Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Efficacy of Secukinumab in Psoriasis: Post Hoc Gender-Wise Analysis of the SUPREME Study
Authors Stingeni L , Malara G, Conti A, Di Costanzo L, Carrera CG, Burlando M, Malagoli P, Musumeci ML, Bardazzi F, Brazzelli V, Amerio P , De Simone C , Trevisini S, Balato A, Megna M, Loconsole F, De Felice C, Bartezaghi M, Rausa A, Aloisi E, Orsenigo R, Costanzo A
Received 11 June 2022
Accepted for publication 2 November 2022
Published 5 January 2023 Volume 2023:16 Pages 27—38
DOI https://doi.org/10.2147/CCID.S378135
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Luca Stingeni,1 Giovanna Malara,2,3 Andrea Conti,4 Luisa Di Costanzo,5 Carlo Giovanni Carrera,6 Martina Burlando,7 Piergiorgio Malagoli,8 Maria Letizia Musumeci,9 Federico Bardazzi,10 Valeria Brazzelli,11 Paolo Amerio,12 Clara De Simone,13,14 Sara Trevisini,15 Anna Balato,16 Matteo Megna,17 Francesco Loconsole,18 Catia De Felice,19 Marta Bartezaghi,20 Alice Rausa,20 Elisabetta Aloisi,20 Roberto Orsenigo,20 Antonio Costanzo21,22 On behalf of the SUPREME Study Group
1Dermatology Section, Medical and Surgical Department, University of Perugia, Perugia, Italy; 2Dermatology Unit, Hospital “Bianchi Melacrino Morelli”, Reggio Calabria, Italy; 3Department of Dermatology, Papardo Hospital, Messina, Italy; 4Dermatologic Unit, Department of Surgery, Infermi Hospital, AUSL Romagna, Rimini, Italy; 5Department of Dermatology, “Gaetano Rummo” Hospital, Benevento UOC Dermatologia, AO G. Rummo, Benevento, Italy; 6U.O. Dermatologia, Fondazione IRCCS Ca’ Granda - Ospedale Maggiore Policlinico, Milano, Italy; 7IRCCS San Martino Polyclinic Hospital, Di.S.Sal. Section of Dermatology, Genoa, Italy; 8Dermatology Unit IRCCS Policlinico San Donato, Milan, Italy; 9Dermatology Clinic, University of Catania, Catania, Italy; 10Division of Dermatology, University Hospital Policlinico “S. Orsola-Malpighi”, Bologna, Italy; 11Institute of Dermatology, Foundation IRCCS Policlinico San Matteo and University of Pavia, Pavia, Italy; 12Dermatologic Clinic, G. D’Annunzio University, Chieti, Italy; 13Istituto di Dermatologia, Università Cattolica del Sacro Cuore, Rome, Italy; 14Fondazione Policlinico Universitario A. Gemelli – IRCCS, Rome, Italy; 15Dermatology Department, University of Trieste, Trieste, Italy; 16Dermatology Unit, University of Campania “Luigi Vanvitelli”, Naples, Italy; 17Section of Dermatology, Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy; 18Department of Biomedical Sciences and Human Oncology, University of Bari, Bari, Italy; 19Department of Clinical Dermatology, Centre for the Study and Treatment of Psoriasis, San Gallicano Dermatological Institute, IRCCS, Rome, Italy; 20Novartis Farma SpA, Origgio, Italy; 21Unit of Dermatology, IRCCS Humanitas Research Hospital, Milan, Italy; 22Department of Biomedical Sciences, Humanitas University, Milan, Italy
Correspondence: Luca Stingeni, Dermatology Section, Medical and Surgical Department, University of Perugia, Perugia, Italy, Tel +39075-5783881, Email [email protected]
Purpose: Psoriasis, a common systemic inflammatory disorder, presents with gender-related differences in the quality of life (QoL) and treatment outcomes. This post hoc analysis from the Phase 3b SUPREME study explored gender-related differences in patient characteristics and efficacy of secukinumab 300 mg on Psoriasis Area and Severity Index (PASI) 75/90/100 and impact on QoL using the Dermatology Life Quality Index (DLQI) in patients with moderate to severe psoriasis through week 24.
Patients and Methods: The proportion of patients achieving PASI 75/90/100 was computed using a nonresponder imputation approach. Differences between cohorts were analyzed using a logistic regression model. The mean change from baseline in DLQI was computed using the Wilcoxon test.
