Back to Journals » Journal of Pain Research » Volume 16
Electroacupuncture Attenuates Neuropathic Pain in a Rat Model of Cervical Spondylotic Radiculopathy: Involvement of Spinal Cord Synaptic Plasticity
Authors Yang P , Chen HY, Zhang X, Wang T, Li L, Su H, Li J , Guo YJ, Su SY
Received 30 March 2023
Accepted for publication 7 July 2023
Published 18 July 2023 Volume 2023:16 Pages 2447—2460
DOI https://doi.org/10.2147/JPR.S415111
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Pu Yang,1,* Hai-Yan Chen,2,* Xi Zhang,2 Tian Wang,1 Ling Li,2 Hong Su,1 Jing Li,1 Yan-Jun Guo,1 Sheng-Yong Su2,3
1Guangxi University of Chinese Medicine, Nanning, Guangxi, People’s Republic of China; 2The First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning, Guangxi, People’s Republic of China; 3Guangxi Key Laboratory of Molecular Biology of Preventive Medicine of Traditional Chinese Medicine, Nanning, Guangxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Sheng-Yong Su, Email [email protected]
Purpose: Cervical spondylotic radiculopathy (CSR) is a common neurologic condition that causes chronic neck pain and motor functions, with neuropathic pain (NP) being the primary symptom. Although it has been established that electroacupuncture (EA) can yield an analgesic effect in clinics and synaptic plasticity plays a critical role in the development and maintenance of NP, the underlying mechanisms have not been fully elucidated. In this study, we explored the potential mechanisms underlying EA’s effect on synaptic plasticity in CSR rat models.
Materials and Methods: The CSR rat model was established by spinal cord compression (SCC). Electroacupuncture stimulation was applied to LI4 (Hegu) and LR3 (Taichong) acupoints for 20 min once a day for 7 days. Pressure pain threshold (PPT) and mechanical pain threshold (MPT) were utilized to detect the pain response of rats. A gait score was used to evaluate the motor function of rats. Enzyme-linked immunosorbent assay (ELISA), Western blot (WB), immunohistochemistry (IHC), immunofluorescence (IF), and transmission electron microscopy (TEM) were performed to investigate the effects of EA.
Results: Our results showed that EA alleviated SCC-induced spontaneous pain and gait disturbance. ELISA showed that EA could decrease the concentration of pain mediators in the cervical nerve root. WB, IHC, and IF results showed that EA could downregulate the expression of synaptic proteins in spinal cord tissues and promote synaptic plasticity. TEM revealed that the EA could reverse the synaptic ultrastructural changes induced by CSR.
Conclusion: Our findings reveal that EA can inhibit SCC-induced NP by modulating the synaptic plasticity in the spinal cord and provide the foothold for the clinical treatment of CSR with EA.
Keywords: electroacupuncture, synaptic plasticity, cervical spondylotic radiculopathy, spinal dorsal horn
Introduction
Cervical spondylotic radiculopathy (CSR) is one of the most common cervical disc degenerative diseases of the spine, accounting for 60–70% of cervical spondylosis cases.1 The past few years have witnessed an increase in the annual incidence of CSR due to population aging, lifestyle changes, life or work pressure, and other factors.2 It has been established that CSR-induced neck and shoulder pain can be categorized as chronic pain,3 which is typically caused by nerve root compression. Neuropathic pain (NP) is characterized by a long course, high incidence, and high recurrence rate, which are the main symptoms of CSR.4 NP refers to chronic pain related to neurological system lesions or diseases with a major impact on quality of life and results in a significant financial burden.5 The treatment of NP is often challenging, with only a small percentage of patients benefiting from several interventions.6 Surgery is the typical treatment for CSR when disc herniation is limited to the level of the disc, but they suffer from several limitations,7 such as the progression of kyphosis and postoperative incision pain. Accordingly, conservative treatment has become the preferred alternative for CSR in recent years, encompassing medical exercise therapy, mechanical cervical tractions, transcutaneous electrical nerve stimulation, pain management education, and cervical collar. However, these strategies are controversial for the long-term treatment of CSR, and there is an urgent need for more effective and convenient therapy.
Chronic NP is characterized by allodynia, hyperalgesia, and spontaneous pain.8,9 The spinal cord is pivotal in pain transmission and modulation.10 Current evidence suggests that synapses located in the dorsal horn of the spinal cord are closely related to nociceptive signal transmission.11 Changes in synaptic plasticity are considered a critical process in the recovery of function after nerve damage.12 A preclinical animal study showed that synaptic plasticity is involved in the development of chronic neuropathic pain through the release of brain-derived neurotrophic factor (BDNF).13 The levels of synaptic proteins that serve as markers of synaptic plasticity have been reported to be increased in the spinal cord, such as postsynaptic density 95 (PSD-95), growth-associated protein 43 (GAP-43), synaptophysin (SYN), and alpha-synuclein (α-syn) in NP model rats.14,15 Meanwhile, a significant decrease in the width of the synaptic cleft and an increase in the thickness of the postsynaptic density were observed in the spinal dorsal horn of NP rats. Taken together, these results substantiate that the regulation of spinal synaptic plasticity directly or indirectly contributes to the progression of NP.
