Back to Journals » Journal of Pain Research » Volume 16
Efficacy of Peripheral Nerve Stimulation with a High Frequency Electromagnetic Coupled (HF-EMC) Powered Implanted Receiver in Treating Different Pain Targets/Neuralgias
Authors Abd-Elsayed A, Moghim R
Received 29 November 2022
Accepted for publication 17 January 2023
Published 23 February 2023 Volume 2023:16 Pages 589—596
DOI https://doi.org/10.2147/JPR.S399532
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Andrea Tinnirello
Alaa Abd-Elsayed,1 Robert Moghim2
1Anesthesiology Department, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA; 2Colorado Pain Care, Denver, CO, USA
Correspondence: Alaa Abd-Elsayed, Department of Anesthesiology and Pain Management, University of Wisconsin School of Medicine and Public Health, 600 Highland Avenue, Madison, WI, 53792-3272, USA, Email [email protected]
Introduction: Chronic pain is a significant global public health problem. Peripheral nerve stimulation (PNS) has been gaining popularity in recent years as it is effective, safe and less invasive than surgery for the treatment of chronic pain. The authors aimed to document and share a collection of patient-reported pain scores before and after implanting a percutaneous PNS lead/s with an external wireless generator at various target nerves.
Methods: The authors designed a retrospective study, reviewing electronic medical records. Statistical analysis was performed using SPSS 26; p-value ≤ 0.05 was considered significant.
Results: The mean baseline pain scores of 57 patients have reduced significantly after the procedure at different follow-up durations. Target nerves included genicular nerves, superior cluneal nerves, posterior tibial nerve ± sural nerve, middle cluneal nerves, radial and ulnar nerves and right common peroneal nerve. In the one-month follow-up group, mean pain score was reduced from 7.44 ± 1.48 pre-procedure to 1.6 ± 1.49, from 7.42 ± 1.5 pre-procedure to 1.6 ± 1.5 7.42 at 3 months, from 7.52 ± 1.5 to 1.72 ± 1.57 at 6 months, from 7.41 ± 1.53 to 1.7 ± 1.55 at 9 months, from 7.41 ± 1.58 to 1.76 ± 1.63 at 12 months, from 7.38 ± 1.59 to 1.69 ± 1.56 at 15 months and from 7.5 ± 1.7 to 1.45 ± 1.57 at 24 months (p ≤ 0.001). Patients also reported significant reduction in morphine milliequivalent, pre-procedure MME 47.75 ± 452.5 to 37.92 ± 43.51 at 6 months (p = 0.002, N = 57), pre-procedure MME 42.72 ± 43.19 to 30.38 ± 41.62 at 12 months (p = 0.003, N = 42), and pre-procedure MME 41.2 ± 46.12 to 21.19 ± 40.88 at 24 months (p ≤ 0.001, N = 27). The only complications occurred post procedure with 2 patients receiving an explant and 1 patient receiving a lead migration.
Conclusion: PNS has been shown to be safe and effective in treating chronic pain at different sites with sustained pain relief for up to 24 months. This study is unique in providing long-term follow-up data.
Keywords: peripheral nerve stimulation, external generator, peripheral nerves, superior cluneal nerves, middle cluneal nerves, ankle pain, knee pain, genicular nerves, low back pain
Introduction
Among adults, chronic pain is one of the most common and difficult-to-treat musculoskeletal diseases and a major source of disability. Not only does it affect a person physically, but it also has a significant socioeconomic impact as there are increasing numbers of workers who miss work. Another problem associated with chronic pain is that, in most cases, the cause is usually nonspecific.1 Further, some individuals do not respond well to conservative and conventional treatments such as anti-inflammatory drugs, analgesics, and physical therapy.
Despite its side effects, the use of opioids for chronic pain has skyrocketed, which has correlated to an increase in abuse.2 Due to its risks and complications, surgery is usually the last resort in managing chronic, refractory pain. In most cases, patients usually opt out of these invasive procedures. These reasons, among many others, cause chronic pain to remain a challenge in the healthcare industry, both in diagnosis and management.
For decades, peripheral nerve stimulation has been used in the treatment of chronic pain. The origins of electrostimulation date back to 47 CE with the use of electrical eels to treat ailments such as arthritis, gout, and headaches.3 Many devices have been implemented over time with various techniques and efficacy, but modern devices have focused on the “gate control” theory set forth by Melzack and Wall in the 1960s.13 The advent of percutaneous insertion of electrodes for effective peripheral nerve stimulation was demonstrated in 1999 by Weiner and Reed.14 This technique was much less invasive than previous procedures and was shown in the coming years to effectively treat many conditions such as lower back, groin, ankle, neck, and abdominal pain along with migraines and CRPS type 2.15–26
This article presents a collection of cases comparing patient-reported pain scores before and after the implant at various sites of a percutaneous peripheral nerve stimulation lead/s with an external generator.