Results: Among the 433 patients (males: 71.6%), females had a higher DLQI than males at baseline (13.1 vs 9.5; P< 0.0001). Males had a slightly higher response for PASI 90 than females at week 16 (80.7% vs 78.1%; P=0.0779) and 24 (83.2% vs 79.7%; P=0.0319). No differences were observed between genders in PASI 100/75 responses at week 24. Both genders showed an improvement in DLQI with secukinumab at week 24 (− 10.9 vs − 8.1, respectively, in females vs males; P=0.0004).
Conclusion: In summary, secukinumab was effective in the treatment of psoriasis, irrespective of gender.
Keywords: plaque psoriasis, PASI, patient-reported outcomes, Dermatology Quality of Life Index, Hospital Anxiety and Depression Scale
Introduction
Psoriasis is a common systemic inflammatory disorder affecting the skin with a significant impact on patients’ quality of life (QoL).1,2 It occurs in both men and women; however, there are gender-specific differences associated with several aspects of this disease. Men have increased disease severity,3,4 whereas women experience more stress and effects on their QoL.2,5 Furthermore, men and women can respond differently to treatment. An observational registry-based study from the United Kingdom enrolling 3110 patients reported that women had lower odds of achieving a Psoriasis Area Severity Index (PASI) 90 response with biologic therapy at 6 and 12 months.6 Moreover, women were found to have significantly higher needs and expectations from treatment outcomes than men as shown by the German and Swiss registry-based data comprising nearly 5000 patients with psoriasis.7 A single-center cross-sectional study in a hospital in Brazil that enrolled 281 patients with psoriasis showed that the proportion of patients affected by depression and anxiety was 19% and 36%, respectively, and that females were affected more than males.8
Secukinumab is a fully human monoclonal antibody that selectively neutralizes interleukin (IL)-17A, a cornerstone cytokine involved in the pathogenesis of psoriatic disease.9 In pivotal randomized trials, secukinumab has demonstrated rapid and sustained efficacy and safety in the long-term treatment of moderate to severe plaque-type psoriasis.10–12 Results from the phase 3 FIXTURE, CLEAR and CLARITY studies have confirmed the superiority of secukinumab in showing a greater and more rapid improvement in patients’ QoL compared with etanercept and ustekinumab.11–14
The phase 3b SUPREME study (NCT02394561) assessed the effect of the psoriasis susceptibility allele human leukocyte antigen (HLA)-Cw6 on the efficacy and safety of secukinumab 300 mg in 434 patients with moderate-to-severe chronic plaque psoriasis (42.6% and 56.7% were HLA-Cw6 positive and negative, respectively).15 The results confirmed that secukinumab shows high and comparable efficacy in both cohorts of patients, with 80.4% and 84.2% of HLA-Cw6–positive and 81.7% and 83.3% of HLA-Cw6–negative patients achieving PASI 90 at week 16 and 24, respectively.16 Post hoc analyses of the SUPREME study to investigate gender-wise patient characteristics and the efficacy of secukinumab in patients with psoriasis in a 24-week period are presented.
Materials and Methods
The SUPREME core study was a 24-week, phase 3b, multicenter, prospective study conducted across 50 centers in Italy. The study design and eligibility criteria of the SUPREME study have been reported previously together with efficacy results.15 Briefly, the study included patients ≥18 years of age with moderate to severe chronic plaque-type psoriasis (defined by PASI score ≥10 or PASI score > 5 but < 10 and Dermatology Life Quality Index [DLQI] ≥10) for at least 6 months, who were candidates for systemic therapy and treatment naïve, or had failed response to other systemic therapies. Patients were treated with subcutaneous secukinumab 300 mg during the induction (0−4 weeks) and maintenance phases (5−24 weeks). Secukinumab was administered as two 150-mg injections per week during the induction phase starting from week 0, and thereafter as two 150-mg injections per month during the maintenance phase. At week 16, the severity of psoriasis was assessed and patients achieving a PASI 50 response were eligible to continue study treatment for up to 24 weeks. This post hoc analysis included 433 patients treated with secukinumab, 310 (71.7%) males and 123 (28.3%) females.
