Back to Journals » Infection and Drug Resistance » Volume 11
Efficiency of gold nanoparticles coated with the antimicrobial peptide indolicidin against biofilm formation and development of Candida spp. clinical isolates
Authors de Alteriis E, Maselli V , Falanga A , Galdiero S , Di Lella FM, Gesuele R, Guida M , Galdiero E
Received 1 February 2018
Accepted for publication 29 March 2018
Published 3 July 2018 Volume 2018:11 Pages 915—925
DOI https://doi.org/10.2147/IDR.S164262
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
Elisabetta de Alteriis,1 Valeria Maselli,1 Annarita Falanga,2 Stefania Galdiero,2 Federica Maria Di Lella,3 Renato Gesuele,1 Marco Guida,1 Emilia Galdiero1
1Department of Biology, University of Naples “Federico II”, Naples, Italy; 2Department of Pharmacy, University of Naples “Federico II”, Naples, Italy; 3Section of Microbiology and Virology, University Hospital “Luigi Vanvitelli” of Naples, Naples, Italy
Background: This article examines the use of a novel nano-system, gold nanoparticles coated with indolicidin (AuNPs–indolicidin), against pathogenic Candida albicans biofilms. Candida species cause frequent infections owing to their ability to form biofilms, primarily on implant devices.
Materials and methods: We used an integrated approach, evaluating the effect of AuNPs-indolicidin on prevention and eradication of Candida biofilms formed in multi-well polystyrene plates, with relative gene expression assays. Four biofilm-associated genes (FG1, HWP1, ALS1 and ALS3, and CDR1 and CDR2) involved in efflux pump were analyzed using reverse transcription polymerase chain reaction.
Results: Treatment with the nano-complex significantly inhibits the capacity of C. albicans to form biofilms and impairs preformed mature biofilms. Treatment with AuNPs–indolicidin results in an increase in the kinetics of Rhodamine 6G efflux and a reduction in the expression of biofilm-related genes.
Conclusion: These data provide a chance to develop novel therapies against nosocomially acquired refractory C. albicans biofilms.
Keywords: antimicrobial activity, anti-biofilm activity, fungal biofilm, drug resistance, fungal infections
Introduction
Candida albicans is a typical component of the gastrointestinal and genitourinary tract flora in healthy people. Alterations in host immunity, bacterial flora, or other environmental factors allow its overgrowth, causing a wide range of infections. Thus, C. albicans is an opportunistic pathogen, and it is the third most commonly isolated fungal agent acquired in hospitals.1
Closely related to nosocomial infections is the capability of pathogenic microorganisms to form biofilms. Approximately 80% of human bacterial infections2 and health-care associated infections related to the implantation of medical devices (such as urinary or intravascular catheters and prosthetic heart valves) are caused by biofilms.3
Candida albicans forms highly structured biofilms, and it is the predominant fungal species isolated from medical devices. Once established on medical devices, Candida biofilms have the potential to disseminate bloodstream infections, leading to systemic infections of tissues and organs.4
Microbial biofilms are much more resistant to antibiotics and immune-system effectors when compared to planktonic cells. The high resistance of biofilms to antimicrobials is also considered responsible for the worldwide increase in multidrug-resistant pathogens.5,6
Resistance of Candida biofilm to antimicrobials is related to several factors, such as the higher frequency of mutation and horizontal gene transmission in the biofilm; the production of reactive oxygen species not being balanced by the antioxidant system, which increases mutability; the reduced penetration of antibiotics into the matrix; and the presence of recalcitrant persister cells.
In addition, the up-regulation of efflux pumps for drug exportation is thought to mediate resistance, even when the antifungal agent is absent, differently from the situation observed in planktonic cells, where the same pumps are up-regulated only in response to drug exposure.7–10
The extreme resistance of Candida biofilms to antifungal agents has a great clinical impact: it implies the requirement to use high antifungal doses and/or remove the colonized medical devices. In this scenario, there is an urgent need to discover new anti-biofilm therapeutics as alternatives to conventional drugs. Ideally, compared to traditional antibiotics, new drugs should have multiple mechanisms of action and lower susceptibility to the development of resistance.
