Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Electroacupuncture Ameliorates Depression-Like Behaviors in Post-Stroke Rats via Activating AMPK-Mediated Mitochondrial Function
Authors Ding Z, Gao J, Feng Y, Wang M, Zhao H, Wu R, Zheng X, Feng X , Lai M
Received 14 September 2023
Accepted for publication 17 November 2023
Published 4 December 2023 Volume 2023:19 Pages 2657—2671
DOI https://doi.org/10.2147/NDT.S436177
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Zhimin Ding,1,* Jing Gao,2,* Yixuan Feng,1,* Mengyu Wang,1 Huandong Zhao,1 Ruonan Wu,1 Xinyue Zheng,1 Xiaodong Feng,1,2 Mingyin Lai3
1School of Rehabilitation Medicine, Henan University of Chinese Medicine, Zhengzhou, People’s Republic of China; 2Department of Rehabilitation Center, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, People’s Republic of China; 3School of Traditional Chinese Medicine, Hainan Medical University, Haikou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaodong Feng; Mingyin Lai, Email [email protected]; [email protected]
Background: Post-stroke depression (PSD) is one of the most common complications of stroke. Electroacupuncture (EA) is an effective traditional Chinese medicine treatment for PSD, which is widely used in clinical settings. EA has a significant therapeutic effect against PSD, but the mechanism is still unclear. This study aimed to determine whether EA ameliorates depression-like behaviors in PSD rats by regulating the adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK)-mediated mitochondrial function.
Methods: Middle cerebral artery occlusion (MCAO) and chronic unpredictable mild stress (CUMS) were used to develop a PSD rat model. To elucidate the role of AMPK in EA treatment, compound C, a selective inhibitor of AMPK, was injected into the lateral ventricle of rats before EA treatment. EA treatment was performed for 14 consecutive days for 30 min per day after PSD modeling. A modified Zea-Longa five-point scale scoring system was used to determine neurologic function in MCAO rats. Behavioral tests were conducted to evaluate depression-like phenotypes in rats. Depression-like behaviors were tested by sucrose preference test (SPT), novelty suppressed feeding test (NSFT), and open-field test (OFT). The structure and morphology of the prefrontal cortex were observed by histopathological hematoxylin-eosin (HE) and Nissl staining. The mitochondrial morphology and function were analyzed by colorimetry, chemiluminescence, Western blotting, and quantitative real-time polymerase chain reaction (qRT-PCR).
Results: EA treatment successfully ameliorated depression-like behaviors, upregulated AMPK expression, and improved mitochondrial function. However, AMPK inhibition by Compound C exacerbated depression-like behaviors and aggravated neuronal and mitochondrial injury in PSD rats.
Conclusion: EA treatment improved depression-like behaviors in PSD rats and promoted mitochondrial function by activating AMPK.
Keywords: electroacupuncture, post-stroke depression, mitochondria, AMPK
Introduction
Post-stroke depression (PSD) is a common psychiatric disorder after a cerebrovascular insult.1 PSD is associated with higher mortality, worse recovery, more pronounced cognitive deficits, and lower quality of life than stroke without depression. A prospective study showed that the prevalence of PSD in the acute phase after stroke was 32.2%.2 In addition, a prospective cohort study from mainland China reported that the prevalence of PSD was 25.4%, 17.6%, and 12.4% at 2 weeks, 3 months, and 12 months after stroke, respectively.3 Although it is now widely accepted that neurobiological and psychosocial factors are important contributors to PSD, the key molecular mechanisms involved in its pathogenesis need to be further explored.
Mitochondria, an important organelle that performs and coordinates various metabolic processes in the cell, is the main site of adenosine triphosphate (ATP) production and is an important contributor to several common cellular processes, such as apoptosis and autophagy. With ongoing research, the central role of mitochondria in the regulation of neuronal activity is becoming increasingly prominent. Previous studies have shown that mitochondrial dysfunction is ubiquitously involved in the development of cerebrovascular disease, neurodegenerative disease, and psychiatric disorders.4–6 Pan et al7 showed that mitochondrial proteins associated with energy metabolism and antioxidants may play a critical role in self-repair after stroke injury and PSD-induced neuronal damage. Moreover, in psychiatric disorders, depression-like behavioral changes due to chronic stress can alter the mitochondrial gene expression and metabolism in different brain regions.8 Therefore, the modulation of neuronal mitochondrial function after stroke may be a key approach in preventing and treating PSD.
Adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK) is widely expressed in the brain and plays an important role in modulating neuronal polarization.9 AMPK functions as a cellular energy sensor as well as a regulator of metabolism and mitochondrial homeostasis.10 When cellular energy levels decrease, AMPK is activated and alleviates tissue damage via a variety of processes, including modulation of cellular autophagy, oxidative stress, and mitochondrial function.11 Several studies have suggested that impaired energy metabolism is an important pathological factor in the development of PSD.12 Exercise promotes ATP production in the hippocampal tissue and alleviates depression-like behavior by modulating AMPK-mediated mitochondrial function.13 These findings suggest that AMPK-mediated mitochondrial function may participate in the pathogenesis of PSD, although further research is warranted.
Electroacupuncture (EA), one of the therapeutic methods in traditional Chinese medicine, is a hot research topic in alternative medicine. A narrative review of clinical guidelines identified 11 countries with positive recommendations for acupuncture for PSD.14 Clinical studies have shown that EA treatment has significant antidepressant efficacy, with few adverse effects.15,16 In addition, some animal studies have found that EA treatment ameliorated depression-like behaviors by modulating the tPA/BDNF/TrkB signaling pathway in the prefrontal cortex.17 However, till now, it is unknown whether AMPK-mediated mitochondrial function participates in the protective effects of EA treatment on PSD.