The objective of this post hoc analysis was to describe the baseline characteristics of patients enrolled in the SUPREME study by gender, and to assess the efficacy of secukinumab in both female and male cohorts using PASI 75/90/100 and Investigator’s Global Assessment (IGA) 0−1 response through week 24. The absolute mean change in PASI from baseline was assessed at week 16 and 24. The time to reach PASI 75/90 was also assessed. Spearman coefficient of correlation between DLQI score at baseline and change in PASI at week 16 was calculated for both genders. The QoL was assessed using DLQI and the Hospital Anxiety and Depression (HAD) scale (HAD-A and HAD-D). Mean changes in DLQI, HAD-A, and HAD-D scores from baseline to week 16 and 24 were calculated. Safety outcomes included assessment of treatment-emergent adverse events (TEAEs), serious TEAEs, TEAEs leading to study drug discontinuation, and death in both patient cohorts through 24 weeks.
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice, and was approved by all competent Ethics Committees and regulatory authorities. Informed consent was obtained by the investigators from all patients enrolled in the study.
Statistical Analysis
The safety population of the SUPREME study was assessed in this post hoc analysis, which was defined as all enrolled patients included in the full analysis set population receiving at least one dose of the study drug. Treatment outcomes were analyzed in both gender cohorts, females versus males, from the overall patient population of the SUPREME study. Patient demographics and baseline characteristics were described using descriptive statistics: continuous variables by mean and standard deviation and categorical variables by absolute and relative frequencies (n and percentage). Differences between cohorts in continuous variables were tested using a t-test or Wilcoxon test based on data distribution, and categorical variables were tested using chi-square analysis. The proportion of patients achieving PASI 75/90/100 and IGA 0/1 responses were computed using a nonresponder imputation approach, and differences between cohorts were analyzed using a logistic regression model considering height, weight, and waist as covariates. P-values for absolute change in PASI from baseline were computed using an analysis of covariance model and relevant baseline variables (ie, those significantly different between cohorts) as covariates. Time to reach PASI 75/90 was presented as a Kaplan-Meier curve and both cohorts were compared using a Log rank test. The mean change from baseline in DLQI was computed using the Wilcoxon test, whereas HAD-A and HAD-D scores were computed using t-tests. Absolute and relative frequencies of patients experiencing safety events were summarized. No multiplicity test adjustments were done, and all P-values should be considered nominal.
Results
Patient Population
The demographics and baseline characteristics were generally balanced and comparable between both cohorts (Table 1). The majority of both male and female patients were Caucasian, with females and males having an average age of 45.6 and 45.1 years and body mass index of 26.6 and 27.6 kg/m2, respectively. Male patients had a larger waist circumference than females (98.9 cm vs 90.4 cm; P<0.0001). For both genders, psoriasis was moderate to severe based on the IGA mod 2011 scores (females: 97.6%, males: 97.1%) with comparable mean PASI scores (females: 20.5 and males: 21.5; P=0.3653). Minor non significant differences in psoriasis localization between genders were present, particularly the scalp type, which was more common (81.3% and 77.7% in females and males, respectively) than the nail type (32.5% and 41.6%, respectively). Both cohorts had a long history of psoriasis with time since the first diagnosis at ~18 years. Metabolic syndrome was present in 19.5% and 15.5% of female and male patients, respectively. The proportion of patients with any comorbidity was 60.2% and 62.9% in females and males, respectively, with no significant differences between genders. Female patients showed more impaired QoL than males (DLQI scores: 13.1 in females vs 9.5 in males; P<0.0001). HAD-A (females: 9.6 vs males: 6.3; P<0.0001) and HAD-D scores (females: 6.7 vs males: 5.2; P=0.0002) of female patients were also significantly higher compared with those of males. A higher proportion of male patients were naïve to psoriasis therapy than females (7.7% vs 1.6%; P=0.0132). The percentage of females and males exposed to biologic and nonbiologic therapies were comparable; however, more females received phototherapy (23.6%) than males (14.5%) prior to secukinumab treatment (P=0.0239; Table 1).