Antimicrobial peptides (AMPs) are produced by a variety of organisms as a first line of defense11 against bacteria and fungi. Their principal mechanism of action involves binding to conserved structural components of the cell envelope followed by an interaction with the cell membrane, which can be rapidly lethal. Some AMPs also bind to intracellular targets and inhibit important biological processes such as cell-wall formation or DNA, RNA, and protein synthesis. These mechanisms of action are rapidly bactericidal/fungicidal and decrease the chances of the development of resistance compared to conventional antibiotics.11–15
AMPs belonging to several classes, from natural sources or from synthesis, have been mainly used against pathogenic bacterial biofilms. Reports concerning the use of AMPs against Candida biofilms are scarce.16 Nonetheless, some AMPs have been used in the prevention of the initial adhesion of cells to the surface and in the removal of the mature biofilm.17 One of the most studied families of anti-biofilm AMPs encompasses the cathelicidins and their derivatives,18 some of which, such as the AS10 peptide, are effective against biofilms of different strains of C. albicans.19
Indolicidin, a cathelicidin-related antimicrobial peptide, has been shown to have good antibacterial activity,20 and a low toxicity profile in vitro and in vivo, as demonstrated in studies on different animal models.21–24 It is a 13-residue peptide with a high content of tryptophan and broad-spectrum antimicrobial and hemolytic activities. As an anti-biofilm compound, indolicidin has been proved to be effective against methicillin-resistant Staphylococcus aureus (MRSA) biofilms.25 So far, indolicidin has not been proposed for the control of fungal biofilm formation.
Moreover, several reports have indicated nanoparticles (NPs) to be effective against bacterial and fungal biofilms. In particular, gold nanoparticles (AuNPs) have resilient properties compared to other inorganic NPs, and thus they could be used as carriers of antitumor drugs, antibodies, antibiotics, or drugs for selective killing of diseased cells and microbes.26–28
Several factors limit the use of AMPs in clinics: potential toxicity, high costs of production, instability to proteases, and lack of knowledge about therapeutic administration. One limitation is represented by the fact that AMPs are composed of L-amino acids, which are recognized by microbial and host cell proteases that break down the peptides, decreasing their activity. Therefore, our research was aimed at overcoming this limitation by functionalizing AuNPs with indolicidin to improve its anti-biofilm activity in vivo. It is widely accepted that multivalence may provide greater efficacy and conjugation on NPs may reduce degradation by peptidases.
In this study, we investigated the efficacy in vitro of a nano-complex of gold nanoparticles coated with indolicidin (AuNPs–indolicidin) in both preventing cell adhesion and eradicating the developed biofilms of some Candida clinical isolates and American Type Culture Collection (ATCC) strains. Furthermore, we investigated the expression levels of four biofilm-associated genes, namely EFG1, HWP1, ALS1, and ALS3.29 The activity of efflux pumps is widely described as the main cause of drug resistance in C. albicans, with an overexpression of CDR1 and CDR2 genes even after short exposure to antifungal drugs.30–32 Therefore, this study also investigated the possible involvement of efflux pumps and the expression of the connected CDR1 and CDR2 genes after the treatment of Candida biofilms.
Materials and methods
Synthesis and characterization of AuNPs–indolicidin
The peptide (Ac-CILPWKWPWWPWRR-CONH2) was synthesized using an automatic synthesizer (Syro I MultiSynThec, Wullener, Germany), as previously reported.33 The peptide was characterized using reversed-phase high-performance liquid chromatography (Shimadzu Corporation, Kyoto, Japan), on a column equipped with an ultraviolet Lambda-Max Model 481 detector, and liquid chromatography–mass spectrometry (Finnigan/Thermo Electron Corporation, San Jose, CA, USA).
Purified peptide was conjugated, in the presence of 10 mmol/L citrate buffer (pH 6), to Au-NPs (Cod. 752.568; Sigma-Aldrich, St Louis, MO, USA) with a diameter of 5 nm (5.47×1013 NPs/mL), as previously reported.34 The functionalized NPs were purified by centrifugation (14,000 g for 30 min) and the pellet was resuspended in PBS in the presence of 0.05% (v/v) Triton X-100. To calculate the amount of peptide coupled to AuNPs, fluorimetric measurements were carried out using a Varian Carry Eclipse spectrometer (Agilent Technologies, Santa Clara, CA, USA). The fluorimetric and zeta potential (ZP) measurements have been previously reported.35 The final peptide concentration coupled to AuNPs was 250 µM.
