Back to Journals » Drug Design, Development and Therapy » Volume 16
Efficiency and Safety of Baofei Granules in Chronic Obstructive Pulmonary Disease (Lung and Spleen Qi Deficiency Syndrome): A Multicenter, Randomized, Double-Blind, Placebo-Controlled Phase II Clinical Trial
Authors Sun Y , Chen X, Zhang L, Yuan WA, Chen Q, Zhang YB, Liu LJ, Zhang W, Sun M
Received 13 July 2022
Accepted for publication 8 November 2022
Published 14 December 2022 Volume 2022:16 Pages 4251—4267
DOI https://doi.org/10.2147/DDDT.S382285
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Yuan Sun,1,* Xuan Chen,1,* Lei Zhang,2 Wei-an Yuan,1 Qi Chen,1 Yi-bao Zhang,1 Lu-jiong Liu,1 Wei Zhang,1 Meng Sun1
1Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, People’s Republic of China; 2Shanghai Innovation Center of TCM Health Service, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Zhang; Meng Sun, Email [email protected]; [email protected]
Background: Baofei Granules (BFGs) have been extensively applied in the clinical treatment of chronic obstructive pulmonary disease (COPD) and significantly have affected COPD patients with lung and spleen qi deficiency syndrome. However, the data from previous small-sample clinical trials are limited. This trial aimed to estimate the efficiency and safety of BFGs in COPD with lung and spleen qi deficiency syndrome.
Methods: It is a multicenter, randomized, double-blind, placebo-controlled phase II clinical trial. The 216 stable COPD patients will be divided randomly in a ratio of 1:1. The whole trial period consists of a 4-week introductory period, a 52-week treatment period and a 48-week follow-up. Study visits occur every 4 weeks during the treatment period and every 12 weeks during the follow-up. All the subjects will receive 10g BFGs or placebo three times per day for 56 weeks and be followed up for 48 weeks. The primary efficiency evaluation outcome will be the frequency and duration of AECOPD, and the secondary efficiency evaluation outcome will be pulmonary function tests (PFTs), modified Medical Research Council (mMRC) dyspnoea scale, six-minute walking test (6MWT), COPD assessment test (CAT) score, traditional Chinese medicine (TCM) syndrome score, the frequency of emergency medication, BODE index, and the time to first Clinically important deterioration (CID). The safety evaluation outcomes will be adverse events (AEs), vital signs, physical examination, twelve-lead electrocardiogram (ECG), and laboratory examinations. All the data will be analyzed by SAS9.4.
Discussion: This is the first and largest clinical trial that evaluates the efficiency and safety of BFGs for COPD with lung and spleen qi deficiency syndrome. It will provide valuable clinical evidence for recommendations on COPD management by the integrated TCM and western medicine.
Trial Registration: CTR20211280. Date: June 09, 2021. http://www.chinadrugtrials.org.cn/clinicaltrials.searchlistdetail.dhtml?id=383a370ecd9f43d7af6f1c8585779e1a.
Keywords: Baofei Granules, chronic obstructive pulmonary disease, efficacy, safety, traditional Chinese medicine
Background
Chronic obstructive pulmonary disease (COPD) is a common disease characterized by persistent respiratory symptoms and airflow limitation. Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) is an essential factor in promoting the continuous disease progression and an independent risk factor for death in most COPD patients.1
Data from the Global Burden of Disease Study estimated the point prevalence of COPD at 3.92% worldwide in 2017, and the estimated COPD-attributable death rate was 42/100,000 (4.72% of all-cause deaths).2 The total number of COPD patients in China is about 100 million. A survey estimated that the overall incidence of COPD in China was 13.7% in residents > 40 years old, while more than 27% in residents > 60 years old. China ranks first in the world in the number of COPD deaths, with nearly 1 million deaths annually, which induced a vast social and economic burden.3
The management of stable COPD aims to reduce current symptoms and future risks of exacerbations with pharmacological treatments and non-pharmacological treatments. Bronchodilators, corticosteroids, and antibiotics are commonly used maintenance medications in COPD, while pulmonary rehabilitation is one of the essential non-pharmacological treatments complementary to pharmacological treatment.4 However, the not-yet-understood mechanism of COPD is a considerable hindrance to radically improving outcomes in patients with COPD. It is difficult to reverse the downtrend in pulmonary function or improve the quality of life for COPD patients with guideline-recommended medications. Moreover, the standard routine medications are always associated with significant side effects such as osteoporosis and pneumonia caused by corticosteroids, tinnitus caused by antibiotics, etc.5–7 In a word, it is urgent for us to explore better treatments and medications to prevent and treat COPD.
