, the content of collagen II in the superficial layer and middle layer of cartilage was significantly decreased in the KOA group (P < 0.01). Compared with the KOA group, the collagen II in the EA group was significantly increased (P < 0.01; Figure 2B and D). These results indicated that EA could help to alleviate the degradation of cartilage ECM and cartilage degeneration in KOA rabbits.
EA Improved Mitochondrial Morphology and Function of KOA Chondrocytes
Transmission electron microscopy (TEM) is the gold index for observing mitophagy.26 In order to further confirm the regulatory effect of EA on mitophagy in KOA rabbit chondrocytes, TEM observation was performed. According to the evaluation criteria of mitochondrial morphology,26 the mitochondrial membrane of the control group was complete, and there were distinct cristae. In the KOA group, autophagic vesicles were rarely seen in chondrocytes, and mitochondria were damaged, with a swollen donut/spherical shape (Figure 3A, blue arrows), complete loss of mitochondrial cristae, and loss of most of the mitochondrial matrix. Mitophagy vesicles were observed in the EA group, and mitochondria had good cristae with relatively complete morphology. Mitochondria are the main source of ATP production. The results showed that the ATP content in the KOA group was significantly lower than that in the control group (P < 0.01), and the EA group was significantly higher than that in the KOA group (P < 0.05; Figure 3B). These results suggested that EA promoted the formation of mitophagy vesicles and improves mitochondrial function.
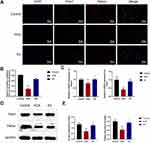
EA Activates Mitophagy of KOA Chondrocyte
To determine the activation of mitophagy, we performed double immunofluorescence staining of TOM20 (mitochondrial marker) and LC3B (autophagy marker). The results showed that the colocalization coefficient of the KOA group was significantly lower than that of the control group (P < 0.01). The colocalization coefficient of the EA group was significantly higher than that of the KOA group (P < 0.01; Figure 4A and B). The expression of mitophagy-related proteins in cartilage was tested by Western Blot, which showed that compared with the control group, the autophagy marker LC3II/I in the KOA group was significantly decreased (P < 0.05), and the expression of mitochondrial markers TOM20 and TIM23 was increased (P < 0.01 and P < 0.05), indicating that the autophagy level was downregulated and the damaged mitochondria may be accumulated. However, the EA group could reverse the changes of KOA group, suggesting that EA could activate mitophagy of chondrocytes (Figure 4C and D).
EA Activated Mitophagy by Upregulating PinK1-Parkin Pathway
Pink1-Parkin is a typical pathway mediating mitophagy, and its co-activation is key in initiating mitophagy. The co-expression of pink1 and parkin was observed by immunofluorescence double staining. The colocalization coefficient of the KOA group was significantly lower than that of the control group (P < 0.01). The colocalization coefficient of the EA group was significantly higher than that of the KOA group (P < 0.01; Figure 5A and B), indicating that this pathway may be activated after EA intervention. RT-PCR and Western blot were used to analyze the expression of Pink1 and Parkin. The mRNA and protein expressions of Pink1 and Parkin in the KOA group were significantly decreased compared with those in the control group (P < 0.01), and the EA group were significantly higher than those in the KOA group (P < 0.01; Figure 5C and E). These results suggested that EA may be able to activate mitophagy through upregulating Pink1-Parkin pathway.
Discussion
Mitochondria are important organelles involved in the pathogenesis of KOA, and mitophagy can maintain mitochondrial homeostasis and effectively protect cartilage.27 In this study, optical microscopy and electron microscopy were used to observe the cartilage and chondrocyte morphology of rabbits in each group, combined with the detection of mitophagy-related genes and proteins, the possible mechanism of EA protecting cartilage was explored.