Growth conditions of microorganisms
Reference strains from ATCC (Manassas, VA, USA), C. albicans 90028, C. albicans 14053, and C. albicans 10231, were used in this study. Moreover, four C. albicans and one Candida tropicalis multi-resistant clinical isolates obtained from University Hospital “Luigi Vanvitelli” (Naples, Italy) were examined. The isolates had been taken as part of routine hospital laboratory procedures. The strains were named C1–C8; C1–C4, Candida albicans strains isolated from central venous catheter, oropharyngeal tract, blood, and oropharyngeal tract, respectively; C5–C7, C. albicans ATCC strains 90028, 10231, and 14053, respectively; C8, Candida tropicalis strain isolated from blood. All strains were maintained on Sabouraud dextrose agar (SDA) medium (1% peptone, 4% glucose, 1.5% agar, pH 5.6) (Difco) at 37°C for 24 h. Then, the yeast cells were inoculated in triptone soya broth (TSB) medium with 1% glucose (Difco) and incubated at 37°C for 24 h under agitation. Yeast cells were harvested by centrifugation, and washed twice in PBS, pH 7.0.
Determination of minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of planktonic cells
The antifungal effects of indolicidin on all C. albicans strains were evaluated using the broth microdilution susceptibility test according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI, 2008 M27-A3). Indolicidin was tested at concentrations ranging from 10 to 300 µM. Amphotericin B (Sigma-Aldrich, St Louis, MO, USA) at concentrations ranging from 0.001 to 100 µg/mL served as the standard antifungal compound. The MIC was determined as the minimal concentration that inhibited fungal growth determined at 590 nm using a microplate reader (Synergy™ H4; BioTek Instruments, Inc., Winooski, VT, USA).
The MFC was determined by subculturing 10 µL of the medium collected from the wells showing no microscopic growth after 48 h, on SDA medium plates. The MFC was the lowest concentration that yielded no colonies after 24 h growth on agar. The MFC/MIC ratio was calculated to determine whether the substance had fungistatic (MFC/MIC ≥4) or fungicidal (MFC/MIC <4) activity.36
Candida biofilm formation
Candida cells were suspended at 107 cells/mL in RPMI 1640 medium. Then, 100 µL of the suspension was added into individual wells of polystyrene 96-well plates and incubated at 37°C to allow biofilm formation. Biofilms were allowed to develop over two time intervals (24 h and 48 h). For 24 h biofilms, 40 ppm AuNPs, 150 mM indolicidin, or 40 ppm AuNPs–indolicidin were added to the wells to prevent cell adherence. For the 48 h biofilms, the medium was renewed after 24 h to allow a mature biofilm formation, and then AuNPs, indolicidin, and AuNPs–indolicidin, at the same concentrations as for the 24 h biofilms, were added to the wells for a further 24 h incubation.
Crystal-violet (CV) staining assay
The total biomass of the biofilm was analyzed using the CV staining method.37–39 Wells were washed with 300 µL of PBS to remove the planktonic cells, then fixation was performed by air drying at 60°C for 60 min. Then, 150 µL of CV (0.2% p/v) was added to each well and incubated for 15 min. After washing, CV bound to the biofilm was detached by adding 150 µL of 30% v/v acetic acid to each well. After incubating the plate for 30 min at room temperature, the absorbance at 570 nm was detected with a DR 5000™ spectrophotometer (Hach Company, Loveland, CO, USA).
XTT reduction assay
A quantitative measurement of C. albicans biofilm formation was obtained by assessing the metabolic activity of the attached cells with a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-(phenylamino) carbonyl)-2H-tetrazolium hydroxide (XTT) colorimetric assay kit (Sigma-Aldrich St Louis, MO, USA).40 In brief, wells were washed with 300 µL of PBS to remove the planktonic cells, then 100 µL of PBS and 10 µL XTT solution were added to each well. Plates were incubated at 37°C for 3 h in the dark and the colorimetric change was measured at 492 nm by a microtiter plate reader (Synergy H4; BioTek Instruments, Inc.).
Efflux of Rhodamine 6G (R6G) of planktonic cells
Candida cells were grown in 20 mL of TSB with glucose 1% p/v at 37°C for 18 h. Then, an aliquot of 1×108 cells was transferred to 5 mL of fresh medium and incubated at 37°C for 4 h. Cells were centrifuged at 4000 rpm for 5 min, and pellets were washed three times with PBS and then resuspended in 0.1 M KCl for 1 h. A stock solution of 10 mM R6G in DMSO was used.41 Then, 5 µL of the 10 mM R6G solution was added to the cell suspension and incubated at 37°C for 10 min. Cells were centrifuged at 10,000 rpm for 2 min, and the pellet was washed with 0.1 M KCl and resuspended in 5 mL of 0.1 M KCl. Then, the AuNPs–indolicidin complex was added to the cell suspension and incubated at 37°C. Every 10, 20, and 30 min, 1 mL of the sample was collected and centrifuged at 10,000 rpm for 2 min, and the supernatant absorbance at 527 nm was determined with a DR 5000™ spectrophotometer (Hach Company).