Siguan is a combination of LI4 and LR3 acupoints used in traditional Chinese medicine to treat pain conditions. The theoretical basis of this therapy revolves around the concept that pain is associated with impaired Qi circulation, and acupuncture at the Siguan point facilitates the flow of Qi and alleviates pain. Thus, selecting the Siguan point can regulate Qi movement and relieve pain. LI4 and LR3 acupoints are commonly used in acupuncture clinics to treat various types of pain, as reported in the published literature.16–18 Electroacupuncture (EA) is a technique that involves applying an electrical current to acupuncture needles. As a promising and beneficial complementary and alternative medicine, it has been widely used to alleviate pain for over 100 years. Some studies indicated that EA could effectively attenuate NP;19,20 the mechanism may be related to peripheral and central sensitization. In terms of peripheral effects, EA has been shown to inhibit the interaction between peripheral TRPV1 and P2X3 receptors.21 At the same time, in the central nervous system, EA can activate the spinal microglial IL-10/β-endorphin pathway22 and inhibit synaptic vesicle protein expression in the spinal cord to exert an analgesic effect.23 Some studies reported that electroacupuncture could improve synaptic plasticity in NP rats model induced by spinal nerve ligation (SNL).24,25 However, the mechanism underlying the regulatory effect of EA on synaptic plasticity for CSR is still poorly understood.
We hypothesized that synaptic plasticity changes in the spinal cord are involved with NP induced by CSR, which EA may mitigate. To explore the underlying mechanistic basis for the analgesic effect of EA on CSR, we established the CSR model via spinal cord compression, which has been shown to induce spontaneous pain and gait disturbance.26 Importantly, we investigated whether electroacupuncture reduced pain perception and expression of synaptic plasticity-related proteins in the spinal cord, regulated the synaptic ultrastructure in the spinal dorsal horn under CSR, and decreased the expression of pain mediators in the cervical nerve root. Our results suggest that electroacupuncture can alleviate NP in CSR rats by reducing the expression of synaptic proteins and regulating synaptic plasticity.
Materials and Methods
Animals
Male Sprague-Dawley (SD) rats, eight weeks old and weighing an average of 250 ± 20 g, were purchased from Changsha Tianqin Biotechnology Co., Ltd. (Changsha, China) [batch number SCXK (Xiang) 2019-0014]. Five rats were housed per cage at a temperature of 23–25°C and maintained under a 12-hour light/12-hour dark cycle, with free access to food and water. The rats were housed at the Guangxi Key Laboratory of Molecular Biology of Preventive Medicine of Chinese Medicine, where they were allowed to adapt for at least one week before the start of experiments. All experimental protocols and procedures were approved by the Animal Care and Welfare Committee of the Guangxi University of Chinese Medicine (Approve No. DW20220430-075) and followed the International Ethical Guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals to ensure adherence to ethical principles.
CSR Model Establishment
Out of the 60 male SD rats, 12 were randomly assigned to the normal group, and another 15 were randomly assigned to the sham operation group. The remaining 33 were selected as CSR models. Spinal cord compression (SCC) was performed for model establishment as previously described in the literature.27,28 First, the model rats were anesthetized by inhaling 5% isoflurane, which was subsequently reduced to 2% to maintain anesthesia during the experiment (RWD, Shenzhen, China). The rats were immobilized in the prone position, and the neck area was shaved and sterilized using 75% ethanol. A 3 cm midline incision was made in the nuchal C5-T2 region, and the subcutaneous tissues and posterior cervical muscles were gently retracted to fully expose the C6-T1 laminae. The cervical laminae were identified, starting from T1, and the ligamentum flavum and connective tissue of C6-C7 and C7-T1 were carefully separated to expose the C6 lamina. A nylon suture (diameter 0.5 mm, length 15 mm) was then implanted underneath the C6-C7 and T1 laminae, ensuring the dura was kept separate from the lamina to prevent cerebrospinal fluid (CSF) leakage and tearing. After the operation, the incision was meticulously closed in layers. To prevent infection, subcutaneous injection of 80 TU of penicillin was administered. The sham group underwent a similar surgical procedure without spinal canal insertion. The rats were kept on a heating pad until they regained consciousness and were then returned to their cages with access to food and water.
Behavioral Test
The behaviors of rats, such as licking, biting, and hoarseness, were observed every morning after the operation. As previously described by Kawakami et al,29 we evaluated gait disturbance by assigning scores based on the presence and severity of foot deformities and motor paresis of the ipsilateral hind paw. A score of 1 was given for a normal gait with no foot deformities, while a gait with a marked foot deformity, such as a plantar flexed toe or inverted foot, was rated 2. Models were deemed successful when the behavioral score reached 2 or higher, indicating significant gait disturbance. These methods allowed us to accurately assess the effects of our intervention on the CSR rat model.