Methods
The current study, retrospective by design, received an IRB exemption from the university of Wisconsin due to the retrospective nature of the study. Data confidentiality and compliance with the Declaration of Helsinki were ensured. This is a single-center study with all implants performed by a physician who was an experienced surgical implanter. Data was collected from electronic medical records and entered into an excel sheet. Data included patient demographics, pre and post-implant pain scores and adverse events. Data were then entered into SPSS 26; statistical analysis was performed using descriptive statistics and paired t-tests for comparing pre- and post-procedure pain scores; the p-value was considered significant if ≤ 0.05.
There were missing follow-ups at different durations, and the mean scores were adjusted to reflect only pain scores for patients with complete follow-ups.
Device Description
The Freedom PNS System (Curonix, formerly StimWave Technologies, Pompano Beach, FL) uses high-frequency electromagnetic coupling technology to power the implanted receiver. Each electrode array has 4 or 8 contacts (1.3 mm in diameter with 4 mm spacing) with embedded electronics and a separate receiver. A small, external rechargeable transmitter attached to a transmitting antenna worn in the clothing supplies the energy to power the implanted device through the skin. The device uses pulsed electrical current to create an electrical field that acts on nerves to inhibit the transmission of pain signals to the brain.
Procedure Methods
The skin was marked over the needle entry location proximally and anesthetized once the location of the target nerve was visualized on imaging. After a small skin incision, an introducer needle was passed through the subcutaneous tissues toward the target nerve through a first incision. The needle was advanced using intermittent imaging placing the needle within five6 mm of the target nerve. After properly positioning the introducer, the electrode array was inserted through the introducer needle cannula and placed near the nerve.
A subcutaneous pocket was created using a second incision. The steering stylet was removed and a receiver was connected to the electrode array. This allows for proper conduction to the wireless transmitter. The electrode array and receiver were tunneled beneath the skin from the first incision to the pocket. The patient was required to verbally confirm stimulation before proceeding. Once confirmation of stimulation was affirmed, a knot was tied after the second channel marker to secure the receiver to the electrode array and prevent receiver migration. The distal portion of the electrode array and receiver were coiled in a circular pattern using non absorbable sutures to secure the integrity of the coil. The electrode array and receiver coil were sutured to the fascia within the pocket to prevent migration. The pocket was then closed with deep and subcutaneous sutures ensuring proper wound closure. The incisions were covered with tegaderm and sterile gauze.
Programming Parameters
Patient Received 3 Programs
Program 1: Width 32us, rate 1499 kHz, pattern 10 on 20 off and 1 MA
Program 2: Width 32us, 1499 kHz, pattern 10 on 20 off and 1.5 MA
Program 3: Width 32us, 1499 kHz, pattern 10 on 20 off and 2 MA
Results
This review included a total of 57 patients (44 females and 13 males) who received PNS (Curonix, formerly StimWave technologies, Pompano Beach, FL) for the treatment of chronic pain in various sites with different durations of follow-up. The average age of patients was 66.6 ± 11 years). Target nerves included genicular nerves for treating chronic knee pain (N= 19), superior cluneal nerves for treating chronic low back pain (N= 15), posterior tibial nerve ± sural nerve for treating ankle pain (N= 14), middle cluneal nerves for treating sacroiliac joint pain (N= 7), radial and ulnar nerves for treating hand pain (N=1) and right common peroneal nerve for treating foot pain (N=1). Five patients were implanted with one lead and 52 patients were implanted with 2 leads. In the one-month follow-up group, mean pain score was reduced from 7.44±1.48 pre-procedure to 1.6±1.49, from 7.42±1.5 pre-procedure to 1.6±1.5 7.42 at 3 months, from 7.52±1.5 to 1.72±1.57 at 6 months, from 7.41±1.53 to 1.7±1.55 at 9 months, from 7.41±1.58 to 1.76±1.63 at 12 months, from 7.38±1.59 to 1.69±1.56 at 15 months and from 7.5±1.7 to 1.45±1.57 at 24 months. (Table 1 and Figure 1) The reduction in pain scores as reported by the patients was statistically significant at all durations of follow up (p≤ 0.001). Patients also reported significant reduction in morphine milliequivalent use of opioids up to the 24 months follow up, pre procedure MME 47.75±452.5 to 37.92±43.51 at 6 months (p=0.002, N=57), pre procedure MME 42.72±43.19 to 30.38±41.62 at 12 months (p=0.003, N=42), pre procedure MME 41.2±46.12 to 21.19±40.88 at 24 months (p≤ 0.001, N=27). Sub-analysis of different targets separately showed similar results to the whole group comparison with statistically significant reduction in pain scores and MME up to the 24 months follow up. The only complications occurred post procedure with 2 patients receiving an explant and 1 patient receiving a lead migration. Patients reported more than 70% improvement in pain at all follow ups (Table 2).
