Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
Efficacy of Personalized Postoperative Epilepsy Management in Patients with Glioblastoma Utilizing IDH1 Gene Assessment
Authors Meng GQ, Chen S, Ye HB, Ma BJ, Tao S, Ye Z
Received 22 November 2023
Accepted for publication 20 March 2024
Published 12 April 2024 Volume 2024:20 Pages 855—862
DOI https://doi.org/10.2147/NDT.S451300
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Gao-Qiang Meng,1,* Shu Chen,2,* Han-Bin Ye,1 Bao-Jun Ma,1 Shuo Tao,3 Zi Ye1
1Department of Neurosurgery, Affiliated Hospital 2 of Nantong University, Nantong First People’s Hospital, Nantong, 226000, People’s Republic of China; 2Department of Endocrinology, Affiliated Hospital 2 of Nantong University, Nantong First People’s Hospital, Nantong, 226000, People’s Republic of China; 3Department of Out-Patient, Affiliated Hospital 2 of Nantong University, Nantong First People’s Hospital, Nantong, 226000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zi Ye, Department of Neurosurgery, Affiliated Hospital 2 of Nantong University, Nantong First People’s Hospital, No. 666 of Shengli Road, Chongchuan District, Jiangsu, Nantong, 226000, People’s Republic of China, Email [email protected] Shuo Tao, Department of Out-patient, Affiliated Hospital 2 of Nantong University, Nantong First People’s Hospital, No. 666 of Shengli Road, Chongchuan District, Jiangsu, Nantong, 226000, People’s Republic of China, Tel +86 0513 81111429, Email [email protected]
Objective: We explored the correlation between the presence of isocitrate dehydrogenase-1 (IDH1) mutations and the incidence of postoperative epilepsy in patients with glioblastoma, as well as assessed the efficacy of preemptive administration of antiepileptic medications in mitigating the occurrence of postoperative epilepsy.
Methods: Fifty-three patients who received a postoperative pathological diagnosis of glioblastoma, were enrolled in this study. Tumor specimens were subjected to IDH1 gene analysis. The patient cohort was stratified based on their IDH1 mutation status and the administration of prophylactic antiepileptic drugs during the postoperative phase. We subsequently conducted a comparative analysis of postoperative epileptic complications within each patient subgroup.
Results: In the cohort of 53 patients under study, the occurrence of epilepsy was observed in 10 out of 21 patients carrying IDH1 mutations, while 5 out of 32 patients with wild-type IDH1 also experienced epilepsy, revealing a statistically significant difference (P < 0.05). Among the 27 patients who received prophylactic antiepileptic drugs, 6 of them developed epilepsy, whereas 9 out of 26 patients who did not receive prophylactic antiepileptic drugs exhibited concurrent epilepsy, with no statistically significant difference (P > 0.05). However, when performing a subgroup analysis, it was found that 3 out of 12 patients with IDH1 mutations who received prophylactic antiepileptic drugs experienced epilepsy, whereas 7 out of 9 patients who did not receive prophylactic antiepileptic drugs developed epilepsy, demonstrating a statistically significant difference (P < 0.05). Furthermore, within the group of 15 patients with wild-type IDH1, 3 patients who received prophylactic antiepileptic drugs developed epilepsy, while 2 cases of epilepsy occurred among the 17 patients who did not receive prophylactic antiepileptic drugs, with no statistically significant difference (P > 0.05).
Conclusion: In individuals with IDH1 mutant glioblastoma who have undergone surgical resection, the implementation of preventive antiepileptic therapy demonstrates a potential to diminish the occurrence of postoperative epilepsy.
Keywords: epilepsy, glioblastoma, isocitrate dehydrogenase-1, prophylactic antiepileptic therapy
Background
Gliomas represent a group of malignant neoplasms that originate from glial cells and constitute approximately 24% of all tumors affecting the central nervous system, with malignant variants accounting for approximately 80.9% of this category. Among gliomas, glioblastoma stands out as the most prevalent subtype, comprising 59.2% of all glioma cases.1 The prevailing therapeutic modalities for glioblastoma predominantly encompass surgical intervention, complemented by comprehensive approaches including postoperative radiotherapy.2
In certain cases, patients diagnosed with glioblastoma may manifest epilepsy as a concurrent manifestation during the diagnostic and therapeutic phases. Epilepsy represents a prevalent clinical manifestation of glioma, with reported incidences reaching up to 80% among individuals afflicted with this malignancy.3–6 Attributes encompassing a high degree of malignancy, unpredictable peritumoral infiltration, unfavorable treatment outcomes, a short median survival period, and postoperative epilepsy, all contribute to a significant decline in the overall quality of life of patients. The question remains a matter of debate regarding whether patients without preoperative epileptic symptoms should receive prophylactic antiepileptic treatment after surgery. Thus, the pursuit of tailored postoperative antiepileptic treatment strategies for individuals diagnosed with glioblastoma holds exceptional importance.
