Back to Journals » Journal of Pain Research » Volume 16
Efficacy of Repeat Percutaneous Endoscopic Lumbar Decompression for Reoperation of Lumbar Spinal Stenosis: A Retrospective Study
Authors Wang L, Wang T, Fan N, Yuan S , Du P, Si F, Wang A, Zang L
Received 3 August 2022
Accepted for publication 17 January 2023
Published 24 January 2023 Volume 2023:16 Pages 177—186
DOI https://doi.org/10.2147/JPR.S384916
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Krishnan Chakravarthy
Lei Wang, Tianyi Wang, Ning Fan, Shuo Yuan, Peng Du, Fangda Si, Aobo Wang, Lei Zang
Department of Orthopedics, Beijing Chaoyang Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Lei Zang, Department of Orthopedics, Beijing Chaoyang Hospital, Capital Medical University, 5 JingYuan Road, Shijingshan District, Beijing, 100043, People’s Republic of China, Tel +86 13601252787, Email [email protected]
Purpose: To evaluate the efficacy of repeat percutaneous endoscopic lumbar decompression (PELD) in lumbar spinal stenosis (LSS) reoperation.
Patients and Methods: This study included patients with LSS who relapsed following treatment with PELD therapy between March 2017 and March 2020. Visual analog scale (VAS) scores and Oswestry Disability Index (ODI) were analyzed preoperatively, postoperatively at 3, 6, 12, and 24 months, and at final follow-up. The modified MacNab criteria were used to assess clinical effects. All complications were recorded.
Results: At a mean follow-up of 3 years, 24 patients with LSS who underwent repeat PELD were identified. The patients’ mean operative time was 122.3± 29.2 min, blood loss was 12.5± 5.3 mL, and mean hospital stay was 7.0± 1.9 days. VAS leg-pain score improved from 6.1± 1.0 to 2.0± 1.2 (P< 0.001), VAS back-pain score improved from 6.2± 0.8 to 2.1± 1.1 (P< 0.001), and ODI improved from 68.9± 6.0 to 20.9± 5.6 (P < 0.001). According to the modified MacNab criteria, the good-to-excellent rate was 83.3%. Postoperative complications, including hematoma, nerve root injury, and dural injury, developed in four patients.
Conclusion: Repeat PELD for reoperation in patients with LSS has a good clinical effect, and is recommended in routine clinical practice. Careful intraoperative manipulation is recommended to prevent complications.
Keywords: lumbar spinal stenosis, percutaneous endoscopic lumbar decompression, reoperation, complication
Introduction
Lumbar spinal stenosis (LSS) is caused by intervertebral disc herniation, facet joint bone hyperplasia, ligamentum flavum hypertrophy, degenerative slippage, and other factors.1,2 Common symptoms of LSS include low back pain, neurogenic claudication, lower extremity pain, and decreased ability to walk, which not only affects daily function, but also significantly impacts overall quality of life.3,4 Previous studies have reported that conservative treatment is ineffective for symptomatic LSS, and that surgical decompression is the preferred treatment option.5,6
Traditional open decompression is the most commonly used nerve decompression technique in clinical practice.7 Despite satisfactory surgical results, deficiencies in open decompression can lead to suboptimal patient outcomes.8,9 With the development of imaging and in-depth understanding of the pathophysiology of LSS occurrence and development, limited surgical principles, namely limited anatomical resection and effective decompression of the spinal canal, have been widely accepted. Minimally invasive surgeries, such as percutaneous endoscopic lumbar decompression (PELD), have several advantages, including less trauma, less bleeding, faster recovery, shorter hospital stay, and strong efficacy.10–12 Since Kambin and Sampson invented the percutaneous posterolateral technique in 1986, PELD has grown in popularity for the treatment of LSS.13 Satisfaction rates of 82–92% have been reported for PELD in the treatment of lateral LSS.11
Whether or not open decompression or PELD is performed for the treatment of LSS, some patients still have a poor postoperative prognosis and require reoperation.14 The recurrence rate of LSS after PELD has been reported to be 3.19%.15 The main reasons for revision include preoperative combined endplate inflammation, incomplete intraoperative nucleus extraction, poor positioning, and excessive postoperative movement. Our prior study found that the revision rate after PELD for LSS was 6.7%, for which the same-segment revision rate was 4.4%, and that age was a risk factor for revision.16 However, few studies have reported the efficacy of reoperation in patients with recurrent LSS. Therefore, this study followed up patients with PELD recurrence due to LSS in our and other hospitals, and evaluated the efficacy of PELD revision surgery.