 |
Table 1 Pre and Post Pain Scores at Different Follow Up Intervals |
 |
Table 2 Reported Percent Improvement in Pain at Different Follow Up Intervals |
 |
Figure 1 Comparison between pre- and post-implant pain scores. |
Fluoroscopy was used in 42 implants and combined fluoroscopy and ultrasound in 15 implants. Examples of cases implanted using fluoroscopy are shown in Figures 2–6 and with ultrasound in Figure 7.
 |
Figure 2 Fluroscopic image showing a 4 contact lead implanted to stimulate the superior cluneal nerves. |
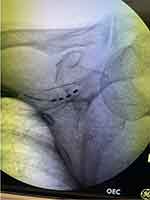 |
Figure 3 Fluroscopic image showing 4 contacts lead implanted to stimulate the suprascapular nerve. |
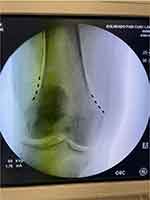 |
Figure 4 Fluroscopic image showing two 4 contact lead implanted to stimulate the superior lateral and medial genicular nerves. |
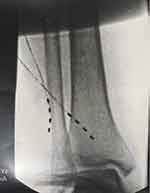 |
Figure 5 Fluroscopic image showing two 4 contact leads implanted to target the posterior tibial and sural nerves. |
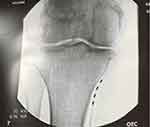 |
Figure 6 Fluroscopic image showing 4 contacts lead implanted to target the infrapatellar saphenous nerve. |
 |
Figure 7 Ultrasound image showing lead placement targeting the sural nerve. |
Discussion
For years, chronic pain continues to be a major global public health concern and has been a major contributor to disability among adults. It also had a significant impact on the economy due to lost work time. More than 85% of patients diagnosed with chronic pain have an unknown or nonspecific cause.4 Depending on the cause, chronic pain is usually managed initially with conventional therapies, including pharmacologic and non-pharmacologic treatments. According to research, the longer an individual endures the pain, the more intense it becomes due to central sensitization.5 This leads to neuropathy regardless of the cause, making it more difficult to treat conventionally. Different medications such as antidepressants, antiepileptics, and opioids have significant side effects and may not be effective in some patients.6 Nerve blocks with and without steroids have been used with success in treating different pain conditions, but one of the major challenges is that they more often than not improve pain only temporarily. PNS can prolong the effect as they are implanted and continuously stimulate the nerve to control certain pain condition.1,7
Recent developments have led to a rise in enthusiasm in the utility of PNS for pain management. Although further investigation is required to determine the exact mechanism underlying PNS, Melzack and Wall’s gate control theory has gained widespread acceptance in recent years.8,11
The evolution of PNS has led to the development of more advanced devices which are less invasive than previous generations.12 With the momentum PNS is gaining, there has been more research regarding its effectiveness in the treatment of chronic pain. However, studies with long term follow-up durations are rare despite their importance in demonstrating their overwhelming effectiveness reported by patients. In this article, we discuss the results of 57 patients with successfully managed chronic pain at various nerve target sites via peripheral nerve stimulation and at different follow-up durations. It has shown to have a sustained effect of pain control even 24 months after the procedure. In addition, our results showed that only 2 patients required an explant due to loss of effectiveness which suggests longevity of the implants.
Our results agree with several previously published studies1,7–10 but is unique in that it included a larger number of patients with long term follow up (up to 24 months). The long term follow up of patients (24 months) makes this study very unique and valuable. Some studies in the spinal cord stimulation field showed pain remission after one year which shows the importance of reporting long term follow ups.27
The study has limitations as this was a retrospective analysis with some missing follow up pain scores. As any other retrospective study, it is not possible to adjust for all confounders.
Conclusion
Our study highlights the efficacy of PNS in treating and managing chronic pain related to different pain conditions associated with certain peripheral nerves. The time frame we examined demonstrates the longevity and safety of the device. The study also illustrated less opioid dosing needed to manage chronic pain when PNS was utilized in these patients. Documentation of long-term pain relief with peripheral nerve stimulation is important and necessary to ensure that patients continue to have access to PNS therapy.