In this study, we conducted an analysis of isocitrate dehydrogenase-1 (IDH1) gene mutation status in surgically resected tumor specimens obtained from patients diagnosed with glioblastoma, and documented postoperative prophylactic antiepileptic treatment and the subsequent occurrence of postoperative epileptic complications. Our primary objective was to investigate the potential association between IDH1 gene mutations and the development of postoperative epileptic complications in patients with glioblastoma. Additionally, we aimed to assess the effectiveness of postoperative prophylactic antiepileptic treatment. The findings from our investigation can serve as a valuable resource for guiding the personalized administration of antiepileptic therapy following glioblastoma surgery, with the goal of reducing the incidence of postoperative epilepsy.
Data and Methods
Data and Follow-Up
In the period spanning from August 2015 to May 2021, a total of 53 patients, comprising 29 males and 24 females, aged between 28 and 78 years, were diagnosed with glioblastoma through postoperative pathological examination following consultations with the neurosurgery department at the First People’s Hospital of Nantong City, Jiangsu Province. These patients all underwent craniotomy for the purpose of tumor resection, achieving complete removal of the tumor as confirmed by intraoperative microscopy, with no observable residual tumor in postoperative imaging. Their excised tumor samples were subjected to IDH1 gene analysis, In this study, the postoperative prophylactic antiepileptic treatment is as follows: postoperative administration of intravenous drip of sodium valproate at a dose of 20–30mg/kg/d. After the return of gastrointestinal function, it is changed to oral sodium valproate sustained-release tablets 0.5g/time, twice a day, and there is a 12–24-hour overlap in the drug-switching process. If the patient takes the medicine regularly without epileptic seizures, and combined with the results of EEG examination, the medicine will be stopped after 3 months. Subsequently, they were categorized based on their IDH1 mutation status and the administration of postoperative prophylactic antiepileptic drugs. We then conducted a comparative analysis of postoperative epileptic complications among these patient groups.
Inclusion criteria: (1) Glioblastoma diagnosis confirmed by postoperative pathological examination; (2) No history of cranial hemorrhage, trauma, intracranial infection, or prior cranial surgery; (3) The New York Heart Association (NYHA) classifies heart function into grades I to II; (4) Preoperative Karnofsky Performance Status (KPS) score exceeding 60; (5) No history of epilepsy prior to the surgery.
Exclusion criteria: (1) Non-compliance with prescribed postoperative medication; (2) Development of severe complications such as intracranial hemorrhage or infection post-surgery; (3) Missed post-surgery follow-up appointments; (4) A pre-existing history of epileptic seizures.
Subsequently, based on the results of postoperative molecular testing, the patients were classified into either the IDH1 mutant or IDH1 wild-type groups. Furthermore, they were classified based on whether prophylactic antiepileptic drugs were administered after surgery into the prophylactic antiepileptic treatment group or the non-prophylactic antiepileptic treatment group. All patients were subject to post-surgery follow-up assessments to determine the occurrence of epilepsy within one year and to document their antiepileptic drug usage.
Extraction and Detection of the IDH1 Gene
In the context of this study, the IDH1 gene within tumor specimens was detected using a two-step process. Initially, DNA extraction and amplification were carried out using tissue-fixing kits and nested PCR, respectively. Following this, the amplified DNA underwent agarose gel electrophoresis to isolate the specific PCR products. Subsequently, the PCR products were subjected to purification through Sanger sequencing. To prepare the samples for electrophoresis, they were denatured at 95 °C for 4 minutes, rapidly cooled on ice for 4 minutes, and then stored at −20 °C. Finally, the obtained results were subjected to analysis using Sequencer software.