Materials and Methods
Patients
This retrospective study analyzed patients who underwent PELD reoperation after LSS recurrence following PELD treatment and were admitted to our and other hospitals between March 2017 and March 2020. The inclusion criteria were as follows: (1) the patient had been treated with PELD for LSS and had been in symptomatic remission for at least 3 months after surgery; (2) patients developed symptoms of low back pain, lower extremity pain, and neurogenic intermittent claudication≥3 months after the initial surgery; (3) unilateral or bilateral symptoms predominantly on one side; (4) the imaging results were consistent with the clinical symptoms and signs; (5) the stenosis-responsible segment of the LSS was the same as the initial surgical segment; and (6) failure of conservative treatment for more than 3 months.
The exclusion criteria were as follows: (1) spinal deformity, spondylolisthesis, or lumbar instability; (2) lumbar infection or tumor; (3) cervical or thoracic spinal disease; (4) severe central canal stenosis; and (5) incomplete or lost data before final follow-up.
The present study was approved by the institutional review board of our institute.
Surgical Procedures
PELD procedures were performed under local anesthesia with the patients placed in the prone position. Patients were able to communicate with the surgeon during the entire operation, which prevented intraoperative nerve root injuries. All procedures were performed by percutaneous transforaminal endoscopic discectomy and foraminoplasty.
The surgical procedure consisted of the following three key steps: (1) Puncture: the entry point was set at 12–14 cm lateral to the spinal midline at the index intervertebral level. A puncture needle was inserted into the superior articular process (SAP) of the targeted segment. (2) Foraminoplasty: Serial cannulated dilators were inserted into the SAP using a puncture needle. The ligamentum flavum and ventral elements of the SAP were removed. A tubular retractor with an outer diameter of 7.9 mm was then passed over the dilators and secured to the upper lamina; burrs were used to enlarge the foramen further, if necessary. (3) Decompression: The herniated disc, parts of the posterior longitudinal ligament, posterior upper margin of the inferior vertebral body, posterior lower margin of the superior vertebral body (if necessary), and the dorsal ligamentum flavum were removed using a rongeur, endoscopic forceps, endoscopic bone knife, or high-speed drill. Finally, the entire nerve root and dural sac were examined to ensure complete decompression. Adequate irrigation and hemostasis were performed to reduce postoperative infection and hematoma formation, respectively.
Clinical Assessment
Clinical efficacy was determined using the visual analog scale (VAS) and Oswestry Disability Index (ODI) scores preoperatively, at 3, 6, 12, and 24 months postoperatively; and at the final follow-up. Satisfaction with clinical outcomes at the most recent follow-up was graded using the modified MacNab criteria and classified into four categories: excellent, good, fair, and poor. All complications were recorded.
Statistical Analysis
The Statistical Package for Social Sciences software (version 21.0) was used to analyze all data. Data are presented as the mean ± standard deviation. The preoperative and postoperative (3, 6, 12, and 24 months) VAS scores for back and leg pain and ODI were analyzed using univariate analysis of variance. P <0.05 was considered statistically significant.