Herein, we examined whether EA treatment could attenuate depression-like behaviors in PSD rats. Then, we investigated the role of AMPK-mediated mitochondrial function in antidepressant-like effects and the improvement of mitochondrial function produced by EA treatment.
Materials and Methods
Animals and Ethics Statement
A total of 126 male Sprague-Dawley rats (6–8-week-old, weighing 230–260 g) were obtained from Ji’nan Pengyue Laboratory Animal Breeding Co., Ltd. The rats were housed in a pathogen-free environment and standard cages with free access to standard food and water. External factors were maintained including a 12h light/dark cycle, 50–70% relative humidity, and temperature of 23–25°C. All experimental protocols complied with the ARRIVE guidelines18 and the Guide for the Care and Use of Laboratory Animals issued by the Institute for Laboratory Animal Research. The Experimental Animal Ethics Committee of Henan University of Chinese Medicine approved these procedures.
Experimental Design
Rats were randomly assigned to groups for two experimental trials, and the experiments were carried out in a blinded method.
Experiment 1 (Figure 1A): To determine the expression of endogenous AMPK and phosphorylated AMPK (p-AMPK) after PSD or EA treatment, rats were randomly assigned to the Sham, middle cerebral artery occlusion (MCAO), PSD, and EA groups (n = 9 rats per group). The sham group involved only incision and suturing but no thread plug. The MCAO group was treated with the MCAO operation. The PSD group was treated with both MCAO and chronic unpredictable mild stress (CUMS). The EA group was treated with EA for 14 consecutive days after the PSD model was established.
Experiment 2 (Figure 1B): After completing the above experiment, we explored the role of AMPK-mediated mitochondrial function in the antidepressant-like effects of EA. Rats were randomly assigned to Sham, MCAO, PSD, Compound C (CC), EA, and EA + CC groups (n = 15 rats per group). The sham group involved only incision and suturing but no thread plug. The MCAO group was treated with the MCAO operation. The PSD group was treated with both MCAO and CUMS. The rats in the CC and EA + CC groups were injected with Compound C using brain stereotactic localization after the successful establishment of the PSD rat model. The rats in the EA and EA + CC groups received EA treatment for 14 consecutive days.
PSD Model Establishment
The PSD model was induced by combining MCAO with CUMS. The MCAO model was established as described previously.19 Rats were anesthetized with 2% isoflurane (RWD life science, Shenzhen, China) inhalation using a small animal anesthesia machine (RWD Life Sciences, Shenzhen, China). Surgery was performed to expose the left common carotid artery, internal carotid artery, and external carotid artery. The external carotid artery was ligated, and a monofilament nylon suture with a silicon tip was inserted through the external carotid artery into the internal carotid artery to occlude the middle cerebral artery, approximately 18–22 mm from the insertion point. After arterial occlusion for 120 min, the suture was removed to begin reperfusion. Successful MCAO was confirmed using Zea-Longa scores. Three days after MCAO, CUMS was arranged for 21 consecutive days with one stressor each day. Seven stimulation methods were used, including food and water deprivation, swimming in ice water at 4°C, tail clamping, humid environment, wrap restraint, and vibration.
EA Treatment
The rats in the EA and EA + CC groups received EA treatment for 14 consecutive days from the end of the 21 days of CUMS. The combinations of acupuncture points, including Baihui (GV20), Yintang (GV29), Hegu (LI4), and Taichong (LR3), were performed (Table 1, Figure 2A). The acupoints were determined by previously described studies.20 The rats were placed on a custom-made acupuncture experimental apparatus (National Utility Model patent of China, NO. ZL 2019 2 1340810.6), which is less confining, allowing some freedom of movement. After disinfection at the acupuncture points, sterile stainless steel disposable acupuncture needles (13 mm in length, 0.25 mm in diameter, Hwato, Jiangsu, China) were inserted obliquely into the acupoints (GV20 and GV29) to a depth of 2 mm, and the needles were kept at the acupoints for 30 min, and manipulated at 15 min intervals. At the LI4 and LR3 acupoints, acupuncture needles were inserted obliquely to a depth of 2 mm and then attached to the positive and negative electrodes of the EA device (Hwato SDZ-II, Jiangsu, China), respectively. The stimulation of the LI4 and LR3 was performed at an intensity of 1 to 1.2 mA and a frequency of 2/100 Hz alternating, for 30 min.21
 |
Table 1 Location of the Acupoints |
Intracerebral Ventricular Injection
Rats were anesthetized with 2% isoflurane inhalation using a small animal anesthesia machine. Rats were placed under anesthesia on a brain stereotaxic device. The target nuclei were identified using Bregma points, and 5 μL of the specific AMPK inhibitor (Compound C) was injected into each lateral ventricle of the rats (GLPBIO, USA, 0.1 μmol). The injection sites were 1.5 mm rostral, 1.1 mm lateral to the bregma, and 4.5 mm deep to the skull surface. The needle was halted and slowly withdrawn after the injection, and the skin lesion was sutured.
Zea-Longa Scoring Criteria
The neurobehavioral scores of MCAO rats were evaluated using a modified Zea-Longa five-point scale scoring system.22 The Zea-Longa scores were measured 2 hours after the MCAO procedure. The following scoring criteria were employed: 0 points: no observable deficit; 1 point: forelimb flexion, with mild neurological impairment; 2 points: unidirectional circling, with moderate neurological impairment; 3 points: falling to the hemiplegia side, with severe neurological impairment; 4 points: inability to walk spontaneously or lack of consciousness.