ZP of planktonic cells
The ZPs of the fungal cell suspensions, treated and not treated with AuNPs–indolicidin, were determined using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) and disposable zeta cells with gold electrodes. Overnight-cultured C. albicans (106 cells/mL of C1 and C5 strains) were suspended in TSB–glucose with and without 40 ppm AuNPs–indolicidin complex, and incubated for 24 h. Then, cells were centrifuged and resuspended in 20 mM phosphate buffer, pH 6.7. Five measurements (70–100 runs each) were performed with a constant voltage of 40 V, and equilibration time was 5 min at 37°C. Three independent experiments were performed.
Scanning electron microscopy (SEM)
To perform SEM observations of Candida biofilms, the biofilm-containing wells were washed with PBS, fixed overnight with 3% glutaraldehyde at 4°C, then washed with PBS and post-fixed in 1% aqueous solution of osmium for 90 min at room temperature. Then, samples were dehydrated in a series of graded alcohols, dried to the critical drying point, and finally coated with gold. A scanning electron microscope (Quanta 200 ESEM; FEI Europe Company, the Netherlands) operating under a high vacuum with 10 kV was used to observe the biofilm structure.
Total RNA extraction and reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was prepared in triplicate for each strain (treated and not treated with the complex) using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). The quality and amount of purified RNA were analyzed with a NanoDrop™ 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). In brief, 1000 ng of RNA was reverse transcribed with the QuantiTect® Reverse Transcription Kit (Qiagen, USA). Afterwards, we performed a real-time PCR using the QuantiTect SYBR Green PCR Kit in a Rotor-Gene Q cycler, following the manufacturer’s instructions (Qiagen, USA). Quantitative RT-PCR analysis was conducted using the 2-(ΔΔCt) method.42 Specific primers were designed (Table 1) for six target genes, and the ACT1 gene was used for normalization of the relative expression.43 At the end of each test, a melting-curve analysis was carried out (plate read every 0.5°C from 55°C to 95°C) to determine the formation of the specific products. Each sample was tested and run in duplicate.
  | Table 1 Primers used in this study Abbreviations: F, forward; R, reverse. |
Statistical analysis
The results reported are the mean values and SDs obtained from three different observations. The control and the treatment groups in various assays were compared and analyzed using Student’s t-test. Data with p-values <0.05 were considered statistically significant.
Results
Characterization of NPs
Indolicidin was covalently conjugated to AuNPs using thiol chemistry and the conjugation reaction was followed by fluorescence spectroscopy as previously reported,35 showing a reaction yield higher than 90%. The ZPs of AuNPs–indolicidin and AuNPs were –14.8±0.5 and –19.1±0.9 mV, respectively, in agreement with previous results. These data clearly showed that the presence of the peptide on the surface did not significantly modify the charge of the NPs.
Antimicrobial assays of indolicidin
To assess the antimicrobial activity of indolicidin against planktonic cells of Candida strains, we performed a susceptibility assay consisting of the determination of MIC and MFC.
Candida albicans strains and one C. tropicalis strain (C1–C8) were analyzed. Three ATCC C. albicans came from our laboratory’s collection (strains C5, C6, C7), four C. albicans strains colonizing different mucosal sites were isolated from hospitalized patients (strains C1, C2, C3, C4) and one C. tropicalis strain was isolated from the blood of a hospitalized patient (strain C8).
As shown in Table 2, MIC values were 150–200 µg/mL, and all of them were also fungicidal. For C1 and C5, indolicidin was fungicidal at their MIC (MIC=MFC), and these strains also had the best MICs.
  | Table 2 MICs and MFCs of indolicidin on Candida spp. Abbreviations: ATCC, American Type Culture Collection; MIC, minimum inhibitory concentration; MFC, minimum fungicidal concentration. |
Anti-biofilm efficiency of indolicidin, AuNPs, and AuNPs–indolicidin
Total biomass formation in the biofilm was evaluated using CV; the efficacy of the treatments with indolicidin, AuNPs, and AuNPs–indolicidin was determined using the XTT assay, with measurement of the biofilm metabolic activity, which is a technique commonly used to examine the impact of anti-biofilm therapies.