Results
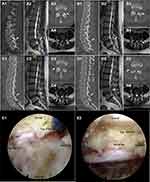
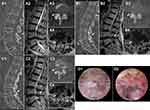
This retrospective analysis included 24 patients (12 men and 12 women) with an average follow-up period of 35.5 months. The mean age of the patients was 63.5±14.1 years, and the mean duration of symptoms was 10.9±5.9 months. Among the 24 patients, nine had central stenosis, eight had lateral recess stenosis, and seven had a combination of the two. One patient had L3–4 stenosis, 19 patients had L4–5 stenosis, and 4 patients had L5–S1 stenosis. Eleven patients underwent contralateral revision (Figure 1) and 13 patients underwent ipsilateral revision (Figure 2). In addition, the mean operative time was 122.3±29.2 min, blood loss was 12.5±5.3 mL, and the mean hospital stay was 7.0±1.9 days (Table 1).
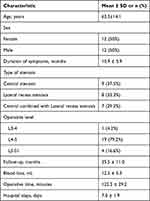 |
Table 1 Demographic Features of Patients |
Clinical Results
According to the modified MacNab criteria, the scores were excellent in eight patients, good in 12, and fair in three, with a good-to-excellent rate of 83.3% (Table 2). Only one patient was rated as poor.
 |
Table 2 Postoperative Modified MacNab Criteria and Outcomes |
The VAS leg pain score decreased from an average of 6.1±1.0 preoperatively to 2.3±1.3 at 6 months and 2.0±1.0 at 24 months, postoperatively. The mean VAS score for leg pain at the final follow-up was 2.0±1.2. All postoperative scores improved significantly compared to the preoperative scores (P<0.001, Table 3). The VAS back pain score also decreased from 6.2±0.8 to 2.3±1.0, decreasing to 2.1±1.1 at the final follow-up. All postoperative VAS back pain scores were significantly different from the preoperative VAS back pain scores (P<0.001; Table 3). Furthermore, the average ODI was reported to be 68.9±6.0 preoperatively and 22.0±5.5 at 2 years postoperatively. At the final follow-up, the ODI (20.9±5.6) was significantly better than the preoperative value (P<0.001).
 |
Table 3 Clinical Outcomes of Percutaneous Endoscopic Lumbar Decompression (PELD) Reoperation |
In addition, we compared the efficacy of ipsilateral and contralateral approaches. There were no significant differences in the VAS-L and VAS-B scores between the ipsilateral and contralateral groups preoperatively and postoperatively (P>0.05, Table 4). Preoperative ODI scores were higher in the contralateral group than in the ipsilateral group (P<0.05), whereas postoperative ODI scores were not significantly different between the ipsilateral and contralateral groups (P>0.05). Moreover, the Macnab results showed no significant difference between the ipsilateral and contralateral groups (Table 4).
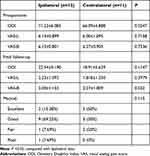 |
Table 4 Comparation Between Ipsilateral and Contralateral |
Complications
Postoperative complications occurred in four patients (Table 5); 1 patient developed numbness and weakness of the lower limbs 6 h after the operation, and the strength of the dorsal extension muscle decreased to grade II. Emergency exploration confirmed hematoma formation in the patient. By removing the hematoma and administering hormones, dehydration, and nutritional nerve symptomatic treatment after surgery, muscle strength recovered to grade IV on the 5th day after surgery. After 3 months of follow-up, the patient’s muscle strength had recovered to grade V, and numbness of the lower limbs had disappeared. One patient experienced nerve root injury during surgery, with pain and numbness of the lateral right calf becoming significantly aggravated. Intravenous glucocorticoid therapy was administered during the operation, and symptomatic treatment with hormones, dehydration, and nutritional nerves was subsequently administered. Pain and numbness were relieved on the 3rd postoperative day. Two patients experienced dural injury of the nerve root sleeve due to scar adhesions. Postoperative abdominal compression was performed in bed for 1 week, and no cerebrospinal fluid leakage was noted.
 |
Table 5 Complications of Percutaneous Endoscopic Lumbar Decompression (PELD) Reoperation |
Discussion
PELD has gradually begun to be widely used in the treatment of LSS; however, there is still a possibility of reoperation,17,18 for which few studies have reported on prognosis. To the best of our knowledge, this is the first study to report the efficacy and safety of repeat PELD for reoperation in LSS. In the present study, we found a good-to-excellent rate of 83.3%. The VAS leg pain score improved from 6.1±1.0 preoperatively to 2.0±1.2 postoperatively, VAS back pain score improved from 6.2±0.8 preoperatively to 2.1±1.1 postoperatively, and ODI improved from 68.9±6.0 preoperatively to 20.9±5.6 postoperatively.