2,3,4-Triphenytetrazolium-Chloride (TTC) Staining
The infarct volume was measured 3 days following the MCAO procedure. After euthanasia, brain tissue was collected and frozen at −20°C for 30 min before being cut into 2-mm-thick coronal sections and incubated in 2% TTC at 37°C for 30 min, with the brain slices being flipped once every 15 min to ensure uniform staining. Image J software (NIH, Bethesda, MD, USA) was used to measure the infarct areas of each brain slice.
Behavioral Tests
The behavioral tests, including sucrose preference test (SPT), novelty suppressed feeding test (NSFT), and open-field test (OFT), were performed on the third day after MCAO (0 day) and on the last day of CUMS (21 days) and EA treatment (35 days).
SPT
To investigate the condition of anhedonia, SPT was performed at 0, 21, and 35 days. On the first day, rats were kept in a single cage with two bottles of 1% sucrose solution. On the second day, one bottle of sucrose solution was changed at random with one bottle of tap water. The rats were denied water and food for 12h on the third day before being subjected to the SPT experiment. The bottles were weighed to calculate the quantity of sugar solution consumed.
NSFT
To assess depression-like behaviors, NSFT was performed at 0, 21, and 35 days. Rats were fed and given free access to water for 24h before the test. During the experiment, the rats were placed in a corner of a plastic box (50 × 50 × 40 cm), with a single pellet of food placed in the center. The feeding latency was defined as the moment when the rats began to chew the food pellet. The test lasted 5 min, and those who did not eat within 5 min had their feeding latency recorded as 5 min.
OFT
The OFT was used to assess the general locomotor activity at 0, 21, and 35 days. Rats were placed individually in the center of a cube (100 × 100 × 40 cm) and allowed to freely explore for 5 min. For the duration of 5 min, the total distance and percentage of time spent in the central area were recorded.
Hematoxylin-Eosin Staining (HE) and Nissl Staining
The rat brain tissues were removed and fixed in 4% formaldehyde for > 24h before being dried and embedded in paraffin. The tissue was subsequently split into 4-µm-thick sections. Later, the slices were stained with HE (Servicebio, Wuhan, China) and Nissl (Servicebio, Wuhan, China) according to the standard protocol. The stained images were analyzed for morphological changes in prefrontal neurons under a microscope (Nikon, Shanghai, China).
Tdt-Mediated Dutp Nick-End Labeling (TUNEL) Staining
Apoptosis was detected by TUNEL staining. After dewaxing the tissue slices, 20 μg/mL of DNase-free proteinase K was added dropwise and incubated at 37°C for 20 min before being removed using the PBS buffer. Finally, terminal deoxynucleotidyl transferase and fluorescein (Servicebio, Wuhan, China) were added to the sections and incubated for 1h at 37°C in a humidified atmosphere. A fluorescence microscope (Nikon, Shanghai, China) was used to detect the fluorescence signal. The number of TUNEL-positive cells was used to calculate the apoptosis rate.
Transmission Electron Microscope (TEM)
TEM was used to examine the mitochondrial ultrastructure alterations in rat neurons. In brief, rats were administered pre-cooled PBS containing 4% paraformaldehyde (Biosharp, Beijing, China) and 2.5% glutaraldehyde (Solarbio, Beijing, China). The brains were promptly removed, and the prefrontal cortex was sliced into 1-mm3 blocks. Then, the tissue blocks were fixed for 1h with 4% glutaraldehyde and then for 90 min with 1% OsO4 in 0.1 mol/L theobromine buffer (pH = 7.4). The tissues were washed, dehydrated in gradient ethanol, and implanted in epoxy resin. Tissues were sectioned into ultrathin (80 nm) sections and stained with uranyl acetate and lead citrate. TEM (JEM-1400 Flash, Tokyo, Japan) was used to evaluate the mitochondrial morphological alterations in neurons.
Colorimetric Method
After the experiment, serum from prepared rats was used to evaluate catalase (CAT), malondialdehyde (MDA), total superoxide dismutase (T-SOD), and glutathione peroxidase (GSH) levels (Elabscience, Wuhan, China) using colorimetric assay kits according to the manufacturer’s protocols.
Chemiluminescence Method
At the end of the experiment, the prefrontal cortex of rats was collected to determine the level of ATP using an ATP colorimetric assay kit (Elabscience, Wuhan, China) according to the manufacturer’s protocols.