 |
Table 1 Demographics and Baseline Characteristics of Patients of Both Genders |
Efficacy Outcomes
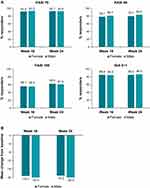
At week 16, PASI 75 response in females and males was comparable (91.9% vs 93.2%, respectively, odds ratio (OR) [95% CI] for males vs females: 2.13 [0.74–6.11]); male patients showed a slightly higher response for PASI 90 (78.1% vs 80.7%; OR [95% CI]: 1.90 [0.93–3.87]) and females for PASI 100 (56.1% vs 55.5%; OR [95% CI]: 1.90 [1.05–3.47]). At week 24, PASI 75 (92.7% vs 92.6%; OR [95% CI]: 1.24 [0.44–3.50]) and PASI 100 (62.6% vs 61.0%; OR [95% CI]: 1.42 [0.77–2.61]) were similar, whereas PASI 90 (79.7% vs 83.2%; OR [95% CI]: 2.28 [1.07–4.84]) was statistically higher in male gender (P=0.0319). The proportion of patients achieving IGA 0−1 scores at week 16 (85.4% vs 84.8% for females and males, respectively; OR [95% CI]: 1.91 [0.87–4.21]) and 24 (85.4% vs 86.8%; OR [95% CI]: 1.61 [0.71–3.64]) was also comparable between both cohorts (Figure 1A).
The mean PASI score decreased from baseline to week 16, corresponding to a mean absolute reduction in scores by 93.0% (−19.3) in females and 94.9% (−20.3) in males, which was sustained up to week 24 as 93.8% (−19.2) in females and 95.5% (−20.5) in males (Figure 1B). A sharp reduction in PASI scores was observed as early as in week 1, which continued until week 4, further decreased by week 8, and was sustained up to week 24. These findings were indistinguishable between both genders (week 1: 16.1 and 16.8; week 4: 4.8 and 5.4; week 8: 2.0 and 2.5; week 16: 1.3 and 1.0; week 24: 1.1 and 0.9 in females and males, respectively; Figure 2). No significant differences in median time to reach PASI 90 were observed between female (57 days) and male patients (58 days; Log rank test, P=0.1721).
 |
Figure 2 Change in absolute PASI score over time. Abbreviation: PASI, Psoriasis Area and Severity Index. |
Patient-Reported Outcomes
Quality of Life
Improvement in dermatological QoL was observed in both cohorts at weeks 16 and 24. Female patients showed a significantly greater improvement in DLQI than males at week 16 (reduction by −10.7 [−83.1%] vs −7.7 [−68.1%] in females vs males, respectively; P=0.0001) and at week 24 (−10.9 [−81.7%] vs −8.1 [−75.4%] in females vs males, respectively; P=0.0004; Figure 3A). The proportion of patients achieving DLQI 0/1 drastically increased from baseline (females: 0.8% vs males: 10.7%; P=0.0002) to week 16 (females: 67.6% vs males: 70.7%; P=0.5292), which was sustained and slightly improved in males at week 24 (females: 67.8% vs 77.2%; P=0.0501) with no statistical difference between cohorts (Figure 3B).
The Spearman correlation coefficient between DLQI at baseline and change in PASI score at week 16 was −0.0682 [P=0.4712] in females and −0.0761 [P= 0.1926] in males, showing no correlation between baseline DLQI and changes in PASI scores in either cohorts (Figure 4).
 |
Figure 4 Correlation between DLQI at baseline and absolute change in PASI score at week 16. Abbreviations: DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area and Severity Index. |
Anxiety and Depression
Anxiety and depression decreased in both female and male patients at weeks 16 and 24. A significant difference was observed between genders for mean change from baseline in HAD-A scores at week 16 (−2.3 vs −1.5 for females and males, respectively; P=0.0345). However, the scores were comparable at week 24 (−2.4 vs −1.9 for females and males, respectively; P=0.2823; Figure 3C). No significant differences were observed between female and male patients in the mean change from baseline in HAD-D scores at weeks 16 (−1.8 vs −1.2; P=0.0913) and 24 (−1.5 vs −1.4; P=0.8075; Figure 3D).