Pressure Pain Threshold (PPT)
The hindlimb pressure pain threshold was assessed by measuring paw withdrawal responses in response to a blunt plexiglass cone source. Tenderness responses were assessed using a YLS-3E electronic pressure apparatus.9 The apparatus was used to apply linearly increasing pressure to the third and fourth metatarsi or lateral plantar regions of the rat. Paw withdrawal, shaking, or screaming were considered pain-like responses, and the pressure value (in grams) automatically recorded was indicative of the pressure pain threshold (PPT) of the rat. More detailed information on the appearance of the YLS-3E and operation is provided in Figure 1.
 |
Figure 1 Details of YLS-3E electronic pressure apparatus. Notes: (A) The appearance of the YLS-3E electronic pressure apparatus. (B) Operation of YLS-3E electronic pressure apparatus. |
Mechanical Pain Threshold (MPT)
The mechanical pain threshold of the hind paw was detected using the up-down paradigm, as described in a previous report.30 The rats were placed in a plexiglass cage with metal mesh at the bottom and allowed to adjust to the environment for 15 min. A set of von Frey filaments (North Coast, USA) was then used to measure the mechanical pain threshold at the plantar region of the right or left hind paw, and the rats were observed for paw withdrawal or licking responses. If there was no response, it was marked as “O”, on the contrary, it was marked as “X”. In cases where a combination of “OX” or “XO” occurred, four additional measurements were taken to obtain a sequence of “O” and “X”. The mechanical pain threshold was calculated and analyzed using the following formula:  , Xf is the number of the last filament, δ is the average difference between the filaments, about 0.224, k is the table reference value according to the above sequence.
, Xf is the number of the last filament, δ is the average difference between the filaments, about 0.224, k is the table reference value according to the above sequence.
Electroacupuncture Treatment
The rats were anesthetized with 2% isoflurane via inhalation prior to electroacupuncture. They were gently immobilized using a self-made retainer. The acupoints LI4 (Hegu, located between the first and second metacarpal bones of the forelimbs) and LR3 (Taichong, located at the depression between the first and second metatarsal junctions of the hindlimbs) were selected bilaterally. Stainless steel acupuncture needles (diameter 0.25 mm, length 13 mm) were inserted into the acupoints at 3–5 mm depth. The ipsilateral needles were connected and stimulated using low-frequency electronic pulse therapy (G6805-I, Qingdao Xinsheng Industrial Co., Ltd., Shandong, China) for 20 minutes at 1 mA and 1.5 Hz. This electroacupuncture procedure was performed once a day for seven consecutive days.
Enzyme-Linked Immunosorbent Assay (ELISA)
To determine the levels of substance P (SP), prostaglandin E2 (PGE2), and neuropeptide Y (NPY) in the cervical nerve root samples, the cervical nerve roots were collected after the last treatment from rats of each group, then centrifuged at 5000 ×g for 10 min at 4°C and the supernatant was collected. The levels of Substance P (SP), prostaglandin E2 (PGE2), and neuropeptide Y (NPY) were determined using commercially available ELISA kits (E-EL-0067c, E-EL-0034c, and E-EL-R0655c, respectively; Elabscience Biotechnology Co., Ltd, Hubei, China). Diluted standards (50 μL) were added to the standard wells, while blank wells received 50 μL of standard & sample diluent. The remaining wells received 50 μL of the sample to be tested. Subsequently, 50 μL of the antibody working solution was added to each well, and the ELISA plate was incubated at 37°C for 45 minutes. The liquid in the wells was then shaken off, and 350 μL of the washing solution was added to each well, which was then allowed to soak for one minute. The liquid in the wells was aspirated from the enzyme plate, and the plate was pat-dried. This washing process was then repeated three times. Next, 100 μL of the working solution of the enzyme conjugate was added to each well, and the ELISA plate was incubated at 37°C for 30 minutes. The liquid in the wells was then shaken off, and 90 μL of substrate solution was added to each well, followed by incubation at 37°C for 15 minutes. Finally, the reaction was terminated by adding 50 μL of termination solution. The concentrations of SP, PGE2, and NPY were expressed in pg/mL.
Western Blot (WB) Analysis
To examine the protein expression levels of the cervical spinal cord, tissues were treated with RIPA lysate (R0020, Solarbio Co., Ltd., Beijing, China) to extract the total protein, and the concentration was determined using the bicinchoninic acid assay as described previously.26 The sample protein (20 μg) was resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride membranes, and blocked for 1h with 5% (w/v) bovine serum albumin in Tris-buffered saline at room temperature. Primary antibodies were rabbit α-syn (AF0402, 1:1000, Affinity), synapsin1 (AF6201, 1:800, Affinity), synapsin2 (AF6202, 1:800, Affinity), and mouse β-actin (AF7018, 1:7000, Affinity) and diluted in Tris-buffered saline with Tween 20 buffer overnight at 4°C. The membranes were then incubated with secondary horseradish peroxidase-conjugated goat anti-rabbit antibody (ZB2301, 1:3000, ZSGB-BIO) for 90 min. Protein signals were visualized using enhanced chemiluminescence (ECL) fluorescence test kit (SC-2048, ZSGB-BIO) at a 1:1 (v/v) ratio. Immunoreactivity was quantified using ImageJ software (National Institutes of Health, Bethesda). The relative protein content was determined by calculating the gray value of the corresponding protein band relative to the β-actin protein band.
Immunohistochemistry (IHC) Analysis
Quantitative IHC is a technique used to measure the density of protein expression in immunohistochemically stained images. This method involves calculating the chromogenic density or color and the associated signal intensity to obtain a quantitative result that can be used to study the amount or trend of protein expression in a cell or tissue. The cervical spinal cord of each group was collected and fixed in 4% paraformaldehyde for 3 days at 4°C. Fixed samples were embedded in paraffin, and 5μm-thick sections were obtained. Slices were deparaffinized in xylene and rehydrated in graded alcohol solutions. The tissue sections were repaired in 3% citric acid repair solution under high pressure and then incubated in 3% H2O2 for 10 min. Next, the sections were washed with distilled water for 5 min and soaked with phosphate-buffered saline (PBS) for 1min. The sections were then incubated with primary antibody to PSD-95 (AF7839, 1:100, Affinity), GAP-43 (DF7766, 1:90, Affinity) at 4°C overnight. The sections were washed three times with PBS buffer for 5 min on the next day, incubated with the secondary antibody at 37°C for 20 min, followed by reaction termination using 3,3’-diaminobenzidine (DAB). BX43 microscope (Olympus, Japan) was used to capture the immunohistochemistry images of the slices, and Image-Pro Plus software was used to quantify the captured image.