Statistical Methods
We employed SPSS 20.0 software to conduct statistical analysis and data processing. The chi-squared test was employed to examine the count data. Using logistic regression analysis to clarify the correlation between prophylactic anti-epileptic treatment and postoperative epilepsy. Differences in postoperative epilepsy incidence between individuals in the IDH1 wild type group and those in the IDH1 mutant group, differences in postoperative epilepsy incidence between patients who received prophylactic antiepileptic therapy and those who did not, differences within the IDH1 mutant treatment group in postoperative epilepsy incidence between individuals who received prophylactic antiepileptic therapy and those who did not, as well as differences within the IDH1 wild type group in postoperative epilepsy incidence between individuals who received prophylactic antiepileptic therapy and those who did not were assessed. P-values were computed, and a significance level of P < 0.05 was considered to indicate statistical significance.
Results
IDH1 Mutation Status and Clinical Characteristics
In the cohort of 53 patients, 21 cases exhibited the presence of an IDH1 mutant, while 32 cases presented with the IDH1 wild type. Chi-squared test was used to assess potential disparities in IDH1 mutation status concerning variables such as gender, age, radiotherapy, and chemotherapy. The results indicate that none of these variables displayed statistically significant differences (P > 0.05).
Notably, among patients who developed epilepsy following surgery, 10 cases were associated with IDH1 mutations, while 5 cases exhibited the IDH1 wild type. Importantly, this difference was found to be statistically significant(P < 0.05) (see Table 1 for details).
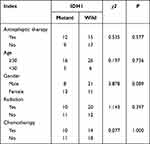 |
Table 1 IDH1 Mutation and Clinical Characteristics |
Evaluation of the Occurrence of Postoperative Epilepsy and Clinical Attributes in Individuals Diagnosed with Glioblastoma
In the cohort of 53 patients, 15 patients experienced postoperative epilepsy complications, while 38 patients did not encounter such complications. To assess variations in IDH1 mutation status, postoperative prophylactic antiepileptic treatment, gender, age, radiotherapy, and chemotherapy among those who developed postoperative epilepsy, a chi-squared test for count data was conducted. The findings revealed that there were no statistically significant differences in postoperative prophylactic antiepileptic treatment, gender, age, radiotherapy, or chemotherapy (P > 0.05). However, a statistically significant difference emerged in the number of patients with postoperative epilepsy between those with an IDH1 mutation (10 cases) and those with the IDH1 wild type (15 cases) (P < 0.05) (see Table 2 for details).
 |
Table 2 Analysis of Postoperative Epilepsy and Its Clinical Features |
Correlation Between Postoperative Prophylactic Antiepileptic Treatment and Postoperative Epilepsy
Among the 53 patients, 27 received preventive antiepileptic treatment and 6 of them experienced epilepsy, while 26 patients did not receive this treatment and 9 of them developed epilepsy. Importantly, there were no statistically significant differences between these two groups (P > 0.05).
The subgroups were further analyzed for postoperative epilepsy. In the IDH1 mutant group, there were 12 patients who received prophylactic antiepileptic treatment, and 3 of them developed epilepsy, while among the 9 patients in the IDH1 mutant group who did not receive this treatment, 7 patients developed epilepsy. Similarly, in the IDH1 wild type group, 15 patients received prophylactic antiepileptic treatment, and 3 of them developed epilepsy, while among the 17 patients in the IDH1 wild type group who did not receive this treatment, 2 patients developed epilepsy. Notably, within the IDH1 wild type group, there were no statistically significant differences in the incidence of epilepsy between those who received and those who did not receive prophylactic antiepileptic therapy (P > 0.05). However, within the IDH1 mutant group, there was a statistically significant difference in the incidence of epilepsy between patients who received and those who did not receive prophylactic antiepileptic therapy (P < 0.05) (see Table 3 for details). Conducting logistic multiple regression analysis confirmed that prophylactic anti-epileptic therapy is an independent risk factor for postoperative epilepsy in patients with IDH1 mutant glioblastoma. (See Table 4 for details)
 |
Table 3 Subgroup Analysis of Epileptic Status After Genetic Testing Combined with Prophylactic Antiepileptic Therapy |
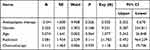 |
Table 4 Postoperative Epilepsy in Patients with IDH1 Mutant Glioblastoma |
Discussion
Gliomas stand as the most prevalent form of primary malignancy affecting the central nervous system,7 and represent the leading cause of tumor-induced epilepsy.8 The prognosis of high-grade gliomas is worse than that of low-grade gliomas,9,10 but the incidence of epilepsy is lower than that of low-grade gliomas, about 40% to 64%.11,12 Gliomas serve as the predominant pathological etiology underlying epilepsy, manifesting in motor and speech impairments that markedly reduce the quality of life of patients. This imposes substantial economic and psychological burdens on both the patients and their families, and the society as a whole.11
Although complete tumor resection or reduction of tumor burden through surgical intervention can effectively control epilepsy in most cases, some individuals still experience postoperative epilepsy complications.13–15 Currently, there remains a debate regarding whether individuals with intracranial tumors lacking preoperative epileptic symptoms should undergo postoperative prophylactic antiepileptic treatment. Many clinicians rely on their personal experience for guidance. The objective of our present study is to provide insights and guidance for the implementation of personalized and selective antiepileptic therapy for patients diagnosed with glioblastoma following surgical procedures.