Cartilage is mainly composed of chondrocytes and extracellular matrix (ECM). The main components of ECM are water (75%), collagen (mainly collagen II, 15%), and proteoglycan (10%), which provide tensile and compressive strength for cartilage.28 The degradation of ECM is an important marker of KOA progress.26 Fmodeling, the pathological results showed obvious defects on the cartilage surface, obvious absence of proteoglycan staining, and significant reduction in the distribution and content of collagen II, which were consistent with our previous modeling effect,19 suggesting that the KOA model established by immobilization method was similar to clinical practice. EA is a treatment that combines traditional acupuncture with electrical stimulation, and there was a high-quality study that demonstrated the efficacy of electroacupuncture in the treatment of knee osteoarthritis.29 Acupoints Xuehai (SP10), Liangqiu (ST34), Neixiyan (EX-LE4), and Waixiyan (EX-LE5) are distributed around the knee joint. A study has proven that electroacupuncture in EX-LE4 and EX-LE5 can strengthen the muscle strength of the quadriceps muscles, promote joint fluid circulation, and nourish joint cartilage.30 Electroacupuncture in SP10 and ST34 can drive quadriceps muscle contraction and improve the muscle strength of quadriceps muscle.31 Therefore, these acupoints for electroacupuncture intervention can correct the abnormal stress environment of the knee joint, promote the repair of knee microcirculation, and alleviate the degeneration of knee cartilage. As expected, after EA intervention, the Lequesne MG score of the knee joint with KOA decreased, the knee pain was reduced and the knee dysfunction was improved (Figure 1). Moreover, the cartilage surface defects were significantly reduced, and the contents of proteoglycan and collagen II in the ECM were increased, indicating that EA could slow down the progression of KOA and effectively protect cartilage (Figure 2).
Damaged mitochondria will lead to decreased ATP production, increased oxidative stress, increased mitochondrial membrane permeability, mitochondrial DNA mutations, and eventually lead to the degradation of proteoglycan and collagen II in the ECM of chondrocytes, and promote the exacerbation of KOA.32 In this study, mitochondria of KOA chondrocytes showed significant damage, mitochondrial swelling and ridge disappearance under the TEM. The determination of ATP level also confirmed the impairment of its function to varying degrees. EA intervention could improve the co-localization level of TOM20 and LC3B, promote the formation of mitophagy vesicles, improve the mitochondrial morphology, remove damaged mitochondria, and improve the energy metabolism of chondrocytes, which may be the reason why EA improves collagen II production and metabolism in ECM.
Pink1-Parkin pathway is a classical pathway regulating mitophagy. In physiological conditions, Pink1 enters the mitochondrial matrix through the action of TOM and TIM. When mitochondria are damaged, the outer membrane depolarizes, so that Pink1 is fixed on the outer mitochondrial membrane, so as to recruit Parkin in the cytoplasm and bind to autophagosomes to initiate mitophagy. The loss of Pink1 or Parkin will directly lead to the inhibition of mitophagy.12,14 Studies have found that Pink1 and Parkin expression are significantly reduced in KOA chondrocytes, and activation of this pathway could effectively activate mitophagy, remove depolarized/damaged mitochondria, and protect chondrocytes.33,34 Therefore, up-regulating the expression of Pink1 and Parkin and restoring their close cooperation is the key to activate mitophagy. In this study, EA might help to upregulate the inhibited Pink1 and Parkin in the chondrocytes of KOA model rabbits and increase their co-localized expression.
Studies have shown that appropriate mechanical stress could activate the Pink1-Parkin pathway and upregulate mitochondria dynamin-related protein 1 (DRP-1), so that damaged mitochondria could be separated from the mitochondrial network and successfully identified and removed.21,35 Excessive mechanical stress will lead to impaired mitochondrial clearance in chondrocytes.36 We have previously found that EA could increase the mechanical properties of muscles through the function of acupoints, thus improving the mechanical environment of the knee joint,37–39 which may be the way EA activates the Pink1-Parkin pathway to regulate mitophagy. Numerous studies have confirmed that EA could activate mitophagy well, and this study complicates the mechanism of EA in KOA.40,41 However, this study has the following limitations. In the study of mitophagy, JC-1 probe is often used to detect mitochondrial membrane potential to evaluate the structure and function of mitochondria. However, this technique can only be used for in vitro cell detection at present and could not be carried out in this study. Therefore, TEM observation and ATP assay were used. It is expected that more appropriate methods will be developed to explain the mechanism of EA in future studies.
Conclusion
In conclusion, we found that EA could help attenuate cartilage matrix degradation in KOA model rabbits, and the mechanism might be related to the activation of mitophagy signaling pathway mediated by Pink1-Parkin, so as to clear damaged mitochondria and play a protective role in cartilage.
Abbreviations
OA, osteoarthritis; KOA, knee osteoarthritis; EA, electroacupuncture; TEM, Transmission electron microscopy; ECM, extracellular matrix.
Ethics Approval
All animal experiments were performed at Beijing University of Chinese Medicine (Beijing China) according to the guidelines of the China Council on Animal Care and Use. This study was approved by the Committee of Experimental Animals of The Beijing University of Chinese Medicine (approval number: BUCM-4-2022-010101-1097). Every effort was made to minimize pain and discomfort to the animals.