Traditional Chinese medicine (TCM) treatment of COPD has been of considerable interest to the medical community. Guided by the Chinese medicine theory, Professor Li Jiansheng at Henan University of Traditional Chinese Medicine summarized the primary Chinese pathogenesis of COPD as an “accumulation of pathogen and damage due to deficiency of vital qi” and proposed that the most common TCM syndrome is “lung and spleen qi deficiency syndrome” in Chinese patients.8–10 Pro. Li has conducted extensive research on the effectiveness and safety of the TCM granules in treating COPD and found that his self-developed granules could significantly reduce the frequency and severity of exacerbations and improve the patients’ symptoms and prognosis.11,12 While, based on pharmacodynamic analyses, the granules made great effects on reducing pulmonary inflammation and remodeling in COPD through its effects on interleukin expression and/or secretion.13–15 Thus, Professor Li developed a new kind of granules called as Baofei Granules (BFGs) for COPD patients with lung and spleen qi deficiency syndrome.
Hitherto, sufficient data on BFGs as therapy in COPD (lung and spleen qi deficiency syndrome) are lacking, and the methodology and sample size limit the present clinical trials. Thus, we aimed to conduct a multicenter, randomized, double-blind, placebo-controlled clinical trial to closely estimate the efficiency and safety of BFGs in COPD (lung and spleen qi deficiency syndrome) and explore the underlying mechanisms.
Methods/Design
Study Design
It is a multicenter, randomized, double-blind, placebo-controlled phase II clinical trial. The trial will be conducted by the ethical principles that have their origin in the Declaration of Helsinki and are consistent with Good clinical practice (GCP) and the applicable regulatory requirements.
216 stable COPD patients fulfilling the eligibility criteria will be enrolled. Subsequently, the subjects will be randomly assigned to two groups (treatment (BFGs) group and control (placebo) group) in a ratio of 1:1. The whole trial period consists of a 4-week introductory period, a 52-week treatment period, and a 48-week follow-up. Study visits occur every 4 weeks during the treatment period and every 12 weeks during the follow-up. All the subjects will receive 10g BFGs or placebo three times per day for 56 (4+52) weeks and their routine medications.
The flowchart of the trial is shown in Figure 1.
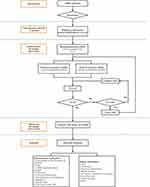 |
Figure 1 The flowchart of the trial. |
Participant Recruitment and Screening
Ten hospitals are selected as the investigational sites in our country (Table 1). The recruitment will be conducted in the outpatient departments or independent reception rooms in each investigational site. Subjects should be informed about the trial before voluntarily signing the informed consent forms.
 |
Table 1 Investigational Sites |
Sample Size
The primary efficiency evaluation outcome of the trial will be the frequency of moderate/severe AECOPD in the 52-week treatment period. According to our pre-experiments, presuming that the number of the exacerbation frequency in treatment group is on average 1.0±1.13, while the number in control group is on average 1.5±1.13. The one-sided alpha level is 0.025, and the beta level is 0.20. It is estimated that the sample size is 86 in each group according to the PASS15 software. Considering a 20% shedding rate, 216 subjects are finally needed in the two groups, with 108 cases in each group.
Diagnostic Criteria of COPD
The diagnosis criteria of COPD are made according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD)4 and Guidelines for the Diagnosis of Chronic Obstructive Pulmonary Disease.16
COPD diagnosis is based on a comprehensive analysis of clinical manifestations, risk factor exposure history, signs and laboratory examinations, etc. The main symptoms of COPD are chronic cough, sputum and/or dyspnoea, and risk factor exposure history. Pulmonary function tests (PFTs) are required to establish a diagnosis of COPD: The presence of a post-bronchodilator forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio (FEV1/FVC) <0.7 confirms the presence of persistent airflow limitation. COPD should be considered in any patient who has dyspnea, chronic cough or sputum production, a history of recurrent lower respiratory tract infections, and/or a history of exposure to risk factors for the disease. Chest X-ray examination helps determine the extent of hyperinflation in the lungs. Patients with bronchial asthma, bronchiectasis, congestive heart, and other lung diseases, such as heart failure and tuberculosis, were excluded.
Diagnostic Criteria of TCM Syndrome
TCM syndrome of “lung and spleen qi deficiency” refers to Diagnostic Criteria of Traditional Chinese Medicine Syndromes for COPD.17
Diagnose of “lung and spleen qi deficiency syndrome”: ①coughing/shortness of breath, aggravated by exercise; ②fatigue or spontaneous perspiration, aggravated by exercise; ③being prone to catching a cold; ④poor appetite or inferior food intake; ⑤bloating in the gastric cavity, or abdominal distension, or loose stools; ⑥an enlarged tongue with a white or greasy fur and deep thready pulse, deep, slow pulse or thready weak pulse.
Subjects who have two of the first three diagnoses (①, ②, ③) and two of the last three diagnoses (④, ⑤, ⑥) will be determined as a TCM syndrome of “lung and spleen qi deficiency”.
Inclusion Criteria
Inclusion criteria include the following:
- Patients clinically diagnosed with COPD for ≥1 year and been classified as GOLD grade II~IV according to the GOLD spirometric criteria;
- Without acute exacerbation of COPD in the past 4 weeks;
- ≥1 moderate/severe AECOPD in the past 1 year;
- Patients with TCM syndrome of “lung and spleen qi deficiency”;
- An age range of 40 to 75 years (≥40 years, ≤75 years);
- Provision of signed informed consent by subjects.