Western Blot
Western blotting analysis was performed as previously described.23 To isolate proteins, the prefrontal brain was lysed. The BCA protein quantification kit (Solarbio, Beijing, China) was used for protein quantification. After quantifying the samples to the same concentration, 20 μg of protein was extracted from each group of samples, and the higher sample buffer was added and cooked in boiling water for 10 min to denature the protein. Equal quantities of protein samples were separated by 10% SDS-PAGE gels and transferred to PVDF membranes. After blocking with 5% nonfat milk at 20–25°C for 2h, samples were incubated overnight at 4°C with primary antibodies against AMPK (1:1000, Abcam, ab27118), p-AMPK (1:1000, CST, 2535S), Drp1 (1:5000, Proteintech, 12957-1-AP), Mfn2 (1:5000, Proteintech, 12186-1-AP), OPA1 (1:4000, Proteintech, 27722-1-AP), and GAPDH (1:5000, Proteintech, 10494-1-AP). The membrane was washed three times with TBST for 5 min each. Then, HRP-goat anti-Rabbit antibody (1:10000, Proteintech, SA00001-2) was added and incubated at room temperature for 1h. The blots were examined using Image J software (NIH, Bethesda, MD, USA) after being exposed to the ECL Western Blotting Substrate (Solarbio, Beijing, China).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from the prefrontal cortex using Trizol reagent (Monad, Wuhan, China), and cDNA was synthesized by reverse transcription using a gDNA Erase Kit (Monad, Wuhan, China). The expressions of Mfn2, OPA1, and Drp1 were detected using a One-Step SYBR RT-PCR kit (Monad, Wuhan, China). To quantify the relative expressions of each gene, the 2–ΔΔCT method was used. The following primers were used: GAPDH, (Forward: 5′-TGTGAACGGATTTGGCCGTA-3′, Reverse: 5′-GATGGTGATGGGTTTCCCGT-3′); MFN2, (Forward: 5′-CTCTGTGCTGGTTGACGAGT-3′, Reverse: 5′-TCGAGGGACCAGCATGTCTA-3′); Drp1, (Forward: 5′-TGGAAAGAGCTCAGTGCTGG-3′, Reverse: 5′-TCAACTCCATTTTCTTCTCCTGT-3′); and OPA1, (Forward: 5′-ATTTCGCTCCTGACCTGGAC-3′, Reverse: 5′-GGTGTACCCGCAGTGAAGAA-3′).
Statistical Analysis
GraphPad Prism 8.0 (San Diego, CA, USA) and SPSS 25.0. (SPSS, IBM, Chicago, IL, USA) were used for statistical analysis. Data are expressed as means ± standard error of mean (SEM). The data from behavioral tests were analyzed using two-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. The remaining data were evaluated using one-way ANOVA followed by Tukey’s post hoc test. P < 0.05 was considered statistically significant.
Results
MCAO Increased Neurological Scores and Cerebral Infarct Volume in MCAO Rats
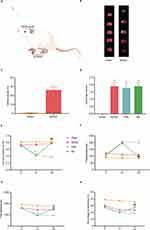
The Zea-Longa score was used to assess the degree of neurological damage, and infarct volume was measured by TTC. The infarct volume and score were significantly higher in the MCAO group than in the sham group, indicating that the neurological impairment in the rats was caused by cerebral ischemia and that the MCAO model was successfully implemented (Figure 2B–D).
EA Treatment Ameliorated Depression-Like Behaviors in PSD Rats
SPT, NFST, and OFT were used to detect changes in depression-like behaviors in each group of rats. Depression-like behaviors were not statistically different among groups on day 0, suggesting a lack of difference between the groups at baseline. After the PSD procedure, rats in the PSD group showed reduced sucrose preference and locomotor activity and increased feeding latency compared with rats in the MCAO group. However, EA treatment prevented the PSD-induced depression-like behaviors (Figure 2E–H), indicating that EA treatment exerted antidepressive effect on PSD rats.
EA Treatment Increased the AMPK Expression in the Prefrontal Cortex of PSD Rats, and Compound C Blocked the Therapeutic Effect of EA
To explore the molecular mechanism underlying the antidepressive effects of EA, Western blotting analysis was used to detect the expression levels of AMPK and p-AMPK in the prefrontal cortex of each group (Figure 3A–C). The results revealed that no significant differences among the groups in the expression level of total AMPK. PSD reduced AMPK phosphorylation, whereas 14 days of EA treatment dramatically increased p-AMPK levels. To explore the role of AMPK in protection by EA, Compound C, a selective AMPK inhibitor, was injected through the lateral ventricles of rats to block the AMPK expression. Trajectory map of the open-field test is shown in Figure 3D. Compared with the PSD group, the CC group demonstrated a further drop in sucrose preference and locomotor activity, while feeding latency was dramatically enhanced. EA treatment significantly increased the sucrose preference and locomotor activity and reduced the feeding latency, which was partially prevented by Compound C administration (Figure 3E–H). As a result of these findings, EA treatment appears to protect against PSD-induced depression-like behaviors by activating AMPK.
EA Treatment Promoted Repair of Prefrontal Neurons in PSD Rats
As neuronal histology affects the structure and function of the brain, we used HE and Nissl staining to observe the morphological changes in prefrontal neurons and investigate the effect of EA treatment on neuronal injury in PSD rats. Figure 4A and B depict the results of HE and Nissl staining. In the sham group, the prefrontal neurons were neatly aligned, with normal morphology and structural integrity. By contrast, the neurons in the PSD group were loosely distributed, with lower cell numbers, condensed nuclei, deep staining, shrinkage, and even necrosis, showing PSD-induced neuronal damage. In the CC group, the number of neurons undergoing necrosis increased. However, EA treatment partially prevented this effect. The outcomes of Nissl staining were similar to those of HE staining. Neurons showed irregular shapes, and Nissl bodies were lightly stained and even lysed in the PSD and CC groups. However, in the EA group, the shape of neurons was improved, and the number of Nissl bodies was significantly increased.
EA Treatment Ameliorated Mitochondrial Injury and Improved Energy Metabolism in PSD Rats
Mitochondria, as highly dynamic intracellular organelles, play a key role in the pathophysiology of neuronal injury. AMPK is an important factor in the regulation of mitochondrial stability. Previous studies have reported that AMPK activation exerts cerebral protective effects by restoring the structural integrity of mitochondria.24 Therefore, the effect of EA treatment on the mitochondrial morphology and function of prefrontal neurons was investigated.