Funding
This work was supported by Beijing Natural Science Foundation(NO.7192110) and National Natural Science Foundation of China (NO.82074523).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Long H, Liu Q, Yin H, et al. Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: findings From the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022;74(7):1172–1183.
2. Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376–387.
3. Otte P. Basic cell metabolism of articular cartilage. Manometric studies. Z Rheumatol. 1991;50(5):304–312.
4. Jahr H, Gunes S, Kuhn AR, Nebelung S, Pufe T. Bioreactor-Controlled Physoxia Regulates TGF-beta Signaling to Alter Extracellular Matrix Synthesis by Human Chondrocytes. Int J Mol Sci. 2019;20(7):1715.
5. Jaiswal N, Maurya CK, Arha D, et al. Fructose induces mitochondrial dysfunction and triggers apoptosis in skeletal muscle cells by provoking oxidative stress. Apoptosis. 2015;20(7):930–947.
6. Lepetsos P, Papavassiliou AG. ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys Acta. 2016;1862(4):576–591.
7. Chen Y, Wu YY, Si HB, Lu YR, Shen B. Mechanistic insights into AMPK-SIRT3 positive feedback loop-mediated chondrocyte mitochondrial quality control in osteoarthritis pathogenesis. Pharmacol Res. 2021;166:105497.
8. Zeng Z, Zhou X, Wang Y, et al. Mitophagy-A New Target of Bone Disease. Biomolecules. 2022;12(10):1420.
9. Reed KN, Wilson G, Pearsall A, Grishko VI. The role of mitochondrial reactive oxygen species in cartilage matrix destruction. Mol Cell Biochem. 2014;397(1–2):195–201.
10. Wang C, Yang Y, Zhang Y, Liu J, Yao Z, Zhang C. Protective effects of metformin against osteoarthritis through upregulation of SIRT3-mediated PINK1/Parkin-dependent mitophagy in primary chondrocytes. Biosci Trends. 2019;12(6):605–612.
11. Gong G, Song M, Csordas G, Kelly DP, Matkovich SJ, Dorn GN. Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science. 2015;350(6265):aad2459.
12. Huang LW, Huang TC, Hu YC, et al. Zinc protects chondrocytes from monosodium iodoacetate-induced damage by enhancing ATP and mitophagy. Biochem Biophys Res Commun. 2020;521(1):50–56.
13. Ansari MY, Khan NM, Ahmad I, Haqqi TM. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthritis Cartilage. 2018;26(8):1087–1097.
14. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020;72(2):220–233.
15. Chen WH. Guidelines for TCM diagnosis and treatment of knee osteoarthritis(2020 edition). J Traditional Chine Orthopedics Traumatol. 2020;32(10):1–14.
16. Ji B, Liu QG, Fu YY, et al. Effect of the acupotomy therapy and electro-acupuncture on the serum contents of IL-1β, IL-6 and TNF-α in rabbits with knee osteoarthritis. Chine J Pathophysiol. 2009;25(06):1165–1169.
17. Zhang Q, Zhang D, Xu Y, Chen XL, Qin LX, Guo CQ. Effect of Acupotomy on Oxidative Stress of Chondrocytes in KOA Rabbit. J Em Traditional Chine Med. 2022;31(10):1708–1712.
18. Liao Y, Li XH, Li N, Zhou J. Electroacupuncture protects against articular cartilage erosion by inhibiting mitogen-activated protein kinases in a rat model of osteoarthritis. Acupunct Med. 2016;34(4):290–295.
19. Shi XW, Yu WJ, Wang T, et al. Electroacupuncture alleviates cartilage degradation: improvement in cartilage biomechanics via pain relief and potentiation of muscle function in a rabbit model of knee osteoarthritis. Biomed Pharmacother. 2020;123.
20. Lin J, Wu G, Chen J, et al. Electroacupuncture inhibits sodium nitroprusside‑mediated chondrocyte apoptosis through the mitochondrial pathway. Mol Med Rep. 2018;18(6):4922–4930.
21. Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20(1):31–42.
22. Luxue Q, Changqing G, Ruili Z, et al. Acupotomy inhibits aberrant formation of subchondral bone through regulating osteoprotegerin/receptor activator of nuclear factor-kappaB ligand pathway in rabbits with knee osteoarthritis induced by modified Videman method. J Tradit Chin Med. 2022;42(3):389–399.
23. Gong YR, Lin QX, Liu J, Guo ZX, Lu LM, Xiu ZB. Effect of akupotomye on stiffness and fibrosis of rectus femoris muscle in rabbits with knee osteoarthritis. China Medical Herald. 2022;19(15):8–11.