Safety
Males and females had a similar duration of exposure to secukinumab (~168 days). The incidence of TEAEs (50.4% and 45.2% in females and males, respectively) and TEAEs related to the study drug (16.3% and 14.2% in females and males, respectively) were comparable between both genders (Table 2). The incidence of TEAEs leading to study discontinuation was low and comparable between both genders. Infections and infestations were the most frequent adverse events (AEs) in both females (22.0%) and males (14.5%). Hypertension (5.5% vs 2.4%) and increased levels of creatine phosphokinase (3.9% vs 0.0%), lipase (2.3% vs 0.8%), and amylase (2.3% vs 1.6%) were more commonly observed in males than females, respectively. The incidence of serious TEAEs was higher in males (5.8%) than in females (2.4%). One male patient died during the 24-week treatment period due to cardiac arrest, which was deemed by the investigator to be related to the study drug;15,16 however, the patient had a history of concomitant active congestive cardiomyopathy.
 |
Table 2 Summary of AEs by Gender |
Discussion
Psoriasis is common in both men and women; however, they may differ in terms of severity, impact on QoL, and response to treatment. This post hoc analysis showed that women experience a greater QoL burden than men, as reflected by higher DLQI, HAD-A, and HAD-D scores. Both genders exhibited moderate to severe psoriasis without any difference in PASI score at baseline.
Secukinumab was efficacious in both patient cohorts in the treatment of moderate to severe chronic plaque psoriasis. The PASI 90 response was significantly higher in men than in women at week 16 and 24; however, it was not clinically relevant, as PASI 75/100 and IGA mod 0–1 responses were similar in both genders at week 24. Likewise, both genders showed a similar absolute mean reduction from baseline in PASI score by at least 92% at week 16 and 24. The results are consistent with those of previously published studies in moderate to severe plaque-type psoriasis where ~70% of patients showed a PASI 90 response with secukinumab treatment as early as week 16, which was sustained over 52 weeks.11,13 A retrospective real-world study in Italian patients with psoriasis also showed an 84% reduction from baseline in PASI score at week 24 with secukinumab treatment.17 A noteworthy finding of this study was that secukinumab was efficacious in both men and women irrespective of the impact of psoriasis on the QoL at baseline.
The QoL of patients of both genders improved with secukinumab over 24 weeks and was in line with those in the CLEAR and PROSE studies, where at least 72% of patients achieved DLQI 0/1 over 52 weeks.13,18 It is interesting to note that at baseline, in terms of QoL, females were more affected than males. However, both genders experienced a significant improvement with secukinumab treatment over 24 weeks.
Secukinumab showed a favorable safety profile, irrespective of gender, and was consistent with findings from previous studies. The incidence of TEAEs leading to study-drug discontinuation was low and comparable in both cohorts. Similar to that reported in previous studies,11 infection was the most commonly observed AE with secukinumab. No new safety signals were observed during the study.
Literature on the effect of psoriasis therapies by gender type is limited. A pooled analysis of two randomized phase 3 studies of risankizumab, an IL-23 inhibitor, showed a higher PASI 90 response compared with ustekinumab regardless of gender at week 16 (risankizumab: 75.4% and 74.9%; ustekinumab: 55.6% and 39.7% in females and males, respectively) and 52 (risankizumab: 81.4% and 81.2%; ustekinumab: 49.2% and 46.3% in females and males, respectively).19 The Netherlands-based prospective, multicenter BioCAPTURE registry data included patients with psoriasis treated with biologics in daily practice care for 1 year. The analysis showed gender differences, wherein women were less satisfied with psoriasis treatment than men, as reflected by the significantly lower values in the Treatment Satisfaction Questionnaire for Medication scores in women in the side-effects and global satisfaction domains.20 A retrospective study of patients with psoriasis enrolled at the Rome Dermatology Centre and treated with ustekinumab up to 8 years reported greater improvements in females than males in PASI 75 (81.6% vs 71.1%, respectively), PASI 90 (67.3% vs 55.4%, respectively), and PASI 100 (55.1% vs 38.6%, respectively) over 5 years.21 A subgroup analysis of two pooled, randomized controlled trials in patients with moderate to severe chronic plaque psoriasis treated with the Janus kinase inhibitor, tofacitinib, showed that efficacy was not influenced by gender. The PASI 75 response rate with 5-mg and 10-mg tofacitinib doses versus placebo was found to be comparable between females and males over week 16 (females: 49.4% and 61.2% vs 10.9%; males: 40.5% and 58.6% vs 7.8%).22 A single-center, retrospective study of patients with moderate to severe plaque psoriasis showed that males responded better than females to tumor necrosis factor inhibitors, as evaluated by mean percentage PASI improvement (77.1% vs 70.2%; P=0.04).23 Compared with existing data, a more comprehensive analysis was conducted that investigated multiple aspects in the treatment of psoriasis with secukinumab in both genders, including the efficacy in achieving PASI 75/90/100, time to achieve PASI 90, and correlation of secukinumab efficacy with the QoL of patients at baseline. Data from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) showed that treatment with biologics, such as ustekinumab, infliximab, etanercept, and adalimumab, is associated with reduced incidence of depressive symptoms as assessed based on HAD-D scores ≥8 (3.0%) compared with those obtained with conventional treatment (5.7%) or phototherapy (5.9%).24 A single-center prospective study in patients with moderate to severe plaque psoriasis showed that etanercept significantly reduced anxiety and depression, as assessed using HAD-A and HAD-D scores (P<0.001) at 1, 3, and 6 months of treatment.25 In this study, secukinumab showed a reduction in anxiety and depression in both genders at week 16 and 24. However, females showed slightly better and early responses in terms of anxiety and depression than males. A possible reason for this finding may be higher HAD-A and HAD-D baseline scores in females than in males.