Parsons et al conducted the sequencing of an extensive array of protein-coding genes,16,17 subsequently pioneering the discovery of IDH1 gene mutations in glioblastoma. In their study, Bleeker et al18 examined histiocytes from various origins and pinpointed the specificity of IDH1 mutations to glioblastoma.19,20 Their findings elucidated a strong correlation between heightened expression of IDH1 gene mutations at the R132 locus and the occurrence of epilepsy.
IDH1 mutations, predominantly found at the R132H site, induce the substitution of arginine with histidine, leading to a reduction in α-ketoglutarate levels, an increase in 2-hydroxyglutarate (2-HG) levels, and a decrease in oxidized nicotinamide adenine dinucleotide phosphate (NADPH) levels.21 A sequence of modifications disrupts cell signaling pathways sensitive to α-ketoglutarate in tumors. Additionally, the loss of IDH1 gene function results in the conversion of α-ketoglutarate into 2-hydroxyglutarate, consequently leading to an elevated production of 2-hydroxyglutarate. This compound bears structural resemblance to glutamic acid and has the potential to activate N-methyl-D-aspartate (NMDA) receptors and isoxazole propionate (AMPA) receptors.22 Furthermore, the excitation of NMDA and AMPA receptors by glutamic acid can trigger the activation of intracellular mTOR, AKT, and MAPK signaling pathways, which play a role in promoting cell growth and the onset of epileptogenesis.23
Glutamic acid plays a vital role as an excitatory neurotransmitter within brain tissue and exhibits a strong connection with the process of epileptogenesis.24 Glioma cells carrying the IDH1 mutant gene exhibit an elevated synthesis of 2-hydroxyglutarate, leading to its release into the cerebrospinal fluid, consequently influencing the neighboring microenvironment. This 2-hydroxyglutarate, acting as an analog to glutamic acid, heightens the incidence of neurons initiating action potentials, disturbs the equilibrium between neuronal excitatory and inhibitory regulation, ultimately giving rise to epileptogenesis.25
Presently, there remains no definitive consensus regarding the necessity of prophylactic antiepileptic treatment following surgery for patients with central nervous system (CNS) tumors who do not manifest preoperative epileptic symptoms.26,27 Glioblastoma, a profoundly aggressive tumor characterized by widespread tissue infiltration, often leads to noticeable peritumoral edema in affected patients.
Interestingly, some individuals exhibit no signs of epilepsy prior to surgical intervention but subsequently develop epilepsy. This phenomenon may be attributed to several factors, including an elevated risk of epilepsy occurrence in the supratentorial region, potential cortical stimulation or damage resulting from the surgical procedure, and the influence of postoperative chemotherapy and radiation therapy.28
In our study, we observed 27 patients in the prophylactic antiepileptic treatment group, and 6 of them developed epilepsy post-surgery, resulting in an epilepsy incidence of 22.22%. In the non-prophylactic antiepileptic treatment group, there were 26 patients, and 9 of them experienced epilepsy, yielding an epilepsy incidence of 34.62%. Importantly, the difference between these two groups did not reach statistical significance (P > 0.05).
Upon conducting subgroup analysis, we found that within the IDH1 mutant cohort, there were 12 patients who received prophylactic antiepileptic treatment, and 3 of them developed postoperative epilepsy. In contrast, the IDH1 mutant group without prophylactic antiepileptic treatment consisted of 9 patients, and 7 of them developed postoperative epilepsy. Notably, there was a statistically significant difference in epilepsy incidence between these two subgroups (P < 0.05).