Open decompression is the gold standard for managing LSS,7 with a success rate of 62–70%. However, this technique has some disadvantages, such as high surgical risk, use of general anesthesia, greater trauma, high blood loss, long hospital stay, destruction of the rear stable structure, and the risk of severe complications, which seriously affect the clinical treatment effect.8,19,20 One study found that patients with spinal instability during open decompression surgery had a high revision rate of 21%.21 Minimally invasive surgeries, such as PELD, have the advantages of less trauma, less bleeding, faster recovery, shorter hospital stay, and significant efficacy.10–12 Moreover, PELD can preserve the disc tissue to the greatest extent and reduce complications in many patients.12 Although PELD has reliable efficacy and high safety in the treatment of LSS, there are still few patients with postoperative recurrence.22,23 It has been reported that the recurrence rate after PELD treatment for LSS was 3.19%.15 Patients with LSS must undergo reoperation after recurrence. Currently, there are few reports on the efficacy of retreatment for LSS recurrence. In this study, we found that PELD had a good efficacy in LSS revision surgery.
Recurrence after LSS is a common complication, and the choice of revision method is key to improving the success rate of revision surgery. Previous studies have focused on revision rates and risk factors leading to revision;16,24–26 however, studies on the efficacy of revision PELD surgery have rarely been reported. Xu et al evaluated the efficacy of reoperation after percutaneous transforaminal endoscopic discectomy for LSS.27 They found that the VAS leg pain score decreased from 7.1±3.9 before revision surgeries to 1.9±1.2 postoperatively, VAS low back pain score decreased from 6.3±3.2 to 1.8±1.3 postoperatively, and ODI decreased from 35±14 to 7.6±5 postoperatively. However, there were many types of revision surgeries in this study, and the efficacy of each surgery has not been separately reported, which limits the clinical value. One study reported the efficacy of previous lumbar laminectomy or decompression fusion surgery using endoscopic transforaminal and lateral recess decompression.28 Significant improvements were observed in VAS scores and clinical outcomes at final follow-up, suggesting that outpatient transforaminal endoscopic decompression surgery is effective in the majority of patients who have undergone primary lumbar spine surgery. Yoshikane et al reported the efficacy of lumbar endoscopic unilateral laminotomy for bilateral decompression in the treatment of postoperative recurrence of LSS, observing excellent pr good results in 69% of the patients according to the Macnab criteria.26 In addition, significant improvements were observed in the numerical rating scale (NRS) for low back pain, NRS-lower leg pain, NRS-lower leg numbness, and walking ability of the Japanese Orthopedic Association Back Pain Evaluation Questionnaire (JOABPEQ). In our study, the results showed that VAS leg pain score improved from 6.1±1.0 preoperatively to 2.0±1.2 postoperatively, VAS back pain score improved from 6.2±0.8 preoperatively to 2.1±1.1 postoperatively, and ODI improved from 68.9±6.0 preoperatively to 20.9±5.6 postoperatively. According to the modified MacNab criteria, the good-to-excellent rate was 83.3%. Therefore, secondary PELD is an effective and advantageous approach for the treatment of recurrent LSS. In addition, PELD does not require general anesthesia and is less traumatic, which is advantageous for older and frail patients.