Immunofluorescence (IF) Analysis
The spinal cord tissues were fixed in 4% paraformaldehyde for a minimum of one week and sliced into sections 5-μm thick using a cryostat, then processed for IF. The sections were initially blocked with normal goat serum at 37°C for 30 minutes and subsequently incubated overnight at 4°C with primary antibodies specific to α-syn (AF0402, 1:100, Affinity) and PSD-95 (AF7839, 1:100, Affinity). After rinsing three times with PBS for 5 min each, the sections were incubated with a secondary antibody, and DAPI was used to stain the cell nuclei for 10 min at room temperature. The tissue slices were encapsulated by water-soluble tablets and viewed using a fluorescence microscope (Olympus, Japan). The area of positive staining was measured using Image-Pro Plus analysis software. The mean fluorescence units were calculated by dividing the total fluorescent intensity by the area of each fluorescent particle.
Transmission Electron Microscopy (TEM)
Spinal cord tissues (1mm×1mm×1mm) were rinsed with PBS for 4 times and then fixed in 1% osmic acid at 4°C for 2h, dehydrated in a graded by ethanol and acetone gradient, and embedded in SPI-Pon812. Ultrathin sections (50–70nm) were obtained using an ultramicrotome. After double staining with uranyl acetate and lead citrate, the ultrastructural changes of synapses in the spinal dorsal horn neurons were observed by HT7700 electron microscope (Hitachi, Tokyo, Japan). ImageJ software was used to analyze the synapse structure.
Statistical Analysis
SPSS 26.0 statistical software (IBM Corp, Armonk, NY, USA) was used for data analysis. All experimental data were presented as mean ± standard deviation (SD). The normality of data distribution was assessed by the Shapiro–Wilk normality test. The multiple comparisons were analyzed using one-way analysis of variance (ANOVA) followed by Least Significant Difference (LSD) post hoc comparisons for data with equal variances. In case of unequal variances, the Dunnett T3 test was used. A P-value <0.05 was statistically significant.
Results
Electroacupuncture Improved Pressure Pain Threshold and Mechanical Pain Threshold in Cervical Spondylotic Radiculopathy Rats
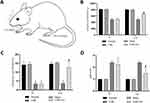
To investigate the analgesic effect of EA on the CSR model induced by cervical spinal canal insertion, the pressure pain threshold and mechanical pain threshold were measured using the YLS-3E electronic pressure apparatus and von Frey filaments. As shown in Figure 2B and C, the pain threshold decreased in CSR model rats after the operation compared with the normal and sham groups (P<0.05). After EA intervention, the decreased pain threshold was reversed compared with the model group (P<0.05). These results demonstrated that EA could alleviate CSR-induced neuropathic pain.
Electroacupuncture Alleviated Gait Disturbance Caused by Cervical Spondylotic Radiculopathy
To assess the gait disturbance effect of EA on CSR model rats, we used the gait score, a widely used measure for rodents known to require forelimb motor function. As shown in Figure 2D, the gait score was increased in the CSR and CSR+EA groups (P<0.05), indicating that motor function was compromised. In addition, the CSR+EA group was significantly lower than the CSR group after intervention (P<0.05), indicating that EA could alleviate CSR-induced gait disturbance.
Electroacupuncture Reduced Nerve Root Expression of Pain Mediators
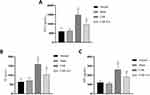
It is widely acknowledged that substance P (SP), prostaglandin E2 (PGE2), and neuropeptide Y (NPY) are key pain mediators involved in the modulation of pain perception and transmission. SP can promote glutamate release and enhance nociceptive signaling, thus contributing to the development and maintenance of pain. Besides, PGE2 can sensitize peripheral nociceptors and enhance subjective pain perception, potentiating pain response. NPY is highly expressed in nerve injury and is thought to play a key role in the development and maintenance of NP. Therefore, we measured the expression of SP, PGE2, and NPY on the cervical nerve root by ELISA. As shown in Figure 3, SP, PGE2, and NPY were higher in the CSR group than in the normal and sham groups (P<0.05). In addition, EA treatment significantly decreased the protein expression of SP, PGE2, and NPY compared to the CSR group (P<0.05). These results showed that CSR could release pain mediators, which could be reversed by EA stimulation.