These findings suggest that a blanket prophylactic antiepileptic therapy approach for patients with glioblastoma does not significantly reduce the overall epilepsy incidence. However, through the utilization of IDH1 gene testing, selective prophylactic antiepileptic treatment for IDH1 mutant patients could effectively lower the occurrence of postoperative epilepsy.
Conclusion
In summary, the presence of an IDH1 mutation appears to be a risk factor for postoperative epilepsy in patients with glioblastoma, leading to the recommendation that routine prophylactic antiepileptic treatment may not be warranted. Nevertheless, it is noteworthy that patients with glioblastoma harboring an IDH1 mutation exhibit a significantly higher incidence of epilepsy compared to those with IDH1 wild type, and in this particular cohort of patients, prophylactic antiepileptic medication can effectively reduce postoperative epilepsy rates. It is important to acknowledge that our current study is limited in scope as a single-center, retrospective analysis, featuring a relatively small dataset and potential biases. As part of our ongoing research efforts, we plan to conduct larger-scale data analyses and prospective studies to reaffirm these conclusions, offering more precise guidance for personalized and selective antiepileptic treatment during the postoperative phase, ultimately aiming to minimize the incidence of postoperative epilepsy.
Abbreviations
IDH1, isocitrate dehydrogenase 1; GBM, glioblastoma multiforme; KPS, Karnofsky; DNA, deoxyribonucleic acid; SPSS, Statistical Package for the Social Sciences; 2-HG, 2-hydroxyglutaric acid; NADPH, nicotinamide adenine dinucleotide phosphate; NMDA, N-Methyl-D-aspartic acid; AMPA(α-amino-3-hydroxy-5-methylisoxazole-4-propionate; mTOR, mammalian target of rapamycin; AKT, protein kinase B; MAPK, mitogen-activated protein kinase.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was conducted with approval from the Ethics Committee of the Second Affiliated Hospital of Nantong University. This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff who implemented the intervention and evaluation components of the study.
Funding
Jiangsu Commisson of Health (No.Z2021017); Jiangsu Commisson of Health (No.QN2023009); Clinical Medical Research Special Project Fund of Nantong University (No.2022LY008).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Ostrom QT, Price M, Neff C, et al. CBTRUS Statistical Report: primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015-2019. Neuro Oncol. 2022;24(Suppl 5):v1–v95. doi:10.1093/neuonc/noac202
2. Nabors LB, Portnow J, Ahluwalia M, et al. Central Nervous System Cancers, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020;18(11):1537–1570. doi:10.6004/jnccn.2020.0052
3. Lange F, Hörnschemeyer J, Kirschstein T. Glutamatergic Mechanisms in Glioblastoma and Tumor-Associated Epilepsy. Cells. 2021;10(5):1226. doi:10.3390/cells10051226
4. Pallud J, Le Van Quyen M, Bielle F, et al. Cortical GABAergic excitation contributes to epileptic activities around human glioma. Sci Transl Med. 2014;6(244):244ra89. doi:10.1126/scitranslmed.3008065
5. Venkatesh HS, Johung TB, Caretti V, et al. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell. 2015;161(4):803–816. doi:10.1016/j.cell.2015.04.012
6. Wang Y, Qian T, You G, et al. Localizing seizure-susceptible brain regions associated with low-grade gliomas using voxel-based lesion-symptom mapping. Neuro Oncol. 2015;17(2):282–288. doi:10.1093/neuonc/nou130
7. Ostrom QT, Patil N, Barnholtz-Sloan JS, et al. CBTRUS Statistical Report: primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013-2017. Neuro Oncol. 2020;22(12 Suppl 2):iv1–iv96. doi:10.1093/neuonc/noaa200
8. Jiang T, Nam DH, Wang Q, et al. Clinical practice guidelines for the management of adult diffuse gliomas. Cancer Lett. 2021;499:60–72. doi:10.1016/j.canlet.2020.10.050