First, TEM revealed morphological alterations in the mitochondria of prefrontal neurons in PSD rats (Figure 4C). The neurons in the sham group were normal and undamaged, and mitochondrial cristae were visible and undamaged. In the MCAO, PSD, and CC groups, neurons developed cell membrane holes, loss of integrity, expanded mitochondria, loss of cristae breaks, and vacuoles. The neurons in the EA group were less damaged than the PSD neurons, with only minor mitochondrial edema and structural integrity. By contrast, Compound C injection into the lateral ventricles partially counteracted the therapeutic effect of EA.
Mitochondrial morphology is essential for the maintenance of mitochondrial function. Fragmented mitochondria lead to disruption of energy metabolism. Next, ATP production and energy metabolism were evaluated using the Chemiluminescence Assay Kit. Figure 5C shows that the ATP level in the PSD group was much lower than in the sham and MCAO groups. Meanwhile, the CC group exhibited a greater energy shortage. By contrast, EA treatment raised the ATP levels in the prefrontal cortex. However, Compound C partially blocked the therapeutic of EA, and ATP levels were lower than those in the EA + CC group.
EA Treatment Reduced Oxidative Stress Injury and Neuronal Death in PSD Rats
Under normal physiological conditions, mitochondria can establish an effective antioxidant system for the body to promptly remove excessive reactive oxygen species and prevent their harmful effects. The colorimetric method was used to determine the levels of GSH, CAT, T-SOD, and MDA in the prefrontal cortex (Figure 5D–G). Compared with the sham group, the levels of GSH, CAT, and T-SOD were considerably lower, while MDA levels were significantly higher, in the PSD and CC groups. However, after EA treatment, these effects were partially reversed.
In addition to their role in cellular energy metabolism and biological oxidation, mitochondria are key manipulators of apoptosis. Therefore, the apoptosis of prefrontal neurons was observed by TUNEL staining (Figure 5A and B). Almost no TUNEL-positive neurons were observed in the sham group, while the rate of neuronal apoptosis was significantly higher in the PSD group, which was further exacerbated by Compound C administration. However, the number of TUNEL-positive neurons was significantly reduced after EA treatment.
EA Treatment Promoted Mitochondrial Fusion–Fission Homeostasis in PSD Rats
Drp1, Mfn2, and OPA1 are markers of mitochondrial fission and fusion, which are essential for maintaining mitochondrial morphology and function. Therefore, the protein and mRNA expressions of Drp1, Mfn2, and OPA1 were detected by Western blotting (Figure 6A–D) and qRT-PCR (Figure 6E–G). Compared with the sham group, the protein and mRNA expressions of Drp1 were significantly higher, and those of Mfn2 and OPA1 were significantly lower, in the PSD and CC groups. EA treatment significantly reduced the protein and mRNA expressions of Drp1 while increasing the expressions of Mfn2 and OPA1, which was reversed by Compound C injection.
Discussion
PSD is the most common mood disorder following stroke and is the main factor limiting recovery and rehabilitation in stroke patients. Understanding the pathophysiological causes of PSD is hampered by the multifaceted nature and complexity of the disease. Selective 5-hydroxytryptamine reuptake inhibitors are the main antidepressants used in clinical practice, but their clinical applications are limited due to their side effects.25 Therefore, further research into PSD is still necessary.
The structural and functional abnormalities of the prefrontal cortex are strongly associated with negative processing biases, anxiety disorders, and depression-like behaviors.26 Experimental studies have shown that unilateral ischemic lesion to the prefrontal cortex leads to pronounced and persistent anxiety and depression phenotypes.27 Clinical studies have shown that frontal lobe activation is lower in PSD patients compared to stroke patients.28 Chen et al29 found in a study that although reduced BDNF in both the prefrontal cortex and hippocampus was associated with the development of PSD, nasal administration exerted an antidepressant effect by increasing BDNF levels in the prefrontal cortex but not in the hippocampus. Recent studies have shown that EA can modulate ATP levels in the prefrontal cortex, which may contribute to its antidepressant effects.30 Therefore, the present study further investigated the relationship between the antidepressant effects of EA and the morphology and function of mitochondria in the prefrontal cortex.