Electroacupuncture Reversed Synaptic Protein Expression in Cervical Spinal Cord
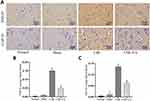
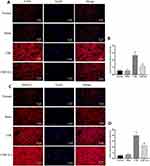
To determine the effect of EA on synaptic proteins, we measured the expression of α-syn, synapsin1, and synapsin2 proteins via WB assays, while the expression of postsynaptic marker postsynaptic density protein 95 (PSD-95) and growth-associated protein 43 (GAP-43) was measured by immunohistochemistry analyses. As shown in Figure 4, WB indicated that α-syn, synapsin1, and synapsin2 protein expression in the cervical spinal cord was higher in the CSR group than in the normal group and the sham group but was decreased in the CSR+EA group (P<0.05). Furthermore, as shown in Figure 5, immunohistochemistry analysis showed the average optical densities of PSD-95 and GAP-43 in the spinal cord were higher in the CSR group than in the normal group and the sham group but were significantly decreased in the CSR+EA group (P<0.05). On the other hand, as shown in Figure 6, the mean fluorescence intensity of PSD-95 was higher in the CSR group than in the normal group and sham group (P<0.05); EA treatment significantly decreased the mean fluorescence intensity (P<0.05), consistent with the WB and immunohistochemistry results. These findings suggested that EA could downregulate the expression of multiple synaptic proteins, thus improving synaptic plasticity.
EA Preserved the Synaptic Ultrastructure of Spinal Dorsal Horn Neurons
To explore whether EA could impact synaptic ultrastructure, TEM was used to observe the synapses of cervical dorsal horn neurons. As shown in Figure 7, in the normal group, the synaptic vesicles in the presynaptic membrane were evenly distributed, while the postsynaptic side exhibited a thinner postsynaptic density (PSD), indicating no substantial accumulation of transduction-related proteins and no significant increase in the synaptic cleft width. At the same time, there was no significant difference between the sham group and the normal group in synaptic ultrastructure. In the CSR group, the synaptic vesicles in the presynaptic membrane gathered, the presynaptic active zone became longer, and the PSD area increased, indicating that many transduction-related proteins accumulated and the synaptic cleft width increased. On the other hand, the CSR+EA group exhibited an equal distribution of synaptic vesicles, shorter presynaptic active zone, thinner PSD zone, and narrower width of synaptic cleft compared with the CSR group.
Discussion
CSR has been associated with foraminal space reduction, degenerative disc disease, and spondylarthrosis. Based on previous animal studies,27,28 we used a compression material to induce nerve root injury in rats, resulting in a clinically relevant model of CSR presenting spontaneous pain behavior and gait disturbance. Our results showed that EA at LI4 and LR3 acupoints for 7 days significantly alleviated neuropathic pain and motor function impairments, consistent with our previous study. Our previous studies revealed that EA could reduce the release of inflammatory factors in CSR rats through the JAK-STAT/SCOS signaling pathway.31 However, the central mechanism of EA in treating CSR remains to be elucidated.
EA is a therapeutic modality incorporating electrical stimulation into traditional acupuncture. By combining the principles of traditional meridian theory with the benefits of low-frequency currents, EA can adjust stimulation intensity and frequency. Several studies have shown that EA effectively alleviates chronic neck pain and movement impairments induced by spondylotic cervical myelopathy.32 The analgesic effect appears to be related to the modulation of spinal synaptic plasticity through the suppression of the TNF-α-TNFR1-caspase-8 signal pathway and increased expression of integrin β1 and Akt, which inhibit the apoptosis of annulus fibrosis cells.33 Our study provided hitherto unreported evidence that LI4 and LR3 acupoints EA intervention effectively alleviated pain and gait impairments by modulating spinal synaptic plasticity. This mechanism has been observed in various pain conditions, such as neuropathic pain,25 trigeminal neuralgia,34 sciatica,35 and irritable bowel syndrome.36 There is an increasing consensus suggesting that the efficacy of EA is frequency-dependent and can improve synaptic plasticity by upregulating basic fibroblast growth factor expression.24 Taken together, our findings indicate that EA has huge potential as a treatment modality for pain and functional impairments associated with various conditions.
It is now understood that surgery-related incisional pain can trigger the release of various pain mediators, including SP, PGE2, and NPY.37 SP is a neurotransmitter that accelerates pain transmission via glutamate release from primary nociceptive afferents in the spinal cord.38 PGE2, produced by cyclooxygenases (COX), promotes inflammatory pain sensitization by promoting synaptic transmission in the spinal cord dorsal horn.39 On the other hand, NPY is reportedly one of the most significantly upregulated neuropeptides in dorsal root ganglion neurons after nerve injury,40 and NPY antagonists or genetic knockdown of NPY expression have been found to alleviate neuropathic pain.41 A previous study reported that electroacupuncture could reduce the spinal levels of pain mediators such as SP, CGRP, COX-1, and PGE2 and alleviate neck-incision pain.42 Our study further validated that EA treatment could reverse SCC-induced upregulation of SP, PGE2, and NPY in the nerve root.