Exclusion Criteria
Exclusion criteria include the following:
- Patients accompanied by asthma, main bronchiectasis, active pulmonary tuberculosis, lung cancer, idiopathic pulmonary fibrosis, diffuse pantothenic bronchiolitis, lung resection, severe bronchiectasis, interstitial lung disease, or other active diseases;
- Patients accompanied by severe cardiovascular disease (congestive heart failure with NYHA Class III or IV), arrhythmias or cardiac valve abnormalities with hemodynamic changes; angina or myocardial infarction in the past 1 month);
- Patients accompanied by severe digestive system diseases, endocrine system diseases, nervous system diseases, and others;
- Patients accompanied by mental illness or depression;
- Patients with moderate/severe AECOPD and/or pneumonia in the past 4 weeks.
- Patients with pneumonectomy or lung volume reduction surgery in the past 12 months;
- Patients need long-term oxygen therapy (daily oxygen inhalation time ≥15h) or mechanical ventilation;
- Patients with the standard treatment of oral glucocorticoid, intravenous glucocorticoid, or inhaled corticosteroids at present;
- Patients combined with conditions which absolute or relative contraindications to the six-minute walking test (6MWT): heart rate >120 bpm, systolic pressure >180mmHg, and diastolic pressure >100mmHg or difficulty in completing the 6MWT with lower extremity mobility limitation;
- Alanine aminotransferase (ALT) or aspartate aminotransferase (AST) >1.5 times the upper limit of normal reference or serum creatinine (Scr) > the upper limit of normal reference;
- Patients with a history of chronic alcoholism or drug abuse in the past 1 year;
- Patients who are allergic or intolerant to the research medication and the emergency medication;
- Pregnant women or breastfeeding women, or women who are exposed to the risk of pregnancy;
- Patients who have participated in other clinical trials in the past 30 days;
- Furthermore, those who be considered inappropriate to participate in this clinical trial by the investigator.
Dropout, Termination, and Removal Criteria
Subjects will be withdrawn if:
- Being pregnant;
- Total duration of AECOPD >10 weeks;
- Patients with poor compliance: The actual dosage was < 80% of the total dosage or >120% of the total dosage with 3 consecutive visits;
- Due to severe complications or changes in the comorbidities or special physiological changes;
- The occurrence of allergic reactions or serious adverse events (SAE), etc.;
- Cases of broken blinding or emergency unblinding;
- Voluntary withdrawal.
- Dropout cases and the reason for dropout should be recorded in the case report forms. Safety reasons for dropout should continue monitoring and recording the patient’s regression as much as possible.
The trial will be terminated if:
- Serious safety problems occur in the trial, and the investigator believes that the rights and interests of the subject may be compromised;
- Significant errors in the clinical trial protocol are identified during the trial or significant deviations in implementation that makes it difficult to evaluate the efficiency and/or safety of the drug;
- Discontinuation by the sponsor (eg, financial reasons, management reasons, etc.);
- National Medical Products Administration withdraws the trial, etc.
The case will be removed if:
- Subjects who failed to receive the investigational products;
- There is no data to evaluate the efficiency or safety after randomization, etc.
Randomization
Randomization will be achieved using an independent interactive web response system (IWRS). A teletherapist will first enter the designed parameters, the site numbers, and the product numbers in IWRS. Then a random sequence of 216 eligible subjects according to a 1:1 ratio will be generated by IWRS. IWRS will assign each subject a subject identification code (SIC). A unique data management and statistical analysis will be responsible for the Establishment and management of IWRS.
Blinding
BFGs and placebo will be made with the same colors, smells, shapes, and tastes according to the Guiding Principles of Clinical Research on New Drugs of Chinese Medicines. Personnel who are irrelevant to this trial’s clinical observation, monitoring, and statistical analysis will put the product code on the outer package based on the random sequence assigned by IWRS. The blind codes will be placed in a closed envelope in duplicate and saved by the principal investigator (PI) and the sponsor without access.
Emergency Unblinding
Unblinding will be allowed only in an occurred medical emergency to determine an appropriate therapy required by the information of the investigational products. The PI and the sponsor should be notified before the emergency unblinding as soon as possible. The treatment allocation and the manual of the investigational products will be provided after entering the SIC in IWRS. The date, the location, and the reasons for the emergency unblinding must be recorded as required by the IWRS. Investigators should contact the sponsor within 24-hour to report the emergency unblinding if the previous contact failed.
Intervention
Basic Treatments
Routine Medications
Based on health education, all subjects will receive routine Western medicine treatment according to the GOLD4 and the Guidelines for the Diagnosis of Chronic Obstructive Pulmonary Disease,16 including:
Moderate COPD (GOLD grade II): Tiotropium, 18μg, inhale once a day.
Severe COPD (GOLD grade III): Budesonide and Formoterol fumarate powder for inhalation, 160μg/4.5μg, inhale twice a day.
Very severe COPD(GOLD grade IV): Tiotropium, 18μg, inhale, once a day + Budesonide and Formoterol fumarate powder for inhalation, 160μg/4.5μg, inhale, twice a day.