24. Guo Y. Experimental Acupuncture Science. China Press of Traditional Chinese Medicine; 2021.
25. Asjid R, Faisal T, Qamar K, Malik S, Umbreen F, Fatima M. Effect of Platelet-rich Plasma on Mankin Scoring in Chemically-induced Animal Model of Osteoarthritis. J Coll Physicians Surg Pak. 2019;29(11):1067–1071.
26. Peng Z, Sun H, Bunpetch V, et al. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials. 2021;268:120555.
27. Sun K, Jing X, Guo J, Yao X, Guo F. Mitophagy in degenerative joint diseases. Autophagy. 2021;17(9):2082–2092.
28. Adarmes H, Donders L, Dorner C, Gonzalez E, Galleguillos M. Glycosaminoglycans (GAGs) determination in healthy and damaged equine articular cartilage. Austral J Vet Sci. 2017;49(2):129–133.
29. Tu JF, Yang JW, Shi GX, et al. Efficacy of Intensive Acupuncture Versus Sham Acupuncture in Knee Osteoarthritis: a Randomized Controlled Trial. Arthritis Rheumatol. 2021;73(3):448–458.
30. Wu WH, Tang J, Wu YP, Chen ZY. Modern rehabilitation evaluation of efficacy of electric acupuncture at Xiyan in treating knee osteoarthritis. Shanghai J Traditional Chine Med. 2015;49(06):63–65.
31. Wirat T, Daranee S, Chaniya T, Chantima C, Aroon C. Comparison of the Effectiveness of Six and Two Acupuncture Point Regimens in Osteoarthritis of the Knee: a Randomised Trial. Acupunct Med. 2009;27(1):1420.
32. Cheleschi S, Cantarini L, Pascarelli NA, et al. Possible chondroprotective effect of canakinumab: an in vitro study on human osteoarthritic chondrocytes. Cytokine. 2015;71(2):165–172.
33. Xin RB, Xu YY, Long DB, et al. Mitochonic Acid-5 Inhibits Reactive Oxygen Species Production and Improves Human Chondrocyte Survival by Upregulating SIRT3-Mediated, Parkin-dependent Mitophagy. Front Pharmacol. 2022;13:911716.
34. Jin ZZ, Chang BH, Wei YL, et al. Curcumin exerts chondroprotective effects against osteoarthritis by promoting AMPK/PINK1/Parkin-mediated mitophagy. Biomed Pharmacother. 2022;151.
35. Scheitlin CG, Nair DM, Crestanello JA, Zweier JL, Alevriadou BR. Fluid Mechanical Forces and Endothelial Mitochondria: a Bioengineering Perspective. Cell Mol Bioeng. 2014;7(4):483–496.
36. Wang YY, Hu YZ, Wang HM, et al. Deficiency of MIF Accentuates Overloaded Compression-Induced Nucleus Pulposus Cell Oxidative Damage via Depressing Mitophagy. Oxid Med Cell Longev. 2021;2021:45.
37. Hu B, Yu JN, Zhang HF, Liu NG, Guo CQ. Effect of Acupotomy Intervention on Contractility of Quadriceps Femoris and Pathological Changes of Articular Cartilage in KOA Rabbits. J Clin Acupuncture Moxibustion. 2018;34(11):50–54.
38. Wang LJ, Shi XW, Zhang W, Wang T, Zhou S, Guo CQ. Effect of needle knife intervention on tensile mechanics of femoral quadriceps tendon in rabbits with knee osteoarthritis. China J Orthopaedics Traumatol. 2019;32(05):462–468.
39. E’Erdun W, Guo CQ, Wang T, et al. Effects of Acupotomy Intervention on Extensor-flexor Atrophy and Elastic Modulus of Muscular Tension in Rabbit Model of Medium-term Knee Osteoarthritis. J Hunan Univ Chine Med. 2019;39(10):1248–1253.
40. Guan R, Li Z, Dai X, et al. Electroacupuncture at GV20‑GB7 regulates mitophagy to protect against neurological deficits following intracerebral hemorrhage via inhibition of apoptosis. Mol Med Rep. 2021;24(1):98.
41. Hsu WT, Chen YH, Yang HB, Lin JG, Hung SY. Electroacupuncture Improves Motor Symptoms of Parkinson’s Disease and Promotes Neuronal Autophagy Activity in Mouse Brain. Am J Chin Med. 2020;48(7):1651–1669.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.