Pain is a complex phenomenon involving both peripheral inputs and central neuronal plasticity. The spinal cord’s synaptic plasticity is widely thought to be crucial for central nervous system function. Numerous rodent studies suggest that nociceptor peripheral sensitization and long-term synaptic plasticity in the spinal cord contribute to chronic pain.43 Central sensitization, a form of synaptic plasticity, reflects the somatosensory nervous system’s response to activity, inflammation, and neural injury.44 This process is modulated by the ascending excitatory pathway and the descending pain modulatory system. Previous studies have suggested that EA may alleviate pain by suppressing neuroglial plasticity-mediated central sensitization.45,46
Synaptic proteins such as α-syn, synapsin1, synapsin2, PSD-95, and GAP-43 are important markers for detecting synaptic plasticity and play a crucial role in the development of pain hypersensitivity.47 For instance, α-syn was found to modulate both short- and long-term synaptic plasticity during in vitro and in vivo studies,48 and its spread from the peripheral to the central nervous system has been associated with sensory nerve degradation and increased pain sensitivity.15 Similarly, it has been shown that synapsin1 and synapsin2 regulate glutamate and GABA release in the spinal cord after nerve injury.49,50 Our study showed that electroacupuncture could reverse SCC-induced upregulation of α-syn, synapsin1, and synapsin2 proteins in the spinal cord. PSD-95, involved in the central sensitization of neuropathic pain, can be knocked down to reverse mechanical and thermal hyperalgesia caused by spinal cord or nerve injury.51 Additionally, GAP-43, a marker related to the occurrence and remodeling of synaptic structure, is upregulated in the spinal dorsal horn and associated with hyperalgesia. Our study provided compelling evidence that EA could suppress the overexpression of PSD-95 and GAP-43 proteins in CSR rats. Furthermore, changes in synaptic vesicles, synaptic cleft, PSD thickness, and length of the synaptic active zone were linked to synaptic function and played an important role in functional plasticity. Our TEM results showed that EA could reverse the effects of CSR on synaptic ultrastructure. Our findings suggest that the modulation of synaptic structural and functional plasticity may mediate the analgesic effects of electroacupuncture in CSR.
Although our study demonstrates the effects of electroacupuncture on synaptic morphology structure, it has some limitations that should be addressed in future research. One aspect that should be explored is the relationship between synaptic plasticity and central sensitization, which is considered a key factor in the development of neuropathic pain.52,53 Additionally, synaptic plasticity is the cellular physiological basis of pain signal transmission, including changes in synaptic structure and function.54 Despite exploring the effects of EA on synaptic morphology structure, our study did not examine long-term potentiation using electrophysiological techniques, which will be assessed in our future studies.
Conclusions
Our findings corroborate that electroacupuncture at LI4 (Hegu) and LR3 (Taichong) effectively alleviates neuropathic pain, improves motor function, and increases the pressure and mechanical pain thresholds in CSR rats. These analgesic effects are related to the downregulation of pain mediators and synaptic proteins and changes in synaptic ultrastructure, which regulate spinal cord synaptic plasticity. These results provide a rationale for the application of electroacupuncture as a therapeutic approach to neuropathic pain management.
Data Sharing Statement
The data used to support the findings of this study are available from the published literature.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81960895, and No. 82160934); the Innovation Project of Guangxi Graduate Education (YCBXJ2023023). We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Pu Yang and Hai-Yan Chen share first authorship. The authors declare that they have no conflicts of interest for this work.
References
1. Hu J, Chen F, Qiu G, et al. Jingshu Keli for treating cervical spondylotic radiculopathy: the first multicenter, randomized, controlled clinical trial. J Orthop Translat. 2021;27:44–56. doi:10.1016/j.jot.2020.10.010
2. Luyao H, Xiaoxiao Y, Tianxiao F, et al. Management of cervical spondylotic radiculopathy: a systematic review. Global Spine J. 2022;12(8):1912–1924. doi:10.1177/21925682221075290
3. Yu CX, Li B, Xu YK, et al. Altered functional connectivity of the periaqueductal gray in chronic neck and shoulder pain. Neuroreport. 2017;28(12):720–725. doi:10.1097/wnr.0000000000000819
4. Liu XH, Du YM, Cong HJ, et al. Effects of continuous epidural injection of dexamethasone on blood glucose, blood lipids, plasma cortisol and ACTH in patients with neuropathic pain. Front Neurol. 2020;11:564643. doi:10.3389/fneur.2020.564643
5. Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. 2021;101(1):259–301. doi:10.1152/physrev.00045.2019
6. Ju ZY, Wang K, Cui HS, et al. Acupuncture for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;12(12):Cd012057. doi:10.1002/14651858.CD012057.pub2