Emergency Medications
Commercially available salbutamol pressurized metered-dose inhalers (pMDIs) and spacers are provided as emergency medications according to the individual requirement. The usage of emergency medications should be recorded in the electronic patient-reported outcome (ePRO) every time by the subjects themselves. Any other short-acting beta2-agonists will not be permitted during the whole clinical trial.
Salbutamol pMDIs and spacers: 100–200μg (1–2 puffs), every 4 to 8 hours as needed, up to 8 puffs in 24 hours. A Salbutamol pMDIs and spacers will be dispensed to the subjects at the baseline date (V0) and is available during the trial. (Shanghai Kaibao Pharmaceutical Co., Ltd provides.).
Investigational Products
Source and Ingredient
Shanghai Kaibao Pharmaceutical Co., Ltd provides all the investigational products with the statement “for clinical trial use only-not for sale” on the outer package. Investigational products are manufactured, handled, and stored following GCP. Investigators will dispense the investigational products to subjects in investigational sites. The quality of the granules is consistent with the required quality standards. Each granule type was given orally, one packet each time, three times daily for 56 weeks.
Dosage form: Granules; Container type: Packet; Unit dose:10 g/ packet.
Main components of BFGs: Radix Astragali Preparata, Alcoholis Rhizoma Polygonat, Codonopsis Radix, Fried Atractylodes, Poria Cocos, Fritillaria Thunbergii, Ginger Houpo, Tangerine Peel, Honey-fried Radix Asteris Decoction, Oil-fried Epimedium, Earthworm and Herba ardisiae Japonicae.
Indications function: tonifying lung and invigorating spleen, resolving Phlegm, and relieving cough and asthma. It is suitable for patients with stable chronic obstructive pulmonary disease of lung and spleen qi deficiency syndrome.
Dispensation and Recovery
Dispensation:
Introductory period: 4 weeks.
For both groups: placebo, 10g/packet, 1 packet each time, three times a day.
The investigational products (placebo) without code-requirement will be dispensed to subjects in a package containing a 4-week dose.
Treatment period:52 weeks.
For treatment group: BFGs, 10g/packet, 1 packet each time, three times a day.
For control group: placebo, 10g/packet, 1 packet each time, three times a day.
Investigational products will be dispensed according to the random sequence. A 4-week+ 7-day dose of either BFGs or placebo will be distributed to subjects at the baseline date (V0). Then, subjects will receive an 8-week+ 7-day dose of investigational products every 8 weeks at the following 6 site visits.
Recovery:
All unused packets of drugs and empty packets will be returned to the investigators at the next visit. Adherence will be determined as follows: Medication adherence% = [actual dose/(specified dose)] ×100%.
Dispensation and recovery of the investigational products please see Table 2
 |
Table 2 Dispensation and Recovery of the Investigational Products |
Comorbidities and Exacerbations Regulation
Concomitant medications and therapies should be recorded in detail, including the name, usage, dose, and reasons in the electronic case report forms (eCRFs) and the subject folders for the final analysis and report on the trial.
Prohibited combination medication and therapy in the trial as follows:
- Additional Western medicine (antibiotics, glucocorticoid, mucolytic, antitussive) for stable COPD.
- Chinese medicine for COPD.
- Non-pharmacological treatments for COPD.
- Any other medicine that may affect the efficiency evaluation.
Subjects are encouraged to make contact with the investigators positively if AECOPD occurs. Investigators should terminate the investigational product treatment and take careful treatments to protect the safety of the subjects until the symptom returns to the stable Investigators also should complete ePRO diaries every day to record the time, degree, and duration of AECOPD, effective treatments, dropout, hospitalization, and results. Hospitalization for a COPD exacerbation will not be defined as an SAE. Investigators will not withdraw the hospitalized subjects if they return to a stable clinical state (symptom returns to the ordinary or near-ordinary level) after hospitalization.
Data Collection and Management
Various parameters are followed up according to the data collection time points. For the data collection time points please see Table 3.
 |
Table 3 Data Collection Time Points |
The baseline is defined as the last non-missing observations before the first medication session, including demographic characteristics, vital signs, medical history, concomitant medications and therapies, laboratory indicators, primary diagnosis basis, etc.
The trial uses the electronic data capture (EDC) system to complete the online data management through electronic data entry, data verification, and data review. Investigators or assistant investigators (AI) should fill in the data in the eCRFs accurately without changes, blanks, or omissions. The data are reviewed by the monitors, data administrators, and medically qualified persons. Investigators should put electronic signatures on the eCRFs.
All records should be well preserved. The PI, the sponsor, statistical experts, and data administrators will finally review the data and deal with the dataset data type, missing value, and outlier value before statistical analysis. The data management will lock the data after the final review. An independent professional statistician will finish the statistical analysis.
Quality Control
All investigators are qualified in GCP and human subjects protection to ensure the consistency of the results. Monitors appointed by the sponsor will be regularly inspected and monitored throughout the process, following the standard protocol.
Outcomes
The Efficiency Evaluation outcomes
- The frequency and duration of AECOPD;
Including time to first AECOPD, time to first moderate/severe AECOPD, and the frequency of moderate/ severe AECOPD (episode/per year).