All the cases included in this study had unilateral or bilateral symptoms predominantly on one side, and the symptomatic side or the side with severe symptoms was selected as the approach side for revision surgery. We compared the efficacies of the ipsilateral and contralateral approaches. The results showed no significant difference between the ipsilateral and contralateral approaches in terms of outcome at final follow-up. However, the operation becomes more difficult owing to anatomical changes and scar management during ipsilateral revision. In addition, patients experienced more pain and discomfort during bilateral revision compared with contralateral revision when dealing with scars around the nerve roots. More people in the contralateral group (5/45.45%) exhibited an Excellent MacNab score compared to the ipsilateral group (2/15.38%); one person in the ipsilateral group was poor, while no one in the contralateral group was classified as such. However, there was no statistical difference between the ipsilateral and contralateral groups in terms of modified MacNab outcomes, which may be due to the fact that this study was a small-sample retrospective study. Statistical differences in the efficacy of ipsilateral versus contralateral revisions may be seen if the sample size is increased. In a follow-up study, we will further expand the relevant research to obtain more research results.
Previous studies have concluded that effective decompression of central canal stenosis is not possible; however, with newer and improved concepts, techniques, instruments, and levels, PELD can now be applied to treat central canal stenosis. Studies have also reported the feasibility and benefits of PELD for central lumbar stenosis.29–31 PELD includes transforaminal endoscopic discectomy and foraminoplasty. Discectomy for central lumbar spinal stenosis must be performed under general anesthesia, which increases the risk of nerve injury as the patient cannot perceive nerve function under general anesthesia.11,32 Foraminoplasty using the paraspinal approach facilitates direct access to the disc lesion with minimal manipulation of the disc joints and less postoperative pain.33 It can be performed under local anesthesia, allowing direct patient feedback to avoid possible nerve damage during and after the procedure.34 Therefore, in this study, the transforaminal approach was adopted for patients with central spinal stenosis combined with lateral stenosis, achieving satisfactory results.
Revision surgery is more challenging than primary surgery; the initial operation leads to the destruction of the anatomical structure and formation of scars, which increases the difficulty and time of revision surgery. In addition, the risk of complications such as dural injury, nerve root injury, and hematoma formation is also increased.35 The incidence of complications after repeated discectomy was reported to be 13.0%, with dural tears and infections being the most common problems.36 A retrospective study by Inada et al reported that the incidence of dural lacerations during revision surgery was 16.7%.37 In the present study, the incidence of dural injury was lower than that in previous studies, with only two patients (8.3%) experiencing dural injury, both of which occurred in the nerve root sleeve. Both cases of dural injury were caused by severe scar adhesion during scar treatment. Postoperative abdominal compression-banded bed rest for 1 week was performed, and no cerebrospinal fluid leakage or wound infection was observed. A retrospective study found that in the treatment of recurrent lumbar disc herniation, nerve damage occurred in 2.9% of patients in the PELD group and 8.3% of patients in the open lumbar microdiscectomy group.38 In our study, the incidence of nerve injury was 4.2% (one patient), which was caused by stretching stimulation during scar treatment. Intravenous glucocorticoid therapy was administered during the operation, and symptomatic treatment with hormones, dehydration, and nutritional nerves was administered after the operation. Pain and numbness were relieved on the 3rd day after surgery. Therefore, to reduce injury to the dura mater and nerve root, scars should be treated from the lateral edge of the SAP. The position was determined through the articular bone surface of the articular process and slowly separated inwards. Careful excision of free scars is necessary. Scar removal was not necessary, and nerve decompression was the ultimate goal. For severe adhesion between the scar and nerve, it is not necessary to completely remove the scar without affecting the nerve decompression.
Hematoma is a rare complication of spinal surgery that can cause nerve compression and lead to nerve damage, which can cause life-long disability in severe cases.39,40 However, the optimal timing of hematoma evacuation during spinal surgery remains controversial. Most investigators recommend performing surgery within 12 h of symptom onset, or as soon as possible within 6 h of the greatest neurological deficit for better neurological outcomes.41,42 However, Foo et al found that the timing of surgery was not associated with the postoperative outcomes.43 In this study, one patient developed hematoma, numbness, weakness of the lower extremities, and decreased muscle strength 6 h after the operation. Surgery was urgently performed to remove the hematoma, and the patient recovered well. In addition, we recommend increasing the irrigation saline bag to increase water pressure during surgery to reduce intraoperative bleeding. Before the end of the operation, the saline bag was lowered to reduce water pressure to detect potential bleeding and to reduce the risk of postoperative bleeding and hematoma formation.