Brain has a high energy expenditure and metabolism, and its function is highly dependent on mitochondrial activity and integrity. Mitochondrial homeostasis is critical for regulating neuronal activity. Ischemia and hypoxia enhance mitochondrial dysfunction, which promotes cell death by initiating a cascade of events involving poor cellular energy metabolism, oxidative stress, and apoptosis. Several investigations have found that mitochondrial dysfunction is important in the pathophysiology of depressive-like behaviors.31 In this study, we used TEM to observe the mitochondrial shape. After PSD, the mitochondria were enlarged, and the mitochondrial cristae were ruptured and vacuolated. The mitochondrial morphology was improved dramatically after EA treatment. Reduced local blood flow as a result of an ischemic stroke is a major contributor to the disruption of neuronal energy metabolism, and aberrant energy metabolism is a crucial pathogenic mechanism involved in the development of depression. Previous research has found that receptor P2X4, the ATP receptor, is chronically deficient in depression following stroke.32 Mitochondria are energy metabolic factories, providing energy support for cellular activities. Here, we detected the ATP content in the prefrontal cortex. The results showed that PSD led to a decrease in ATP levels, and EA treatment attenuated this alteration. Meanwhile, mitochondrial dysfunction is directly associated with increased levels of oxidative stress and apoptosis. Accumulating evidence has suggested that both stroke and depression in rats lead to high MDA and nitric oxide levels.23,33 Similarly, clinical studies have confirmed the role of oxidative stress in PSD based on plasma metabolomics studies.34 The levels of CAT, GSH, T-SOD, and MDA were determined using the colorimetric technique in this study. EA reduced the increase in oxidative stress caused by PSD. In addition, Peng et al35 showed that CUMS increased the apoptosis rate, which is consistent with our findings. In addition, mitochondria are extremely active organelles that are continually changing shape due to fission and fusion. The dynamic balance between fission and fusion is required for mitochondrial activity and addressing the cell’s metabolic and energy needs.36 Mfn2, Drp1, and OPA1 are essential proteins that govern mitochondrial fusion and fission, as well as mitochondrial architecture and function. They are also linked to certain cerebrovascular and mental diseases.37 Ren et al38 found that cerebral ischemia worsened the neuronal injury by increasing the Drp1 expression and decreasing the Mfn2 expression. Furthermore, after cerebral ischemia/reperfusion injury, OPA1 was observed to be significantly cleaved in vitro and in vivo.19 Previous research has also revealed that Mfn2 modulates anxiety and depression-like behaviors via its impact on mitochondrial function.39 Therefore, the protein and mRNA expressions of Drp1, Mfn2, and OPA1 in the prefrontal cortex were determined by Western blotting and qRT-PCR. The Drp1 expression was significantly increased while the expressions of Mfn2 and OPA1 were significantly decreased in the PSD group, suggesting that the mitochondrial functions were altered by the PSD procedure. EA treatment restored mitochondrial fusion and fission homeostasis. In addition, not only is Mfn2 involved in the regulation of mitochondrial morphology and function, but its low expression is also closely related to oxidative stress and apoptosis.40 This study supports our findings that PSD decreased the Mfn2 expression while increasing oxidative stress and apoptosis.
AMPK is a cellular energy sensor that is activated under energy stress and is essential for maintaining energy homeostasis. Under energy stress, AMPK phosphorylates certain enzymes and growth control nodes to increase ATP production.10 Previous studies have shown that AMPK activation is essential for normal mitochondrial metabolic functions.41 Further research has revealed that AMPK regulates mitochondrial function in part by directly modulating the production of proteins involved in mitochondrial dynamics. Dong et al42 showed that AMPK is activated during energy stress to maintain mitochondrial function and reduce endothelial cell injury following cerebral ischemia via modulating Mfn2 protein expression. Wu et al43 revealed that activation of the AMPK signaling pathway promoted Drp1-mediated mitochondrial fission to protect against neuronal damage during ischemic stroke. Our findings confirmed this mechanistic relationship by showing that endogenous p-AMPK expression was dramatically lowered after the PSD procedure and was elevated following EA treatment. To confirm that EA treatment can modulate mitochondrial function through AMPK, we suppressed AMPK expression following the PSD process by injecting Compound C into the lateral ventricles. The therapeutic effects of EA were largely reversed by this intervention, which also partially decreased the ATP levels, raised oxidative stress and apoptosis levels, downregulated the protein and mRNA expressions of Mfn2 and OPA1, and upregulated the Drp1 expression. The relationship between EA and AMPK was verified by this mechanistic investigation.
Previous research has indicated that EA may be an effective and safe treatment for PSD.15 Our findings suggest that EA treatment significantly increased sucrose preference and locomotor activity and decreased feeding latency. Furthermore, EA treatment significantly alleviated prefrontal neuronal damage. Basic research has demonstrated that EA improves neuronal injury by altering mitochondrial function.44 Although many studies have reported a correlation between PSD and mitochondria, fewer studies have been conducted on the relationship between EA and PSD-induced mitochondrial dysfunction. The expression of mitochondrial function-related proteins was found to be decreased in PSD rats, whereas EA treatment reversed these changes.17 Based on this, we investigated the involvement of AMPK-mediated mitochondrial activity in the antidepressant effects of EA for the first time. Our results suggest that EA increased the endogenous p-AMPK expression and alleviated PSD-induced mitochondrial dysfunction, whereas blocking AMPK expression partially blocked the therapeutic effect of EA.
Conclusion
AMPK-mediated mitochondrial function is involved in the antidepressant effects of EA. Our results provided a novel perspective on the mechanisms underlying the effects of EA in the treatment of PSD.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics Statement
This animal study was reviewed and approved by the Ethics Committee of Laboratory Animals of the Henan University of Chinese Medicine (DWLL202110013).
Acknowledgments
Transmission electron microscopy images were kindly provided by the TEM Center of Henan University of Chinese Medicine.
Funding
The National Natural Science Foundation of China (82104973), Henan province science and Technology Project (222102310715), Special subject of scientific research on traditional Chinese medicine in Henan province (2022ZY1162), Special Research Project of national clinical research base of traditional Chinese medicine of health committee of Henan Province (2022JDX087), Henan province traditional Chinese medicine “Double first-class” to create a scientific research project (HSRP-DFCTCM-2023-1-33), The Joint Fund of Science and Technology R&D Program of Henan Province (Cultivation Category of Advantageous Disciplines) (232301420072).
Disclosure
All authors declare that there are no potential conflicts of interest.