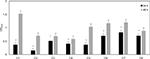
In Figure 1, the biofilm quantification of all the Candida strains after 24 h and 48 h is reported. The figure shows that all strains were able to develop both intermediate (24 h) and mature biofilms (48 h). Biofilms of ATCC strains (C5, C6, C7) at 48 h, compared with those of clinical isolates (C1, C2, C3, C4, C8), showed a biomass formation more similar to each other.
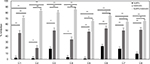
The results of inhibitory experiments with AuNPs–indolicidin on the biofilm formation at 24 h are reported in Figure 2. For all strains, the nano-complex presented an inhibitory effect on the adhesion which was more evident and significant than for indolicidin and AuNPs alone. In particular, it is possible to observe a significant inhibition (more than 50%) for all strains treated with AuNPs–indolicidin, with a more evident effect obtained for clinical strains (strains C1, C2, C3, C4). Figure 2 also shows that the peptide indolicidin alone was able to inhibit biofilm formation, reaching 40% inhibition in most cases.
XTT assays were performed to determine whether the AuNPs–indolicidin was able to eradicate the 48 h biofilms. Figure 3 shows that the major removal of mature biofilm was achieved for all strains with the complex AuNPs–indolicidin, although in some cases indolicidin alone was also effective. The results showed that the metabolic activity of Candida biofilms decreased for all the strains examined after treatment with AuNPs–indolicidin, above all for ATCC strains (C5, C6, C7), which showed a percentage of eradication of 55–65%.
Microscopy analysis
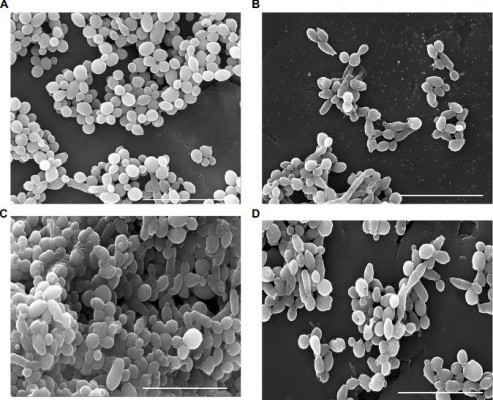
The structure of Candida biofilms before and after treatment with AuNPs–indolicidin was analyzed by SEM. In Figure 4, the representative microphotographs of strain C1 biofilms after 24 h and 48 h, and the corresponding biofilms after treatment are reported. The 24 h biofilm appeared relatively scarce and mostly composed of yeast-like cells (Figure 4A). In contrast, the 48 h biofilm (Figure 4C) showed a characteristic dense network of cells, with elongated forms surrounded by an abundant polymeric matrix. The occurrence of hyphae and a polymeric matrix is indicative of biofilm maturation, and represents a main virulence factor of Candida species.
The treatment impaired the formation of the 24 h biofilm (Figure 4B) and was also able to eradicate the developed one (Figure 4D).
The main structural differences visualized under SEM after treatment were major changes in the cell-surface appearance, from smooth to rough (Figure 4B), probably indicating outer cell-wall damage.
Kinetics of R6G efflux
The kinetics of R6G efflux was determined to evaluate the capacity of planktonic Candida cells to activate the drug efflux pumps following the nano-complex treatment.
Figure 5 reports the kinetics of R6G efflux by planktonic Candida cells of the two representative strains C1 and C5 after treatment with AuNPs–indolicidin. A significant increase in R6G release compared to controls, in a time-dependent manner, was observed.
ZP analysis
The ZPs of fungal cell suspensions (strains C1 and C5) treated and not treated with the AuNPs–indolicidin were determined. The results show that the treatment of cells with AuNPs–indolicidin did not significantly change the charge of cells. The ZP of planktonic cells after treatment with the nano-complex was similar to that of untreated ones (Table 3), and also similar to the ZP value of cells treated with AuNPs alone (–15.3 mV). These results show that there was no clear electrostatic effect of the nano-complex that could be attributed to damage to the membrane surface.
Real-time PCR
The effects of AuNPs–indolicidin on the expression of four biofilm-associated genes (EFG1, HWP1, ALS1, ALS3) and two drug-transporter encoding genes (CDR1, CDR2) were evaluated by quantitative RT-PCR. ALS1 and ALS3 encode cell-surface glycoproteins (adhesins) that mediate cell-to-cell adherence, EFG1 is one of the main transcription factors for biofilm formation, and HPW1 encodes a cell-wall fungal protein for hyphal development. As expected, the four biofilm-associated genes were down-regulated in the planktonic cells of all the strains examined with respect to the corresponding cells of the biofilms (data not shown).