This study has several limitations which should be noted. First, this was a single-center retrospective study with a small sample size and a short follow-up period. Second, some patients underwent their first surgery at other hospitals, so their condition during the initial surgery was unknown. Third, this study did not compare other revision procedures such as open decompression.
Conclusion
PELD has an obvious curative effect in patients with recurrent LSS after initial PELD treatment, and postoperative recovery is fast without serious complications. Clinically, it is a worthy treatment option for patients with LSS after relapse of PELD.
Data Sharing Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study design was approved by the Ethics Review Committee of Beijing Chaoyang Hospital (Registration number: 2021-8-5-5), and no administrative permission was required to access the raw data for this study. In addition, all subjects provided informed consent prior to participation. All methods in the study were carried out in accordance with the Helsinki guidelines and declaration.
Funding
There is no funding that supported this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ. 2016;352:h6234. doi:10.1136/bmj.h6234
2. Suzuki A, Nakamura H. Microendoscopic lumbar posterior decompression surgery for lumbar spinal stenosis: literature review. Medicina 2022;58(3):384. doi:10.3390/medicina58030384
3. Djurasovic M, Glassman SD, Carreon LY, Dimar JR. Contemporary management of symptomatic lumbar spinal stenosis. Orthop Clin North Am. 2010;41(2):183–191. doi:10.1016/j.ocl.2009.12.003
4. Siebert E, Pruss H, Klingebiel R, Failli V, Einhaupl KM, Schwab JM. Lumbar spinal stenosis: syndrome, diagnostics and treatment. Nat Rev Neurol. 2009;5(7):392–403. doi:10.1038/nrneurol.2009.90
5. Grotle M, Smastuen MC, Fjeld O, et al. Lumbar spine surgery across 15 years: trends, complications and reoperations in a longitudinal observational study from Norway. BMJ Open. 2019;9(8):e028743. doi:10.1136/bmjopen-2018-028743
6. Minamide A, Yoshida M, Maio K. The natural clinical course of lumbar spinal stenosis: a longitudinal cohort study over a minimum of 10 years. J Orthop Sci. 2013;18(5):693–698. doi:10.1007/s00776-013-0435-9
7. Jacobs WC, Rubinstein SM, Koes B, van Tulder MW, Peul WC. Evidence for surgery in degenerative lumbar spine disorders. Best Pract Res Clin Rheumatol. 2013;27(5):673–684. doi:10.1016/j.berh.2013.09.009
8. Mobbs RJ, Li J, Sivabalan P, Raley D, Rao PJ. Outcomes after decompressive laminectomy for lumbar spinal stenosis: comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: clinical article. J Neurosurg Spine. 2014;21(2):179–186. doi:10.3171/2014.4.Spine13420
9. Yone K, Sakou T, Kawauchi Y, Yamaguchi M, Yanase M. Indication of fusion for lumbar spinal stenosis in elderly patients and its significance. Spine. 1996;21(2):242–248. doi:10.1097/00007632-199601150-00016
10. Ahn Y. Transforaminal percutaneous endoscopic lumbar discectomy: technical tips to prevent complications. Expert Rev Med Devices. 2012;9(4):361–366. doi:10.1586/erd.12.23
11. Ahn Y. Percutaneous endoscopic decompression for lumbar spinal stenosis. Expert Rev Med Devices. 2014;11(6):605–616. doi:10.1586/17434440.2014.940314
12. Pan M, Li Q, Li S, et al. Percutaneous endoscopic lumbar discectomy: indications and complications. Pain Physician. 2020;23(1):49–56.