There are a few limitations of this study that are worth highlighting. This is a post hoc analysis and future investigations may be warranted. There was an imbalance in the proportion of female and male patients in the SUPREME study, which may limit the interpretation of our findings. The current study lacked the inclusion of placebo and an active comparator and was of a short duration of 24 weeks. Nevertheless, the findings from this study are consistent with those from previous studies investigating secukinumab treatment over 52 weeks. Finally, our study included an Italian population, which was predominantly Caucasian; thus, this may limit the generalizability of our results to other ethnic groups or populations from other geographical regions.
Conclusion
Results of the SUPREME study post hoc analysis confirmed that secukinumab, irrespective of gender, is effective in patients with moderate to severe plaque-type psoriasis and has a favorable safety profile during 24 weeks of treatment. No clinically relevant differences were observed between females and males in PASI 75/90/100 responses. Both genders showed an improvement in the QoL and a reduction in anxiety and depression with secukinumab over 24 weeks of treatment. Further extension of this study will corroborate our findings and help dermatologists in the therapeutic decision-making process in treating psoriasis.
Abbreviations
AE, Adverse event; CI, Confidence interval; DLQI, Dermatology Life Quality Index; HAD, Hospital Anxiety and Depression; HLA, human leukocyte antigen; IGA, Investigator’s Global Assessment; IL, Interleukin; OR, Odds ratio; PASI, Psoriasis Area and Severity Index; QoL, Quality of life; TEAE, Treatment emergent adverse event.
Data Sharing Statement
The datasets generated or analyzed during this study are not publicly available. Novartis is committed to sharing with qualified external researchers the access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved on the basis of scientific merit. All data that may be provided will be anonymized to respect the privacy of patients who have participated in the trial, in line with applicable laws and regulations. The data may be requested from the corresponding author of the manuscript.
Ethics Approval and Informed Consent
The study was approved by all competent Ethics Committees and regulatory authorities (Supplementary Table 1). Informed consent was obtained by the investigators from all patients enrolled in the study.
Acknowledgments
The authors thank Sarabjeet Kaur, Khushboo Patel, and Mohammad Fahad Haroon of Novartis for providing medical writing support/editorial support, which was funded by Novartis Farma SpA, Origgio, Italy, in accordance with Good Publication Practice guidelines (http://www.ismpp.org/gpp3).
SUPREME Study Group: Bianchi L, Bonifati C, Buligan C, Caldarola G, Campanati A, Cantoresi F, Cattaneo A, Chiricozzi A, Cusano F, Dapavo P, Dastoli S, Del Giglio M, Di Lernia V, Di Nuzzo S, Dusi D, Fabbrocini G, Fargnoli MC, Fidanza R, Flori ML, Franchi C, Gaiani FM, Galluzzo M, Ghilardi A, Girolomoni G, Gisondi P, Giuliani F, Graceffa D, Hansel K, Mastrandrea V, Mercuri SR, Micali G, Naldi L, Narcisi A, Natalini Y, Offidani AM, Orsini D, Pagnanelli G, Papini M, Parodi A, Patrizi A, Pau M, Pellacani G, Peris K, Persechino S, Pescitelli L, Piaserico S, Pietroleonardo L, Potenza C, Prignano F, Reseghetti A, Romanelli M, Rongioletti F, Russo F, Scuderi R, Sirna R, Skroza N, Stinco G, Talamonti M, Venturini M, Zane C, Zichichi L, and Zini A.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was funded by Novartis Farma SpA, Origgio, Italy.