References
1. Guo J, Wang J, Sun W, Liu X. The advances of post-stroke depression: 2021 update. J Neurol. 2022;269(3):1236–1249. doi:10.1007/s00415-021-10597-4
2. Schöttke H, Gerke L, Düsing R, Möllmann A. Post-stroke depression and functional impairments - a 3-year prospective study. Compr Psychiatry. 2020;99:152171. doi:10.1016/j.comppsych.2020.152171
3. Wang Z, Zhu M, Su Z, et al. Post-stroke depression: different characteristics based on follow-up stage and gender-a cohort perspective study from Mainland China. Neurol Res. 2017;39(11):996–1005. doi:10.1080/01616412.2017.1364514
4. Kim Y, Vadodaria KC, Lenkei Z, et al. Mitochondria, metabolism, and redox mechanisms in psychiatric disorders. Antioxid Redox Signal. 2019;31(4):275–317. doi:10.1089/ars.2018.7606
5. Monzio Compagnoni G, Di Fonzo A, Corti S, Comi GP, Bresolin N, Masliah E. The role of mitochondria in neurodegenerative diseases: the lesson from alzheimer’s disease and parkinson’s disease. Mol Neurobiol. 2020;57:2959–2980. doi:10.1007/s12035-020-01926-1
6. He Z, Ning N, Zhou Q, Khoshnam SE, Farzaneh M. Mitochondria as a therapeutic target for ischemic stroke. Free Radic Biol Med. 2020;146:45–58. doi:10.1016/j.freeradbiomed.2019.11.005
7. Pan J, Liu H, Zhou J, et al. Ipsilateral hippocampal proteomics reveals mitochondrial antioxidative stress impairment in cortical-lesioned chronic mild stressed rats. Curr Mol Med. 2014;14:1186–1196. doi:10.2174/1566524014666141021143333
8. Głombik K, Budziszewska B, Basta-Kaim A. Mitochondria-targeting therapeutic strategies in the treatment of depression. Mitochondrion. 2021;58:169–178. doi:10.1016/j.mito.2021.03.006
9. Amato S, Liu X, Zheng B, Cantley L, Rakic P, Man HY. AMP-activated protein kinase regulates neuronal polarization by interfering with PI 3-kinase localization. Science. 2011;332(6026):247–251. doi:10.1126/science.1201678
10. Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–135. doi:10.1038/nrm.2017.95
11. Lin S-C, Hardie DG. AMPK: sensing glucose as well as cellular energy status. Cell Metab. 2018;27(2):299–313. doi:10.1016/j.cmet.2017.10.009
12. Villa RF, Ferrari F, Moretti A. Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol Ther. 2018;184:131–144. doi:10.1016/j.pharmthera.2017.11.005
13. Sun L, Ye R, Liang R, Xing F. Treadmill running attenuates neonatal hypoxia induced adult depressive symptoms and promoted hippocampal neural stem cell differentiation via modulating AMPK-mediated mitochondrial functions. Biochem Biophys Res Commun. 2020;523(2):514–521. doi:10.1016/j.bbrc.2019.12.036
14. Birch S, Robinson N. Acupuncture as a post-stroke treatment option: a narrative review of clinical guideline recommendations. Phytomedicine. 2022;104:154297. doi:10.1016/j.phymed.2022.154297
15. Cai W, Ma W, Li YJ, Wang GT, Yang H, Shen WD. Efficacy and safety of electroacupuncture for post-stroke depression: a randomized controlled trial. Acupunct Med. 2022;40:434–442. doi:10.1177/09645284221077104
16. Wang H, Li Y. A pilot controlled trial of a combination of electroacupuncture and psychological intervention for post-stroke depression. Complement Ther Med. 2022;71:102899. doi:10.1016/j.ctim.2022.102899
17. Hu G, Zhou C, Wang J, et al. Electroacupuncture treatment ameliorates depressive-like behavior and cognitive dysfunction via CB1R dependent mitochondria biogenesis after experimental global cerebral ischemic stroke. Front Cell Neurosci. 2023;17:1135227. doi:10.3389/fncel.2023.1135227
18. Kilkenny C, Browne WJ, Cuthi I, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Vet Clin Pathol. 2012;41:27–31. doi:10.1111/j.1939165X.2012.00418.x
19. Lai Y, Lin P, Chen M, et al. Restoration of L-OPA1 alleviates acute ischemic stroke injury in rats via inhibiting neuronal apoptosis and preserving mitochondrial function. Redox Biol. 2020;34:101503. doi:10.1016/j.redox.2020.101503
20. Gao J, Lai M, Fu W, et al. Electroacupuncture ameliorates depressive-like state and synaptic deficits induced by hyper-cholinergic tone during chronic stress in rats. Med Sci Monit. 2021;27:e933833. doi:10.12659/MSM.933833
21. Yang L, Ding W, Dong Y, et al. Electroacupuncture attenuates surgical pain-induced delirium-like behavior in mice via remodeling gut microbiota and dendritic spine. Front Immunol. 2022;13:955581. doi:10.3389/fimmu.2022.955581
22. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi:10.1161/01.STR.20.1.84
23. Yu J, Wang WN, Matei N, et al. Ezetimibe attenuates oxidative stress and neuroinflammation via the AMPK/Nrf2/TXNIP pathway after MCAO in rats. Oxid Med Cell Longev. 2020;2020:4717258. doi:10.1155/2020/4717258
24. Cai Y, Yang E, Yao X, et al. FUNDC1-dependent mitophagy induced by tPA protects neurons against cerebral ischemia-reperfusion injury. Redox Biol. 2021;38:101792. doi:10.1016/j.redox.2020.101792