9. Le VH, Minh TNT, Le NQK, et al. A transfer learning approach on MRI-based radiomics signature for overall survival prediction of low-grade and high-grade gliomas. Med Biol Eng Comput. 2023;61(10):2699–2712. doi:10.1007/s11517-023-02875-2
10. Lam LHT, Chu NT, Le NQK, et al. A Radiomics-Based Machine Learning Model for Prediction of Tumor Mutational Burden in Lower-Grade Gliomas. Cancers. 2022;14(14):3492. doi:10.3390/cancers14143492
11. Shan X, Fan X, Jiang T, et al. Clinical characteristics associated with postoperative seizure control in adult low-grade gliomas: a systematic review and meta-analysis. Neuro Oncol. 2018;20(3):324–331. doi:10.1093/neuonc/nox130
12. Liang S, Fan X, Jiang T, et al. Clinical practice guidelines for the diagnosis and treatment of adult diffuse glioma-related epilepsy. Cancer Med. 2019;8(10):4527–4535. doi:10.1002/cam4.2362
13. Shin JY, Kizilbash SH, Robinson SI, et al. Seizures in patients with primary brain tumors: what is their psychosocial impact? J Neurooncol. 2016;128(2):285–291. doi:10.1007/s11060-016-2108-y
14. Song L, Quan X, Chen C, et al. Correlation Between Tumor Molecular Markers and Perioperative Epilepsy in Patients With Glioma: a Systematic Review and Meta-Analysis. Front Neurol. 2021;12:692751. doi:10.3389/fneur.2021.692751
15. Li L, Li G, Jiang T, et al. New-Onset Postoperative Seizures in Patients With Diffuse Gliomas: a Risk Assessment Analysis. Front Neurol. 2021;12:682535. doi:10.3389/fneur.2021.682535
16. Yan H, Parsons DW, Bigner DD, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. doi:10.1056/NEJMoa0808710
17. Parsons DW, Jones S, Kinzler KW, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi:10.1126/science.1164382
18. Bleeker FE, Lamba S, Bardelli A, et al. IDH1 mutations at residue p.R132 (IDH1(R132)) occur frequently in high-grade gliomas but not in other solid tumors. Hum Mutat. 2009;30(1):7–11. doi:10.1002/humu.20937
19. Liang R, Fan Y, Liu Y, et al. The significance of IDH1 mutations in tumor-associated seizure in 60 Chinese patients with low-grade gliomas. ScientificWorldJournal. 2013;2013:403942. doi:10.1155/2013/403942
20. Stockhammer F, Misch M, Hartmann C, et al. IDH1/2 mutations in WHO grade II astrocytomas associated with localization and seizure as the initial symptom. Seizure. 2012;21(3):194–197. doi:10.1016/j.seizure.2011.12.007
21. Fathi AT, Nahed BV, Chi AS, et al. Elevation of Urinary 2-Hydroxyglutarate in IDH-Mutant Glioma. Oncologist. 2016;21(2):214–219. doi:10.1634/theoncologist.2015-0342
22. Chen H, Judkins J, Horbinski C, et al. Mutant IDH1 and seizures in patients with glioma. Neurology. 2017;88(19):1805–1813. doi:10.1212/WNL.0000000000003911
23. Lim JS, Kim WI, Lee JH, et al. Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy. Nat Med. 2015;21(4):395–400. doi:10.1038/nm.3824
24. Albrecht J, Zielińska M. Mechanisms of Excessive Extracellular Glutamate Accumulation in Temporal Lobe Epilepsy. Neurochem Res. 2017;42(6):1724–1734. doi:10.1007/s11064-016-2105-8
25. Mortazavi A, Fayed I, Zaghloul KA, et al. IDH-mutated gliomas promote epileptogenesis through d-2-hydroxyglutarate-dependent mTOR hyperactivation. Neuro Oncol. 2022;24(9):1423–1435. doi:10.1093/neuonc/noac003
26. Englot DJ, Chang EF, Vecht CJ. Epilepsy and brain tumors. Handb Clin Neurol. 2016;134:267–285. doi:10.1016/B978-0-12-802997-8.00016-5
27. Liang S, Zhang J, Fu X, et al. Epilepsy in Adults with Supratentorial Glioblastoma: incidence and Influence Factors and Prophylaxis in 184 Patients. PLoS One. 2016;11(7):e0158206. doi:10.1371/journal.pone.0158206
28. Armstrong TS, Grant R, Norden AD, et al. Epilepsy in glioma patients: mechanisms, management, and impact of anticonvulsant therapy. Neuro Oncol. 2016;18(6):779–789. doi:10.1093/neuonc/nov269
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