13. Kambin P, Sampson S. Posterolateral percutaneous suction-excision of herniated lumbar intervertebral discs. Report of interim results. Clin Orthop Relat Res. 1986;207:37–43. doi:10.1097/00003086-198606000-00008
14. Fan N, Yuan S, Du P, et al. Complications and risk factors of percutaneous endoscopic transforaminal discectomy in the treatment of lumbar spinal stenosis. BMC Musculoskelet Disord. 2021;22(1):1041. doi:10.1186/s12891-021-04940-z
15. Gao Z, Jia T, Chang C, Chen B. Analysis of the efficacy of intervertebral foramen in the treatment of degenerative lumbar spinal stenosis and analysis of postoperative recurrence factors. Med Inform. 2019;32(1):115–118.
16. Wang T, Wang A, Zang L, et al. Reoperations after percutaneous endoscopic transforaminal decompression for treating lumbar spinal stenosis: incidence and predictors. Global Spine J. 2022:21925682221081030. doi:10.1177/21925682221081030
17. Tang S, Jin S, Liao X, Huang K, Luo J, Zhu T. Transforaminal percutaneous endoscopic lumbar decompression by using rigid bendable burr for lumbar lateral recess stenosis: technique and clinical outcome. Biomed Res Int. 2018;2018:2601232. doi:10.1155/2018/2601232
18. Lv Z, Jin L, Wang K, et al. Comparison of effects of PELD and fenestration in the treatment of geriatric lumbar lateral recess stenosis. Clin Interv Aging. 2019;14:2187–2194. doi:10.2147/cia.S226295
19. Guha D, Heary RF, Shamji MF. Iatrogenic spondylolisthesis following laminectomy for degenerative lumbar stenosis: systematic review and current concepts. Neurosurg Focus. 2015;39(4):E9. doi:10.3171/2015.7.Focus15259
20. Zhang J, Liu TF, Shan H, et al. Decompression using minimally invasive surgery for lumbar spinal stenosis associated with degenerative spondylolisthesis: a review. Pain Ther. 2021;10(2):941–959. doi:10.1007/s40122-021-00293-6
21. Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303(13):1259–1265. doi:10.1001/jama.2010.338
22. Hu D, Fei J, Chen G, Yu Y, Lai Z. Treatment for lumbar spinal stenosis in elderly patients using percutaneous endoscopic lumbar discectomy combined with postoperative three-dimensional traction. Expert Rev Med Devices. 2019;16(4):317–323. doi:10.1080/17434440.2019.1599282
23. Li H, Ou Y, Xie F, Liang W, Tian G, Li H. Linical efficacy of percutaneous endoscopic lumbar discectomy for the treatment of lumbar spinal stenosis in elderly patients: a retrospective study. J Orthop Surg Res. 2020;15(1):441. doi:10.1186/s13018-020-01968-0
24. Melcher C, Paulus AC, Roßbach BP. Lumbar spinal stenosis - surgical outcome and the odds of revision-surgery: is it all due to the surgeon? Technol Health Care. 2022;30(6):1423–1434. doi:10.3233/thc-223389
25. Pérez-López JC, Olivella G, Cartagena M, et al. Associated factors of patients with spinal stenosis who undergo reoperation after a posterior lumbar spinal fusion in a Hispanic-American population. Eur J Orthop Surg Traumatol. 2021;32(8):1491–1499. doi:10.1007/s00590-021-03127-5
26. Yoshikane K, Kikuchi K, Okazaki K. Clinical outcomes of selective single-level lumbar endoscopic unilateral laminotomy for bilateral decompression of multilevel lumbar spinal stenosis and risk factors of reoperation. Global Spine J. 2021;21925682211033575. doi:10.1177/21925682211033575
27. Baoshan X, Feng C, Liujun Z, et al. Clinical report of revision surgery after percutaneous transforaminal endoscopic surgery for lumbar stenosis. Chin J Orthop. 2018;38(8):485–496.