Disclosure
L. Stingeni has acted as a speaker and board member for AbbVie, Eli Lilly, Novartis, Almirall, Celgene, Sanofi, and Janssen; G. Malara has acted as a speaker and consultant for AbbVie, Janssen, Amgen, Sanofi, Novartis, Eli Lilly, and UCB Pharma; A. Conti has acted as a consultant for AbbVie, Abbott, Amgen, Celgene, Eli Lilly, Janssen Cilag, Leo Pharma, MSD, Novartis, Sandoz, Schering Plough, UCB Pharma, and Wyeth; C.G. Carrera has reported collaboration with Novartis, AbbVie, Janssen, Lilly, and Leo Pharma; M. Burlando has served as a speaker and consultant for AbbVie, Janssen, Amgen, Novartis, Eli Lilly, and UCB Pharma; P. Malagoli has acted as a consultant or had advisory board agreements for Janssen, Amgen, AbbVie, UCB, Novartis, Lilly, Almirall, and Leo Pharma; M.L. Musumeci has served as a consultant/investigator for AbbVie, Eli Lilly, Janssen Cilag, Novartis, and Biogen; F. Bardazzi has acted as a speaker and/or consultant for Almirall, AbbVie, Celgene, Janssen, Eli Lilly, UCB Pharma, and Leo Pharma; V. Brazzelli has acted as a consultant and congress guest for Janssen Cilag SpA, Novartis, and Sanofi Genzyme; P. Amerio has received honoraria and is on the advisory board of Sanofi, Jansen, Novartis, Pfizer, Celgene, and Eli Lilly; C. De Simone has acted as a speaker and consultant for Almirall, AbbVie, Janssen, Celgene, Leo Pharma, Novartis, Eli Lilly, and UCB Pharma; S. Trevisini has served as a speaker for AbbVie, Novartis, and Janssen; A. Balato has served as speaker and/or consultant for AbbVie, Lilly, and Sanofi; M. Megna has served as a speaker or consultant for AbbVie, Eli Lilly, Novartis, Janssen, and Leo Pharma; M. Bartezaghi employees of Novartis; A. Rausa was an employee of Novartis during study conduct and manuscript submission. She approved the final version to be submitted while she was working for Novartis; E. Aloisi and R. Orsenigo are employees of Novartis; A. Costanzo has served as a speaker and consultant for AbbVie, Eli Lilly, Novartis, Almirall, Celgene, Sanofi, Janssen, and Pfizer. The authors report no other conflicts of interest in this work.
References
1. Boehncke WH, Schon MP. Psoriasis. Lancet. 2015;386(9997):983–994. doi:10.1016/S0140-6736(14)61909-7
2. Obradors M, Blanch C, Comellas M, Figueras M, Lizan L. Health-related quality of life in patients with psoriasis: a systematic review of the European literature. Qual Life Res. 2016;25(11):2739–2754. doi:10.1007/s11136-016-1321-7
3. Hägg D, Sundström A, Eriksson M, Schmitt-Egenolf M. Severity of psoriasis differs between men and women: a study of the clinical outcome measure psoriasis area and severity index (PASI) in 5438 Swedish register patients. Am J Clin Dermatol. 2017;18(4):583–590. doi:10.1007/s40257-017-0274-0
4. Salah LA, Gillstedt M, Osmancevic A. A retrospective study of patients with psoriasis treated with biologics: relation to body mass index and gender. Acta Derm Venereol. 2016;96(7):974–975. doi:10.2340/00015555-2438
5. Napolitano M, Mastroeni S, Fania L, et al. Sex- and gender-associated clinical and psychosocial characteristics of patients with psoriasis. Clin Exp Dermatol. 2020;45(6):705–711. doi:10.1111/ced.14218
6. Warren RB, Marsden A, Tomenson B, et al. Identifying demographic, social and clinical predictors of biologic therapy effectiveness in psoriasis: a multicentre longitudinal cohort study. Br J Dermatol. 2019;180(5):1069–1076. doi:10.1111/bjd.16776
7. Maul JT, Navarini AA, Sommer R, et al. Gender and age significantly determine patient needs and treatment goals in psoriasis - a lesson for practice. J Eur Acad Dermatol Venereol. 2019;33(4):700–708. doi:10.1111/jdv.15324
8. Pollo CF, Miot HA, Matos TDS, et al. Prevalence and factors associated with depression and anxiety in patients with psoriasis. J Clin Nurs. 2021;30(3–4):572–580. doi:10.1111/jocn.15577