Acute exacerbation of COPD (AECOPD) is defined as acute worsening of respiratory symptoms (typically dyspnoea, cough, increased sputum, and/or purulent sputum) in patients with COPD, exceeding normal day-to-day variations that may require a change in medication even hospitalization.
According to the Guidelines for the Diagnosis and Management of Chronic Obstructive Pulmonary Disease (revised version 2021),18 the clinical indications of AECOPD: ①dyspnoea, profuse sputum, and purulent sputum; ②purulent sputum and dyspnoea/profuse sputum; ③need for invasive mechanical ventilation or noninvasive mechanical.
The interval of AECOPD between two episodes is defined as at least 5 days. The duration of AECOPD is defined as the duration from acute exacerbation to the apparent improvement of the symptoms (symptom returns to the normal or near-normal level).
Exacerbations are classified as:1
Mild (treated with short-acting bronchodilators (SABDs) only);
Moderate(treated with SABDs plus antibiotics and/or oral corticosteroids) or
Severe (patient requires hospitalization or visits the emergency room). Severe exacerbations may also be associated with acute respiratory failure.
The evaluation criteria for the TCM syndrome score are shown in Table 4.
 |
Table 4 (6) TCM Syndrome Score |
Efficacy index (n)=[(preintervention scores–postintervention scores)/preintervention scores]×100%.
Clinical recovery: TCM clinical symptoms and signs disappeared or approximately disappeared; TCM scores decreased ≥95%.
Markedly effective: TCM clinical symptoms and signs are significantly improved; TCM scores decreased ≥70%.
Effective: TCM clinical symptoms and signs are improved; TCM scores decreased ≥30%.
Invalid: No TCM clinical symptoms or signs significantly improved and even aggravated; TCM scores decreased < 30%.
It includes the following aspects: B: Body mass index (BMI), O: Degree of airflow obstruction, E: Evaluation of dyspnoea, and D: Dynamic ability. Subjects will undergo the Body mass index (BMI), PFTs, 6MWT, and mMRC dyspnoea scale. Investigators will add the four scores together to obtain the BODE score. Higher scores often indicate poorer outcomes.
For details, please see the Supplementary Table 1.
The time to first CID is the time to the first occurrence of at least one of the following: ①decrease in trough FEV1 from baseline ≥100 mL;②increase in CAT score from baseline ≥2 units; ③moderate/severe exacerbation.
The Data Collection Time Points of the Efficiency Evaluation Outcomes
- AECOPD occurrence:Make records when it happens;
- PFTs:Screening period, V0, V3, V5, V9, V13;
- mMRC dyspnoea scale:V0, V3, V5, V9, V13;
- 6MWT:V0, V3, V5, V9, V13;
- CAT score:V0, V3, V5, V9, V13;
- TCM syndrome score:V0, V3, V5, V9, V13.
The Safety Evaluation Outcomes
- Adverse events (AEs)
Adverse Events (AEs) in the trial are classified according to the Common terminology for adverse event (CTCAE) v5.0, which are not thought to be necessarily causally related to trial medications. All AEs should be recorded and monitored in the eCRF and relevant folder until they disappear. The record of AEs includes the time, severity, and duration, the measures adopted, treatment measures, and the outcome.
AEs are assigned as mild AE, moderate AE, severe AE (SAE), life-threatening or disabling AE, and death related to AE according to CTCAE v5.0. The causal judgment indicators according to the Consensus of Experts on Drug Management of Drug Clinical Trial in Guangdong.21
(2) Vital signs (body temperature, heart rate, breathing, and blood pressure);
(3) Physical examination;
(4) Blood routine (red blood cell (RBC), hemoglobin (HGB), white blood cell (WBC), blood platelet (PLT));
(5) Urinalysis+sediment microscopy (leukocyte (LEU), protein (PRO), glucosuria (GLU), RBC);
(6) Liver function (ALT, AST, total bilirubin (TBil), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT));
(7) Renal function (serum creatinine (Scr), blood urea nitrogen (BUN), urinary N-acetyl-β-D-glucosaminidase (NAG));
(8) Twelve-lead electrocardiogram (ECG).
The Data Collection Time Points of the Safety Evaluation Outcomes
1. AEs:Make records when it happens;
2. Vital signs:Screening period, V0, V1, V3, V5, V7, V9, V11, V13;
3. Physical examination:Screening period, V0, V1, V3, V5, V7, V9, V11, V13;
4. Routine blood test:V0, V1, V3, V5, V9, V13;
5. Routine urine test+examination urinary sediments:V0, V1, V3, V5, V9, V13;
6. Liver function:V0, V1, V3, V5, V9, V13;
7. Renal function:V0, V1, V3, V5, V9, V13;
8. Twelve-lead ECG:V0, V1, V3, V5, V9, V13.
Planned Analysis
Statistical analysis will be performed by SAS V.9.4 software. Subjects’ characteristics, medication, and treatments will be descriptively analyzed using Full Analysis Set (FAS). The efficiency evaluation will be analyzed using FAS and Per Protocol Set (PPS), and the safety evaluation will be analyzed using Safety Analysis Set (SS).