28. Lewandrowski KU, Transforaminal E. Lateral recess decompression after previous spinal surgery. Int J Spine Surg. 2018;12(2):98–111. doi:10.14444/5016
29. Bao BX, Zhou JW, Yu PF, Chi C, Qiang H, Yan H. Transforaminal endoscopic discectomy and foraminoplasty for treating central lumbar stenosis. Orthop Surg. 2019;11(6):1093–1100. doi:10.1111/os.12559
30. Yang JS, Chu L, Chen CM, et al. Foraminoplasty at the tip or base of the superior articular process for lateral recess stenosis in percutaneous endoscopic lumbar discectomy: a multicenter, retrospective, controlled study with 2-year follow-up. Biomed Res Int. 2018;2018:7692794. doi:10.1155/2018/7692794
31. Wen BT, Zhang XF, Wang Y, et al. 经皮内窥镜治疗腰椎间盘突出症的并发症及其处理 [Complication and treatment of the lumbar intervertebral disc herniation using percutaneous endoscopic lumbar discectomy]. Zhonghua Wai Ke Za Zhi. 2011;49(12):1091–1095. Chinese.
32. Ahn Y. Endoscopic spine discectomy: indications and outcomes. Int Orthop. 2019;43(4):909–916. doi:10.1007/s00264-018-04283-w
33. Chang SB, Lee SH, Ahn Y, Kim JM. Risk factor for unsatisfactory outcome after lumbar foraminal and far lateral microdecompression. Spine. 2006;31(10):1163–1167. doi:10.1097/01.brs.0000216431.69359.91
34. Chun EH, Park HS, Modified A. Approach of Percutaneous Endoscopic Lumbar Discectomy (PELD) for far lateral disc herniation at L5-S1 with foot drop. Korean J Pain. 2016;29(1):57–61. doi:10.3344/kjp.2016.29.1.57
35. Ahn Y, Lee SH, Park WM, Lee HY, Shin SW, Kang HY. Percutaneous endoscopic lumbar discectomy for recurrent disc herniation: surgical technique, outcome, and prognostic factors of 43 consecutive cases. Spine. 2004;29(16):E326–E332. doi:10.1097/01.brs.0000134591.32462.98
36. Ebeling U, Kalbarcyk H, Reulen HJ. Microsurgical reoperation following lumbar disc surgery. Timing, surgical findings, and outcome in 92 patients. J Neurosurg. 1989;70(3):397–404. doi:10.3171/jns.1989.70.3.0397
37. Inada T, Nishida S, Kawaoka T, Takahashi T, Hanakita J. Analysis of revision surgery of microsurgical lumbar discectomy. Asian Spine J. 2018;12(1):140–146. doi:10.4184/asj.2018.12.1.140
38. Lee JS, Kim HS, Pee YH, Jang JS, Jang IT. Comparison of percutaneous endoscopic lumbar diskectomy and open lumbar microdiskectomy for recurrent lumbar disk herniation. J Neurol Surg A Cent Eur Neurosurg. 2018;79(6):447–452. doi:10.1055/s-0037-1608870
39. Amiri AR, Fouyas IP, Cro S, Casey ATH. Postoperative spinal epidural hematoma (SEH): incidence, risk factors, onset, and management. Spine J. 2013;13(2):134–140. doi:10.1016/j.spinee.2012.10.028
40. Yi S, Yoon DH, Kim KN, Kim SH, Shin HC. Postoperative spinal epidural hematoma: risk factor and clinical outcome. Yonsei Med J. 2006;47(3):326–332. doi:10.3349/ymj.2006.47.3.326
41. Lawton MT, Porter RW, Heiserman JE, Jacobowitz R, Sonntag VK, Dickman CA. Surgical management of spinal epidural hematoma: relationship between surgical timing and neurological outcome. J Neurosurg. 1995;83(1):1–7. doi:10.3171/jns.1995.83.1.0001
42. Ikuta K, Tono O, Tanaka T, et al. Evaluation of postoperative spinal epidural hematoma after microendoscopic posterior decompression for lumbar spinal stenosis: a clinical and magnetic resonance imaging study. J Neurosurg Spine. 2006;5(5):404–409. doi:10.3171/spi.2006.5.5.404
43. Foo D, Rossier AB. Preoperative neurological status in predicting surgical outcome of spinal epidural hematomas. Surg Neurol. 1981;15(5):389–401. doi:10.1016/0090-3019(81)90178-6
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.