9. Krueger JG, Wharton KA Jr, Schlitt T, et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol. 2019;144(3):750–763. doi:10.1016/j.jaci.2019.04.029
10. Bissonnette R, Luger T, Thaçi D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE extension study). J Eur Acad Dermatol Venereol. 2018;32(9):1507–1514. doi:10.1111/jdv.14878
11. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. doi:10.1056/NEJMoa1314258
12. Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–409. doi:10.1016/j.jaad.2015.05.013
13. Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76(1):60–69.e9. doi:10.1016/j.jaad.2016.08.008
14. Bagel J, Blauvelt A, Nia J, et al. Secukinumab maintains superiority over ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: 52-week results from a double-blind phase 3b trial (CLARITY). J Eur Acad Dermatol Venereol. 2021;35(1):135–142. doi:10.1111/jdv.16558
15. Costanzo A, Bianchi L, Flori ML, et al. Secukinumab shows high efficacy irrespective of HLA-Cw6 status in patients with moderate-to-severe plaque-type psoriasis: SUPREME study. Br J Dermatol. 2018;179(5):1072–1080. doi:10.1111/bjd.16705
16. Papini M, Cusano F, Romanelli M, et al. Secukinumab shows high efficacy irrespective of HLA-Cw6 status in patients with moderate-to-severe plaque-type psoriasis: results from extension phase of the SUPREME study. Br J Dermatol. 2019;181(2):413–414. doi:10.1111/bjd.18013
17. Megna M, Di Costanzo L, Argenziano G, et al. Effectiveness and safety of secukinumab in Italian patients with psoriasis: an 84 week, multicenter, retrospective real-world study. Expert Opin Biol Ther. 2019;19(8):855–861. doi:10.1080/14712598.2019.1622678
18. Augustin M, Dauden E, Mrowietz U, et al. Secukinumab treatment leads to normalization of quality of life and disease symptoms in psoriasis patients with or without prior systemic psoriasis therapy: the PROSE study results. J Eur Acad Dermatol Venereol. 2021;35(2):431–440. doi:10.1111/jdv.16632
19. Strober B, Menter A, Leonardi C, et al. Efficacy of risankizumab in patients with moderate-to-severe plaque psoriasis by baseline demographics, disease characteristics and prior biologic therapy: an integrated analysis of the Phase III UltIMMa-1 and UltIMMa-2 studies. J Eur Acad Dermatol Venereol. 2020;34(12):2830–2838. doi:10.1111/jdv.16521
20. van der Schoot LS, van den Reek JMPA, Groenewoud JMM, et al. Female patients are less satisfied with biological treatment for psoriasis and experience more side-effects than male patients: results from the prospective BioCAPTURE registry. J Eur Acad Dermatol Venereol. 2019;33(10):1913–1920. doi:10.1111/jdv.15733
21. Galluzzo M, D’Adamio S, Silvaggio D, et al. Ustekinumab treatment for moderate-to-severe plaque psoriasis: eight-year real-life experience. Expert Opin Biol Ther. 2020;20(1):95–104. doi:10.1080/14712598.2020.1684472
22. Menter MA, Papp KA, Cather J, et al. Efficacy of tofacitinib for the treatment of moderate-to-severe chronic plaque psoriasis in patient subgroups from two randomised phase 3 trials. J Drugs Dermatol. 2016;15(5):568–580.
23. De Simone C, Caldarola G, Maiorino A, et al. Clinical predictors of nonresponse to anti-TNF-α agents in psoriatic patients: a retrospective study. Dermatol Ther. 2016;29(5):372–376. doi:10.1111/dth.12364
24. Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78(1):70–80. doi:10.1016/j.jaad.2017.08.051
25. Yang A, Xin X, Yang W, et al. Etanercept reduces anxiety and depression in psoriasis patients, and sustained depression correlates with reduced therapeutic response to etanercept. Ann Dermatol Venereol. 2019;146(5):363–371. doi:10.1016/j.annder.2019.03.002
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.