Descriptive statistics and normality tests will be used on the quantitative data. The T-test (normal distribution) or Mann–Whitney test (non-normal distribution) will be used for differences between groups. In contrast, Paired T-test or Wilcoxon signed-rank test will be used for within-group differences. We will use an Analysis of covariance (ANOVA) to get the point estimates, and a 95% confidence interval (CI) for the least-squares (LS) mean change within each group, considering the confounding factors (baseline, within-hospital clustering, or other factors). We will use a generalized estimating equation (GEE) to account for correlation over time and within-hospital clustering.
Categorical data will be compared by Chi-square test, Fisher exact test, Wilcoxon rank-sum test, and Wilcoxon signed-rank test. The CMHΧ2 test will analyze dichotomous data and ranking data. We will use Logistic regression to avoid confounding bias from baseline characteristics.
For survival analysis, a quartile of time-to-event variables with two-sided 95% CI will be calculated following a Kaplan-Meier curve with a statistical comparison between the two groups using the Log rank test. We will use Cox regression analysis due to the influence of important confounding factors (baseline characteristics).
Ethics and Dissemination
The Clinical Research Ethics Committee should approve the trial protocol. All the changes in the trial protocol should be maintained, and the revised protocol should be submitted to the Ethics Committee for re-review. Subjects will receive research medication and physical and chemical examinations for free during the trial. Investigators of each investigational site are responsible for the medical treatment of the subjects. They will provide free medical treatment if trial-related adverse events occur or condition changes.
The sponsor is entitled to publish or distribute any results of this trial. Investigators’ opinions and suggestions can be collected before publishing or distributing the results. After the trial ends, investigators are entitled to publish or distribute the clinical trial results only after getting the written consent of the sponsor. The organizations responsible for the clinical trial are permitted to publish summary reports in thesis papers, and investigators have the right to authorship. Still, both also need prior written consent from the sponsor.
Discussion
COPD represents a significant public health challenge as an essential cause of chronic morbidity and mortality. TCM has notable advantages in preventing and treating COPD through multi-pathway and multi-target points based on TCM characteristic theory “treatment based on syndrome differentiation” and “treating both principal and secondary aspect of disease”. The patients are treated with TCM to “eliminate phlegm and expel blood stasis”, “strengthen the spleen and tonify the lung”, and “warm kidney and benefit qi”. In modern times, TCM has been widely used in patients with COPD, especially in combination with routine Western medicine. Researchers have found that TCM combined with routine Western medicine may improve COPD symptoms and clinical efficiency.22,23 A large systematic review and meta-analysis also confirm this finding.24 A nationwide retrospective cohort study has pointed out that combining TCM with Western medicine might reduce the risk of AECOPD and the incidence of mortality in COPD patients.25 Huiting Huang designed a study that included 234 stable COPD patients and found that the TCM granules could decrease CAT scores and mMRC grading.26 This evidence shows that TCM with Western medicine improved the quality of life compared with Western medicine alone. Meanwhile, TCM could inhibit the fibrosis process and reduce airway remodelling, improved by preclinical studies and clinical trials.27,28
It is noteworthy that adherence to therapy and self-management is essential for COPD patients. A previous report has shown that Chinese patent medicine and TCM decoctions are preferred options for complementary medicines to combine with routine pharmacotherapy for COPD treatment according to the patients’ preferences.29 Therefore, we could speculate that BFGs may promote the patients’ medication adherence.
Previous small-sample clinical studies have shown that BFGs are effective as an adjunct therapy for COPD. Therefore, we designed the multicenter, randomized, double-blind, placebo-controlled phase II clinical trial with follow-up as long as 48 weeks to closely study the efficiency and safety of BFGs in COPD (lung and spleen qi deficiency syndrome) with the expectation. We hope that the advent of TCM brings renewed hope to COPD patients that are not satisfied with the efficacy of the routine medications.
The advantages of the trial protocol are apparent: (1) The report may serve as evidence-based medicine (EBM) evidence for the efficacy and safety of BFGs. (2) The trial with long-term follow-up enables us to verify the efficacy and safety of BFGs. (3) The data gathered will shed new light on TCM for COPD. (4) We add the TCM syndrome score to verify the efficacy further.
While the protocol also has potential limitations: (1) We only focus on one TCM syndrome of COPD while the result is hard to extend to the entire patient. (2) A 48-week follow-up may exacerbate the loss of some subjects. (3) We cannot predict the impact of COVID-19 on subjects and the study progress.
In conclusion, this trial aims to provide credible clinical evidence for the future combination of TCM and Western medicines for COPD, spurring the integration of traditional Chinese medicine and Western medicine. We sincerely hope for the trial’s success and believe the result will provide a new option to improve the symptoms and disease progression.
Trial Status
Recruitment has started and is ongoing since June 2021.