25. Xu J, Zhou Y, Yan C, et al. Neurosteroids: a novel promise for the treatment of stroke and post-stroke complications. J Neurochem. 2022;160(1):113–127. doi:10.1111/jnc.15503
26. Pizzagalli DA, Roberts AC. Prefrontal cortex and depression. Neuropsychopharmacology. 2022;47:225–246. doi:10.1038/s41386-021-01101-7
27. Vahid-Ansari F, Lagace DC, Albert PR. Persistent post-stroke depression in mice following unilateral medial prefrontal cortical stroke. Transl Psychiatry. 2016;6(8):e863. doi:10.1038/tp.2016.124
28. Koyanagi M, Yamada M, Higashi T, Mitsunaga W, Moriuchi T, Tsujihata M. The usefulness of functional near-infrared spectroscopy for the assessment of post-stroke depression. Front Hum Neurosci. 2021;15:680847. doi:10.3389/fnhum.2021.680847
29. Chen C, Dong Y, Liu F, et al. A study of antidepressant effect and mechanism on intranasal delivery of BDNF-HA2TAT/AAV to rats with post-stroke depression. Neuropsychiatr Dis Treat. 2020;16:637–649. doi:10.2147/NDT.S227598
30. Zheng Y, Pan L, He J, et al. Electroacupuncture-modulated extracellular ATP levels in prefrontal cortex ameliorated depressive-like behavior of maternal separation rats. Behav Brain Res. 2023;452:114548. doi:10.1016/j.bbr.2023.114548
31. Simon MS, Schiweck C, Arteaga-Henríquez G, et al. Monocyte mitochondrial dysfunction, inflammaging, and inflammatory pyroptosis in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2021;111:110391. doi:10.1016/j.pnpbp.2021.110391
32. Verma R, Cronin CG, Hudobenko J, Venna VR, McCullough LD, Liang BT. Deletion of the P2X4 receptor is neuroprotective acutely, but induces a depressive phenotype during recovery from ischemic stroke. Brain Behav Immun. 2017;66:302–312. doi:10.1016/j.bbi.2017.07.155
33. Lamtai M, Zghari O, Azirar S, et al. Melatonin modulates copper-induced anxiety-like, depression-like and memory impairments by acting on hippocampal oxidative stress in rat. Drug Chem Toxicol. 2022;45(4):1707–1715. doi:10.1080/01480545.2020.1858853
34. Wang M, Gui X, Wu L, et al. Amino acid metabolism, lipid metabolism, and oxidative stress are associated with post-stroke depression: a metabonomics study. BMC Neurol. 2020;20(1):250. doi:10.1186/s12883-020-01780-7
35. Peng Z, Zhang C, Yan L, et al. EPA is more effective than DHA to improve depression-like behavior, glia cell dysfunction and hippcampal apoptosis signaling in a chronic stress-induced rat model of depression. Int J Mol Sci. 2020;21(5):1769. doi:10.3390/ijms21051769
36. An H, Zhou B, Ji X. Mitochondrial quality control in acute ischemic stroke. J Cereb Blood Flow Metab. 2021;41(12):3157–3170. doi:10.1177/0271678X211046992
37. Qin YY, Pan SY, Dai JR, et al. Alleviation of ischemic brain injury by exercise preconditioning is associated with modulation of autophagy and mitochondrial dynamics in cerebral cortex of female aged mice. Exp Gerontol. 2023;178:112226. doi:10.1016/j.exger.2023.112226
38. Ren KD, Liu WN, Tian J, et al. Mitochondrial E3 ubiquitin ligase 1 promotes brain injury by disturbing mitochondrial dynamics in a rat model of ischemic stroke. Eur J Pharmacol. 2019;861:172617. doi:10.1016/j.ejphar.2019.172617
39. Gebara E, Zanoletti O, Ghosal S, et al. Mitofusin-2 in the nucleus accumbens regulates anxiety and depression-like behaviors through mitochondrial and neuronal actions. Biol Psychiatry. 2021;89(11):1033–1044. doi:10.1016/j.biopsych.2020.12.003
40. Yuan M, Gong M, Zhang Z, et al. Hyperglycemia induces endoplasmic reticulum stress in atrial cardiomyocytes, and mitofusin-2 downregulation prevents mitochondrial dysfunction and subsequent cell death. Oxid Med Cell Longev. 2020;2020:6569728. doi:10.1155/2020/6569728
41. Drake JC, Wilson RJ, Laker RC, et al. Mitochondria-localized AMPK responds to local energetics and contributes to exercise and energetic stress-induced mitophagy. Proc Natl Acad Sci U S A. 2021;118(37). doi:10.1073/pnas.2025932118
42. Dong H, Zhou W, Xin J, et al. Salvinorin A moderates postischemic brain injury by preserving endothelial mitochondrial function via AMPK/Mfn2 activation. Exp Neurol. 2019;322:113045. doi:10.1016/j.expneurol.2019.113045
43. Wu Q, Liu J, Mao Z, et al. Ligustilide attenuates ischemic stroke injury by promoting Drp1-mediated mitochondrial fission via activation of AMPK. Phytomedicine. 2022;95:153884. doi:10.1016/j.phymed.2021.153884
44. Wang H, Chen S, Zhang Y, Xu H, Sun H. Electroacupuncture ameliorates neuronal injury by Pink1/Parkin-mediated mitophagy clearance in cerebral ischemia-reperfusion. Nitric Oxide. 2019;91:23–34. doi:10.1016/j.niox.2019.07.004
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.