Figure 6 reports transcription profiles of the biofilms of the two representative strains C1 and C5 after treatment with the AuNPs–indolicidin complex. The four biofilm-associated genes ALS1, ALS3, EFG1, and HPW1 were down-regulated in the early stages of biofilm development (Figure 6A and B) after treatment with the nano-complex, confirming that AuNPs–indolicidin inhibited to some extent the surface adherence of Candida cells. The analysis of the 48 h biofilm samples (Figure 6C and D), also shows a down-regulation of ALS1, ALS3, and EFG1 following the nano-complex treatment, indicating the susceptibility of the mature biofilms of the strains to AuNPs–indolicidin. Only HPW1 expression increased in 48 h biofilms, being equal to the control in strain C1 and up-regulated in C5.
A reduced expression of CDR1 and CDR2 was observed in both C1 and C5 early biofilms treated with AuNPs–indolicidin (Figure 6A and B), indicating a possible impairment of drug transport pumps. These data suggest that the inhibition of the initial phases of biofilm formation could be partly mediated through a mechanism of suppression of the expression of these genes. Differently from the C1 cells of the 48 h biofilm, where CDR1 and CDR2 were down-regulated (Figure 6C), in the C5 cells of the 48 h biofilm (Figure 6D), CDR1 showed no difference from the control and CDR2 was up-regulated, indicating an increased activity of the efflux pumps in the mature biofilm of this strain after treatment with the nano-complex.
Discussion
Biofilms of C. albicans formed on medical devices not only provide protection from environmental stress, but also enhance the resistance to antifungal agents compared to planktonic cells.
Both AMPs and NPs of different nature (organic, inorganic, or hybrid) represent a potential alternative as a therapeutic strategy for biofilm control.44 In particular, drug-functionalized NPs can be developed to enhance penetration, to selectively target or release drugs locally after microbial attachment or within the biofilms.26 Furthermore, the use of NPs as carriers allows drug efficacy to be enhanced by overcoming resistance mechanisms, such as degradation of β-lactamase, efflux pumps, or wide cell walls.45
In this work, we have provided sound evidence that the AuNPs–indolicidin complex can easily penetrate the biofilm matrix and the cells, altering fungal membrane permeability as well as reducing the cell population.
The results obtained clearly demonstrate that the functionalization of AuNPs with the peptide indolicidin produced a very effective compound able to inhibit the early formation of biofilms and to eradicate mature biofilms of ATCC strains and even of clinical isolates.
The XTT assay revealed the high potential of the nano-complex for prevention and treatment of C. albicans biofilms compared to indolicidin and AuNPs alone, the latter showing less pronounced effects.
In our experiments, AuNPs show a lower activity compared to indolicidin alone on Candida biofilm formation; these data suggest that AuNPs enhance indolicidin activity rather than inhibiting biofilm formation directly. This could be due to the enhanced stability of the nano-complex towards protease degradation occurring in the matrix and/or inside the cells. It has been reported that functionalization of NPs with AMPs overcomes problems such as proteolytic degradation and peptide self-aggregation, maintaining their antimicrobial properties.46
We found that the membrane surface was not significantly modified after treatment with AuNPs–indolicidin, as shown by ZP experiments. Instead, the different kinetics of R6G efflux in cells treated with the nano-complex was indicative of the activation of efflux pumps after treatment. We thus hypothesize that the mechanism of inhibition and eradication of the biofilm was correlated not with damage to the membrane surface, but with penetration of the nano-complex, which allows access to intracellular targets. We found that metabolic activity was impaired.
Moreover, after 24 h treatment, we found a significant down-regulation of the genes involved in biofilm formation (ALS1, ALS3, EFG1, and HWP1), as well as of the drug transporter encoding genes (CDR1, and CDR2), suggesting that the nano-complex increased the susceptibility of C. albicans, through a mechanism of suppression of gene expression. In the mature biofilm, after treatment, we observed a down-regulation for ALS1, ALS3, and EFG1 genes, encoding for molecules essential to C. albicans adhesion and biofilm formation, whereas HPW1 showed more variable behavior.
Conclusion
The results presented in this paper constitute a preliminary study for the possible use of the complex AuNPs–indolicidin as a therapeutic alternative to combat Candida biofilms in clinical applications, avoiding the adverse effects caused by the use of high doses of drugs. Nonetheless, further investigation is required to test the efficacy of this nano-complex on medical implants and to unravel its mechanisms of action.