7. Bai LL, Wang WT, Wang JF, et al. Anterior cervical discectomy and fusion combined with foraminotomy assisted by high-definition 3-dimensional exoscope in the treatment of cervical spondylotic radiculopathy secondary to bony foraminal stenosis. Orthop Surg. 2021;13(8):2318–2326. doi:10.1111/os.13040
8. Zhou LJ, Peng J, Xu YN, et al. Microglia are indispensable for synaptic plasticity in the spinal dorsal horn and chronic pain. Cell Rep. 2019;27(13):3844–3859.e3846. doi:10.1016/j.celrep.2019.05.087
9. Obata K, Yamanaka H, Kobayashi K, et al. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci. 2004;24(45):10211–10222. doi:10.1523/jneurosci.3388-04.2004
10. Sun L, Peng C, Joosten E, et al. Spinal cord stimulation and treatment of peripheral or central neuropathic pain: mechanisms and clinical application. Neural Plast. 2021;2021:5607898. doi:10.1155/2021/5607898
11. Gómez de San José N, Massa F, Halbgebauer S, et al. Neuronal pentraxins as biomarkers of synaptic activity: from physiological functions to pathological changes in neurodegeneration. J Neural Transm. 2022;129(2):207–230. doi:10.1007/s00702-021-02411-2
12. Rothwell JC. Plasticity in the human motor system. Folia Phoniatr Logop. 2010;62(4):153–157. doi:10.1159/000314030
13. Zhang L, Wang G, Ma J, et al. Brain-derived neurotrophic factor (BDNF) in the rostral anterior cingulate cortex (rACC) contributes to neuropathic spontaneous pain-related aversion via NR2B receptors. Brain Res Bull. 2016;127:56–65. doi:10.1016/j.brainresbull.2016.08.016
14. Zhang Z, Ding X, Zhou Z, et al. Sirtuin 1 alleviates diabetic neuropathic pain by regulating synaptic plasticity of spinal dorsal horn neurons. Pain. 2019;160(5):1082–1092. doi:10.1097/j.pain.0000000000001489
15. Ferreira N, Gonçalves NP, Jan A, et al. Trans-synaptic spreading of alpha-synuclein pathology through sensory afferents leads to sensory nerve degeneration and neuropathic pain. Acta Neuropathol Commun. 2021;9(1):31. doi:10.1186/s40478-021-01131-8
16. Dias PA, Guimarães AB, Albuquerque Ade O, et al. Short-term complementary and alternative medicine on quality of life in women with fibromyalgia. J Integr Med. 2016;14(1):29–35. doi:10.1016/S2095-4964(16)60235-2
17. Liu J, Huang Z, Zhang GH. Involvement of NF-κB signal pathway in acupuncture treatment of patients with rheumatoid arthritis. Zhen Ci Yan Jiu. 2020;45(11):914–919. doi:10.13702/j.1000-0607.190848
18. Li JN, Li J, Liu J, et al. Acupuncture with Tiaochong Shugan method by stages for menstrual headache based on syndrome differentiation: a randomized controlled trial. Zhongguo Zhen Jiu. 2022;42(10):1108–1112. doi:10.13703/j.0255-2930.20211203-k0005
19. Lang PM, Stoer J, Schober GM, et al. Bilateral acupuncture analgesia observed by quantitative sensory testing in healthy volunteers. Anesth Analg. 2010;110(5):1448–1456. doi:10.1213/ANE.0b013e3181d3e7ef
20. Zheng Z, Feng SJ, Costa C, et al. Acupuncture analgesia for temporal summation of experimental pain: a randomised controlled study. Eur J Pain. 2010;14(7):725–731. doi:10.1016/j.ejpain.2009.11.006
21. Liu Y, Du J, Fang J, et al. Electroacupuncture inhibits the interaction between peripheral TRPV1 and P2X3 in rats with different pathological pain. Physiol Res. 2021;70(4):635–647. doi:10.33549/physiolres.934649
22. Ali U, Apryani E, Wu HY, et al. Low frequency electroacupuncture alleviates neuropathic pain by activation of spinal microglial IL-10/β-endorphin pathway. Biomed Pharmacother. 2020;125:109898. doi:10.1016/j.biopha.2020.109898
23. Wan J, Nan S, Liu J, et al. Synaptotagmin 1 is involved in neuropathic pain and electroacupuncture-mediated analgesic effect. Int J Mol Sci. 2020;21(3):968. doi:10.3390/ijms21030968
24. Zhou K, Wu Q, Yue J, et al. Electroacupuncture suppresses spinal nerve ligation-induced neuropathic pain via regulation of synaptic plasticity through upregulation of basic fibroblast growth factor expression. Acupunct Med. 2022;40(4):379–388. doi:10.1177/09645284211066499
25. Xing GG, Liu FY, Qu XX, et al. Long-term synaptic plasticity in the spinal dorsal horn and its modulation by electroacupuncture in rats with neuropathic pain. Exp Neurol. 2007;208(2):323–332. doi:10.1016/j.expneurol.2007.09.004
26. Cai HQ, Lin XY, Chen HY, et al. Direct moxibustion exerts an analgesic effect on cervical spondylotic radiculopathy by increasing autophagy via the Act A/Smads signaling pathway. Brain Behav. 2022;12(4):e2545. doi:10.1002/brb3.2545
27. Shen WS, Li CF, Zhou ZS, et al. MicroRNA-204 silencing relieves pain of cervical spondylotic radiculopathy by targeting GDNF. Gene Ther. 2020;27(6):254–265. doi:10.1038/s41434-019-0114-3
28. Sun W, Zheng K, Liu B, et al. Neuroprotective potential of gentongping in rat model of cervical spondylotic radiculopathy targeting PPAR-γ pathway. J Immunol Res. 2017;2017:9152960. doi:10.1155/2017/9152960
29. Kawakami M, Weinstein JN, Spratt KF, et al.; Experimental lumbar radiculopathy. Immunohistochemical and quantitative demonstrations of pain induced by lumbar nerve root irritation of the rat. Spine. 1994;19(16):1780–1794. doi:10.1097/00007632-199408150-00001
30. Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi:10.1016/0165-0270(94)90144-9
31. Chen S, Su SY, Huang YL, et al. Effect of electroacupuncture at distal acupoints on expression of JAK1 and STAT3 proteins in local spinal cord tissue of CSR rats. Chin Archiv Trad Chin. 2018;36(05):1154–1156. doi:10.13193/j.issn.1673-7717.2018.05.033
32. Zhou Y, Wang W, Tian K, et al. Efficacy and safety of electroacupuncture in treatment of cervical spondylosis: a protocol of randomized controlled trial. Medicine. 2021;100(18):e25570. doi:10.1097/md.0000000000025570
33. Liao J, Zhang L, Zheng J, et al. Electroacupuncture inhibits annulus fibrosis cell apoptosis in vivo via TNF-α-TNFR1-caspase-8 and integrin β1/Akt signaling pathways. J Tradit Chin Med. 2014;34(6):684–690. doi:10.1016/s0254-6272(15)30083-2
34. Jia YZ, Li HT, Zhang GM, et al. Electroacupuncture alleviates orofacial allodynia and anxiety-like behaviors by regulating synaptic plasticity of the CA1 hippocampal region in a mouse model of trigeminal neuralgia. Front Mol Neurosci. 2022;15:979483. doi:10.3389/fnmol.2022.979483
35. Xu Q, Liu T, Chen S, et al. Correlation between the cumulative analgesic effect of electroacupuncture intervention and synaptic plasticity of hypothalamic paraventricular nucleus neurons in rats with sciatica. Neural Regen Res. 2013;8(3):218–225. doi:10.3969/j.issn.1673-5374.2013.03.003
36. Lv PR, Su YS, He W, et al. Electroacupuncture alleviated referral hindpaw hyperalgesia via suppressing spinal Long-Term Potentiation (LTP) in TNBS-induced colitis rats. Neural Plast. 2019;2019:2098083. doi:10.1155/2019/2098083
37. Song X, Shao X. Effect of annular external fixator-assisted bone transport on clinical healing, pain stress and joint function of traumatic massive bone defect of tibia. Comput Math Methods Med. 2022;2022:9052770. doi:10.1155/2022/9052770
38. Sindrup SH, Graf A, Sfikas N. The NK1-receptor antagonist TKA731 in painful diabetic neuropathy: a randomised, controlled trial. Eur J Pain. 2006;10(6):567–571. doi:10.1016/j.ejpain.2005.08.001
39. Zeilhofer HU. The glycinergic control of spinal pain processing. Cell Mol Life Sci. 2005;62(18):2027–2035. doi:10.1007/s00018-005-5107-2
40. Chen L, Hu Y, Wang S, et al. mTOR-neuropeptide Y signaling sensitizes nociceptors to drive neuropathic pain. JCI Insight. 2022;7(22):e159247. doi:10.1172/jci.insight.159247
41. Sapunar D, Vukojević K, Kostić S, et al. Attenuation of pain-related behavior evoked by injury through blockade of neuropeptide Y Y2 receptor. Pain. 2011;152(5):1173–1181. doi:10.1016/j.pain.2011.01.045
42. Qiao LN, Wang JY, Yang YS, et al. Effect of electroacupuncture intervention on expression of CGRP, SP, COX-1, and PGE2 of dorsal portion of the cervical spinal cord in rats with neck-incision pain. Evid Based Complement Alternat Med. 2013;2013:294091. doi:10.1155/2013/294091
43. Bliss TV, Collingridge GL, Kaang BK, et al. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci. 2016;17(8):485–496. doi:10.1038/nrn.2016.68
44. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi:10.1016/j.jpain.2009.06.012
45. Dai WJ, Sun JL, Li C, et al. Involvement of Interleukin-10 in Analgesia of Electroacupuncture on Incision Pain. Evid Based Complement Alternat Med. 2019;2019:8413576. doi:10.1155/2019/8413576
46. Lyu Z, Guo Y, Gong Y, et al. The Role of neuroglial crosstalk and synaptic plasticity-mediated central sensitization in acupuncture analgesia. Neural Plast. 2021;2021:8881557. doi:10.1155/2021/8881557
47. Su DJ, Li LF, Wang SY, et al. Pra-C exerts analgesic effect through inhibiting microglial activation in anterior cingulate cortex in complete Freund’s adjuvant-induced mouse model. Mol Pain. 2021;17:1744806921990934. doi:10.1177/1744806921990934
48. Cheng F, Vivacqua G, Yu S. The role of α-synuclein in neurotransmission and synaptic plasticity. J Chem Neuroanat. 2011;42(4):242–248. doi:10.1016/j.jchemneu.2010.12.001
49. Schmidtko A, Luo C, Gao W, et al. Genetic deletion of synapsin II reduces neuropathic pain due to reduced glutamate but increased GABA in the spinal cord dorsal horn. Pain. 2008;139(3):632–643. doi:10.1016/j.pain.2008.06.018
50. Bogen IL, Jensen V, Hvalby Ø, et al. Glutamatergic neurotransmission in the synapsin I and II double knock-out mouse. Semin Cell Dev Biol. 2011;22(4):400–407. doi:10.1016/j.semcdb.2011.07.004
51. Le S, Xu L, Wen C, et al. PSD95 gene specific siRNAs attenuate neuropathic pain through modulating neuron sensibility and postsynaptic CaMKIIα phosphorylation. Chin Med Sci J. 2011;26(4):201–207. doi:10.1016/s1001-9294(12)60001-7
52. Zhou NB, Wang KG, Fu ZJ. Effect of morphine and a low dose of ketamine on the T cells of patients with refractory cancer pain in vitro. Oncol Lett. 2019;18(4):4230–4236. doi:10.3892/ol.2019.10750
53. Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9(8):807–819. doi:10.1016/s1474-4422(10)70143-5
54. Dai Y, Zhang Y, Yang M, et al. Electroacupuncture Increases the hippocampal synaptic transmission efficiency and long-term plasticity to improve vascular cognitive impairment. Mediators Inflamm. 2022;2022:5985143. doi:10.1155/2022/5985143
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.