Abbreviations
AE, Adverse event; AECOPD, Acute exacerbations of chronic obstructive pulmonary disease; AI, Assistant investigators; ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; BFGs, Baofei Granules; BMI, Body mass index; BODE, Body Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity; BUN, Blood urea nitrogen; CAT, Chronic obstructive pulmonary disease assessment test; CI, Confidence interval; CID, Clinically important deterioration; COPD, Chronic obstructive pulmonary disease; EBM, Evidence-based medicine; ECG, Electrocardiogram; eCRFs, Electronic case report forms; EDC, Electronic data capture; ePRO, Electronic patient-reported outcome; FAS, Full Analysis Set; FEV1, Forced expiratory volume in the first second; FEV1%, Forced expiratory volume in the first second as a percentage of the estimated value; FVC, Forced vital capacity (exhale); GCP, Good clinical practise; GEE, Generalized estimating equation; GGT, Gamma-glutamyl transferase; GLU, Glucosuria; HGB, Hemoglobin; IL-10, Interleukin-10; IL-6, Interleukin-6; IL-7, Interleukin-7; IWRS, Interactive web response system; LEU, Leukocyte; LS, Least-squares; mMRC, Modified Medical Research Council; NAG, Urinary N-acetyl-β-D-glucosaminidase; PFTs, Pulmonary function tests; PI, Principal investigator; PLT, Blood platelet; pMDIs, Pressurized metered-dose inhalers; PPS, Per Protocol Set; PRO, Protein; RBC, Red blood cell; SABD, Short-acting bronchodilator; SAE, Serious adverse event; Scr, Serum creatinine; SIC, Subject identification code; 6MWT, Six-minute walking test; SS, Safety Analysis Set; TBil, Total bilirubin; TCM, Traditional Chinese medicine; WBC, White blood cell.
Data Sharing Statement
The datasets used and analyzed during the current study will be available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The protocol of this study has been approved by ethic committee: IRB of Shuguang Hospital affiliated with Shanghai University of TCM(Permit Number:2021-978-53-01). Written informed consent will be obtained from all participants prior inclusion into the study by attending investigators and research assistants. This study have been performed in accordance with the Declaration of Helsinki.
Author Contributions
SY and CX were co-first authors of this manuscript, and ZW reviewed the whole study protocol. This study protocol was also the protocol of a sub-topic responsible by CX. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Shanghai Committee of Science and Technology, China (Grant No. 21S21903002); Shanghai Key Clinical Specialty (No.SHSLCZDZK05101) and Shanghai Key Laboratory of Traditional Chinese Clinical Medicine (20DZ2272200).
Disclosure
The authors declare that they have no competing interests.
References
1. Chinese expert panel in acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Chinese Expert Consensus on the Diagnosis and Treatment of Acute Exacerbation of Chronic Obstructive Pulmonary Disease(AECOPD)(2017 Update). Int J Respir. 2017;37(14):1041–1057. doi:10.3760/cma.j.issn.1673-436X.2017.14.001
2. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–1158. doi:10.1016/S0140-6736(19)30427-1
3. Writing Group of Expert Consensus on Anti-infective Therapy for Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Expert consensus on anti-infective therapy for acute exacerbation of chronic obstructive pulmonary disease in China. Int J Respir. 2019;39(17):1281–1296.
4. GOLD.Global Strategy for the Diagnosis, Management, and Preventi on of Chronic Obstructive Pulmonary Disease[EB/OL]. Available from: https://goldcopd.org/2021-gold-reports/.
5. Janson C, Lisspers K, Ställberg B, et al. Osteoporosis and fracture risk associated with inhaled corticosteroid use among Swedish COPD patients: the Arctic study. Eur Respir J. 2021;57(2):2000515. doi:10.1183/13993003.00515-2020
6. Herath SC, Normansell R, Maisey S, et al. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2018;10(10):CD009764. doi:10.1002/14651858.CD009764.pub3
7. Chen H, Sun J, Huang Q, et al. Inhaled Corticosteroids and the Pneumonia Risk in Patients With Chronic Obstructive Pulmonary Disease: a Meta-analysis of Randomized Controlled Trials. Front Pharmacol. 2021;12:691621. doi:10.3389/fphar.2021.691621
8. Wang Z, Jiansheng L. Study Based Literature on Distribution Regularity of Essential Elements of Syndrome of COPD at Stable Phase. Liaoning J Traditional Chine Med. 2008;(4):513–514. doi:10.13192/j.ljtcm.2008.04.37.wangzhw.014
9. Wang Z, Jiansheng L. Correlation between the Pulmonary Function and the TCM Syndrome Distribution in the Stationary Stage of the Chronic Obstructive Pulmonary Diseases. J Traditional Chine Med. 2011;52(16):1376–1378. doi:10.13288/j.11-2166/r.2011.16.010