Acknowledgment
The authors would like to thank Sergio Sorbo for the preparation and processing of samples for scanning electron microscopy.
Disclosure
The authors report no conflicts of interest in this work.
References
Yapar N. Epidemiology and risk factors for invasive candidiasis. Ther Clin Risk Manag. 2014;10:95–105. | ||
Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8:881–890. | ||
Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17:255–267. | ||
Nobile CJ, Johnson AD. Candida albicans Biofilms and Human Disease. Annu Rev Microbiol. 2015;69:71–92. | ||
Fey PD. Modality of bacterial growth presents unique targets: how do we treat biofilm-mediated infections? Curr Opin Microbiol. 2010;13:610–615. | ||
Spížek J, Novotná J, Řezanka T, Demain AL. Do we need new antibiotics? The search for new targets and new compounds. J Ind Microbiol Biotechnol. 2010;37:1241–1248. | ||
Baillie GS, Douglas LJ. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J Antimicrob Chemother. 2000;46:397–403. | ||
LaFleur MD, Kumamoto CA, Lewis K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob Agents Chemother. 2006;50:3839–3846. | ||
Ramage G, Bachmann S, Patterson TF, Wickes BL, López-Ribot JL. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J Antimicrob Chemother. 2002;49:973–980. | ||
Kuhn DM, Ghannoum MA. Candida biofilms: antifungal resistance and emerging therapeutic options. Curr Opin Investig Drugs. 2004;5:186–197. | ||
Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19:491–511. | ||
Lohner K. New strategies for novel antibiotics: peptides targeting bacterial cell membranes. Gen Physiol Biophys. 2009;28:105–116. | ||
Galdiero S, Falanga A, Berisio R, Grieco P, Morelli G, Galdiero M. Antimicrobial peptides as an opportunity against bacterial diseases. Curr Med Chem. 2015;22:1665–1677. | ||
Falanga A, Nigro E, De Biasi MG, et al. Cyclic Peptides as Novel Therapeutic Microbicides: Engineering of Human Defensin Mimetics. Molecules. 2017;22:E1217. | ||
Falanga A, Lombardi L, Franci G, et al. Marine Antimicrobial Peptides: Nature Provides Templates for the Design of Novel Compounds against Pathogenic Bacteria. Int J Mol Sci. 2016;17:E785. | ||
Di Luca M, Maccari G, Maisetta G, Batoni G. BaAMPs: the database of biofilm-active antimicrobial peptides. Biofouling. 2015;31:193–199. | ||
Moreno MG, Lombardi L, Di Luca M. Antimicrobial peptides for the control of biofilm formation. Curr Top Med Chem. 2017;17:1965–1986. | ||
Duplantier AJ, van Hoek ML. The Human Cathelicidin Antimicrobial Peptide LL-37 as a Potential Treatment for Polymicrobial Infected Wounds. Front Immunol. 2013;4:143. | ||
De Brucker K, Delattin N, Robijns S, et al. Derivatives of the mouse cathelicidin-related antimicrobial peptide (CRAMP) inhibit fungal and bacterial biofilm formation. Antimicrob Agents Chemother. 2014;58:5395–5404. | ||
Yan H, Hancock REW. Synergistic interactions between mammalian antimicrobial defense peptides. Antimicrob Agents Chemother. 2001;45:1558–1560. | ||
Maselli V, Siciliano A, Giorgio A, et al. Multigenerational effects and DNA alterations of QDs-Indolicidin on Daphnia magna. Environ Pollut. 2017;224:597–605. | ||
Galdiero E, Siciliano A, Maselli V, et al. An integrated study on antimicrobial activity and ecotoxicity of quantum dots and quantum dots coated with the antimicrobial peptide indolicidin. Int J Nanomedicine. 2016;11:4199–4211. | ||
Galdiero E, Falanga A, Siciliano A, et al. Daphnia magna and Xenopus laevis as in vivo models to probe toxicity and uptake of quantum dots functionalized with gH625. Int J Nanomedicine. 2017;12:2717–2731. | ||
Jorge P, Lourenço A, Pereira MO. New trends in peptide-based anti-biofilm strategies: a review of recent achievements and bioinformatic approaches. Biofouling. 2012;28:1033–1061. | ||
Mataraci E, Dosler S. In vitro activities of antibiotics and antimicrobial cationic peptides alone and in combination against methicillin-resistant Staphylococcus aureus biofilms. Antimicrob Agents Chemother. 2012;56:6366–6371. | ||
Han G, Ghosh P, Rotello VM. Functionalized gold nanoparticles for drug delivery. Nanomedicine. 2007;2:113–123. | ||
Pissuwan D, Valenzuela SM, Miller CM, Cortie MB. A golden bullet? Selective targeting of Toxoplasma gondii tachyzoites using antibody-functionalized gold nanorods. Nano Lett. 2007;7:3808–3812. | ||
Huang W, Tsai P, Chen Y. Functional gold nanoparticles as photothermal agents for selective-killing of pathogenic bacteria. Nanomedicine. 2007;2:777–787. | ||
Nobile CJ, Mitchell AP. Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol. 2006;8:1382–1391. | ||
Rocha MFG, Bandeira SP, de Alencar LP, et al. Azole resistance in Candida albicans from animals: Highlights on efflux pump activity and gene overexpression. Mycoses. 2017;60:462–468. | ||
Cannon RD, Lamping E, Holmes AR, et al. Efflux-Mediated Antifungal Drug Resistance. Clin Microbiol Rev. 2009;22:291–321. | ||
Gonçalves SS, Souza ACR, Chowdhary A, Meis JF, Colombo AL. Epidemiology and molecular mechanisms of antifungal resistance in Candida and Aspergillus. Mycoses. 2016;59:198–219. | ||
Galdiero S, Capasso D, Vitiello M, D’Isanto M, Pedone C, Galdiero M. Role of surface-exposed loops of Haemophilus influenzae protein P2 in the mitogen-activated protein kinase cascade. Infect Immun. 2003;71:2798–2809. | ||
Takatsuji Y, Ikeno S, Haruyama T. Gold nanoparticles functionalized with peptides for specific affinity aggregation assays of estrogen receptors and their agonists. Sensors (Basel). 2012;12:4952–4961. | ||
de Alteriis E, Falanga A, Galdiero S, Guida M, Maselli V, Galdiero E. Genotoxicity of gold nanoparticles functionalized with indolicidin towards Saccharomyces cerevisiae. J Environ Sci (China). 2018;66:138–145. | ||
Peixoto LR, Rosalen PL, Ferreira GLS, et al. Antifungal activity, mode of action and anti-biofilm effects of Laurus nobilis Linnaeus essential oil against Candida spp. Arch Oral Biol. 2017;73:179–185. | ||
Monteiro DR, Gorup LF, Takamiya AS, de Camargo ER, Filho ACR, Barbosa DB. Silver distribution and release from an antimicrobial denture base resin containing silver colloidal nanoparticles. J Prosthodont. 2012;21:7–15. | ||
Monteiro DR, Gorup LF, Silva S, et al. Silver colloidal nanoparticles: antifungal effect against adhered cells and biofilms of Candida albicans and Candida glabrata. Biofouling. 2011;27:711–719. | ||
Silva S, Pires P, Monteiro DR, et al. The effect of silver nanoparticles and nystatin on mixed biofilms of Candida glabrata and Candida albicans on acrylic. Med Mycol. 2013;51:178–184. | ||
Jothiprakasam V, Sambantham M, Chinnathambi S, Vijayaboopathi S. Candida tropicalis biofilm inhibition by ZnO nanoparticles and EDTA. Arch Oral Biol. 2017;73:21–24. | ||
Niemirowicz K, Durnás B, Tokajuk G, et al. Magnetic nanoparticles as a drug delivery system that enhance fungicidal activity of polyene antibiotics. Nanomedicine. 2016;12:2395–2404. | ||
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. | ||
Li X, Yu C, Huang X, Sun S. Synergistic effects and mechanisms of budesonide in combination with fluconazole against resistant Candida albicans. PLoS One. 2016;11:e0168936. | ||
Koo H, Allan RN, Howlin RP, Stoodley P, Hall-Stoodley L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol. 2017;15:740–755. | ||
Delattin N, De Brucker K, De Cremer K, Cammue BPA, Thevissen K. Antimicrobial peptides as a strategy to combat fungal biofilms. Curr Top Med Chem. 2017;17:604–612. | ||
Torres LMFC, Braga NA, Gomes IP, et al. Nanobiostructure of fibrous-like alumina functionalized with an analog of the BP100 peptide: Synthesis, characterization and biological applications. Colloids Surf B Biointerfaces. 2018;163:275–283. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.