10. Jiansheng L, Zhang H. Clinical Survey on Syndrome Evolution Characteristics in Chronic Obstructive Pulmonary Disease Patients. J Traditional Chine Med. 2017;58(9):772–776. doi:10.13288/j.11-2166/r.2017.09.014
11. Suyun L, Jiansheng L, Wang M-H, et al. Effects of comprehensive therapy based on traditional Chinese medicine patterns in stable chronic obstructive pulmonary disease: a four-center, open-label, randomized, controlled study. BMC Complement Altern Med. 2012;12(1):197. doi:10.1186/1472-6882-12-197
12. Jiansheng L, Su-yun L, Xie Y, et al. The effective evaluation on symptoms and quality of life of chronic obstructive pulmonary disease patients treated by comprehensive therapy based on traditional Chinese medicine patterns. Complement Ther Med. 2013;21(6):595–602. doi:10.1016/j.ctim.2013.09.006
13. Jiansheng L, Li Y, Li S-Y, et al. Long-term effects of Tiaobu Feishen therapies on systemic and local inflammation responses in rats with stable chronic obstructive pulmonary disease. Zhong Xi Yi Jie He Xue Bao. 2012;10(9):1039–1048. doi:10.3736/jcim20120913
14. Ya L, Jiansheng L, Li -W-W, et al. Long-term effects of three Tiao-Bu Fei-Shen therapies on NF-κB/TGF-β1/smad2 signaling in rats with chronic obstructive pulmonary disease. BMC Complement Altern Med. 2014;14:140. doi:10.1186/1472-6882-14-140
15. JianSheng L, Liu X, Dong H-R, et al. Effective-constituent compatibility-based analysis of Bufei Yishen formula, a traditional herbal compound as an effective treatment for chronic obstructive pulmonary disease. J Integr Med. 2020;18(4):351–362. doi:10.1016/j.joim.2020.04.004
16. Chronic Obstructive Pulmonary Disease Group of Chinese Thoracic Society. Guidelines for the Diagnosis of Chronic Obstructive Pulmonary Disease (revised version 2013). Chine J Front Med Sci. 2014;1(2):67–80.
17. Professional Committee of Pulmonary Diseases of China Association of Chinese Medicine. Diagnostic criteria of traditional Chinese medicine syndromes for diffuse interstitial lung disease (2011 Edition). Zhong Yi Za Zhi. 2012;53(1):80–84.
18. Chronic Obstructive Pulmonary Disease Group of Chinese Thoracic Society. Chronic Obstructive Pulmonary Disease Committee of Chinese Association of Chest Physician. [Guidelines for the diagnosis and management of chronic obstructive pulmonary disease (revised version 2021)], Zhonghua Jie He He Hu Xi Za Zhi. 2021;44(3):170–205. Chinese. doi:10.3760/cma.j.cn112147-20210109-00031
19. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi:10.1164/ajrccm.166.1.at1102
20. Celli B R, Cote C G, Marin J M, Casanova C, Montes de Oca M, Mendez R A, Pinto Plata V and Cabral H J. (2004). The Body-Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity Index in Chronic Obstructive Pulmonary Disease. N Engl J Med, 350(10), 1005–1012. 10.1056/NEJMoa021322
21. Cao Y, Wan B. Consensus of Expert on Safety Evaluation of Drug Clinical Trial in GuangDong(Version 2020). Pharmacy Today. 2020;30(11):731–740.
22. Hong M, Hong C, Chen H, et al. Effects of the Chinese herb formula Yufeining on stable chronic obstructive pulmonary disease: a randomized, double-blind, placebo-controlled trial. Medicine. 2018;97(39):e12461. doi:10.1097/MD.0000000000012461
23. Ma JF, Zheng JP, Zhong N, et al. Effects of YuPingFeng granules on acute exacerbations of COPD: a randomized, placebo-controlled study. Int J Chron Obstruct Pulmon Dis. 2018;13:3107–3114. doi:10.2147/COPD.S170555
24. Chan KH, Tsoi YYS, McCall M, et al. The Effectiveness of Traditional Chinese Medicine (TCM) as an Adjunct Treatment on Stable COPD Patients: a Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2021;2021:5550332. doi:10.1155/2021/5550332
25. Hung J, Chen W-C, Lin M-C, et al. Associations of Chinese Herbal Medicine Use with the Risks of Acute Exacerbation and Mortality in Patients with Chronic Obstructive Pulmonary Disease: a Nationwide Retrospective Cohort Study. J Integr Complement Med. 2022;28(1):77–86. doi:10.1089/jicm.2021.0103
26. Huang H, Zhou Y, Liu XH, et al. Effect of Feikang Granules on Pulmonary Function and Quality of Life in Patients with Stable Chronic Obstructive Pulmonary Disease. J Guangzhou Univ Traditional Chine Med. 2019;36(9):1305–1311. doi:10.13359/j.cnki.gzxbtcm.2019.09.001
27. Song SL. Effect of Corbrin Capsule combined with routine western medicine on the airway remodeling process in patients with stable COPD. J Hainan Med Univ. 2017;23(15):2033–2036.
28. Shi L, Yan J. Intervention Effect of Bufei Huoxue Capsule on Airway Remodeling in A Rat Model of Chronic Obstructive Pulmonary Disease and Its Mechanism. J Anhui Univ Chine Med. 2022;41(1):59–65.
29. Liu S, Lai J, Wu L, et al. Chinese Medicine for Chronic Obstructive Pulmonary Disease: a Pilot Study on Patient Preferences. Patient Prefer Adherence. 2021;15:1529–1535. doi:10.2147/PPA.S316872
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