Disclosure
Dr Alaa Abd-Elsayed is Consultant of StimWave. Dr Robert Moghim reports personal fees from Curonix, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Gilmore CA, Kapural L, McGee MJ, Boggs JW. Percutaneous Peripheral Nerve Stimulation (PNS) for the treatment of chronic low back pain provides sustained relief. Neuromodulation. 2019;22(5):615–620. doi:10.1111/ner.12854
2. Vowles, Kevin EA, McEntee MLA, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain. PAIN. 2015;156(4):569–576. doi:10.1097/01.j.pain.0000460357.01998.f1
3. Cambiaghi M, Sconocchia S. Scribonius Largus (probably before 1CE–after 48CE). J Neurol. 2018;265(10):2466–2468. doi:10.1007/s00415-018-8739-5
4. Finucane LM, Downie A, Mercer C, et al. International framework for red flags for potential serious spinal pathologies. J Orth Sports Phys Ther. 2020;50(7):350–372.
5. Roussel NA, Nijs J, Meeus M, Mylius V, Fayt C, Oostendorp R. Central sensitization and altered central pain processing in chronic low back pain: fact or myth? Clin J Pain. 2013;29(7):625–638. doi:10.1097/AJP.0b013e31826f9a71
6. Martel MO, Finan PH, Dolman AJ, et al. Self-reports of medication side effects and pain-related activity interference in patients with chronic pain: a longitudinal cohort study. Pain. 2015;156(6):1092–1100. doi:10.1097/j.pain.0000000000000154
7. Pingree MJ, Hurdle MF, Spinner DA, Valimahomed A, Crosby ND, Boggs JW. Real-world evidence of sustained improvement following 60-day peripheral nerve stimulation treatment for pain: a cross-sectional follow-up survey. Pain Manag. 2022;12(5):611–621. doi:10.2217/pmt-2022-0005
8. Nayak R, Banik RK. Current Innovations in Peripheral Nerve Stimulation. Pain Res Treat. 2018;2018:9091216. doi:10.1155/2018/9091216
9. Picaza JA, Cannon BW, Hunter SE, Boyd AS, Guma J, Maurer D. Pain suppression by peripheral nerve stimulation. Part II. Observations with implanted devices. Surg Neurol. 1975;4:1.
10. Mobbs RJ, Nair S, Blum P. Peripheral nerve stimulation for the treatment of chronic pain. J Clin Neurosci. 2007;14(3):216–21; discussion 222–3. doi:10.1016/j.jocn.2005.11.007
11. Lin T, Gargya A, Singh H, Sivanesan E, Gulati A. Mechanism of peripheral nerve stimulation in chronic pain. Pain Med. 2020;21(Suppl 1):S6–S12. doi:10.1093/pm/pnaa164
12. Kaye AD, Ridgell S, Alpaugh ES, et al. Peripheral nerve stimulation: a review of techniques and clinical efficacy. Pain Ther. 2021;10(2):961–972. doi:10.1007/s40122-021-00298-1
13. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–979. doi:10.1126/science.150.3699.971
14. Weiner RL, Reed KL. Peripheral neurostimulation for control of intractable occipital neuralgia. Neuromodulation. 1999;2(3):217–221. doi:10.1046/j.1525-1403.1999.00217.x
15. Popeney CA, Alo KM. Peripheral neurostimulation for the treat- ment of chronic, disabling transformed migraine. Headache. 2003;43(4):369–375. doi:10.1046/j.1526-4610.2003.03072.x
16. Monti E. Peripheral nerve stimulation: a percutaneous minimally invasive approach. Neuromodulation. 2004;7(3):193–196. doi:10.1111/j.1094-7159.2004.04195.x
17. Paicius RM, Bernstein CA, Lempert-Cohen C. Peripheral nerve field stimulation in chronic abdominal pain. Pain Physician. 2006;9(3):261–6.
18. Krutsch JP, McCeney MH, Barolat G, Al Tamimi M, Smolenski A. A case report of subcutaneous peripheral nerve stimulation for the treatment of axial back pain associated with postlaminectomy syndrome. Neuromodulation. 2008;11(2):112–115.
19. Falco FJE, Berger J, Vrable A, Onyewu O, Zhu J. Cross talk: a new method for peripheral nerve stimulation. An observational report with cadaveric verification. Pain Physician. 2009;12(6):965–983.
20. Reverberi C, Bonezzi C, Demartini L. Peripheral subcutaneous neurostimulation in the management of neuropathic pain: five case reports. Neuromodulation. 2009;12(2):146–155.
21. Lipov EG, Joshi JR, Sanders S, Slavin KV. Use of peripheral subcutaneous field stimulation for the treatment of axial neck pain: a case report. Neuromodulation. 2009;12(4):292–295.
22. Fiala KJ, Kim RB, Martens JM, Abd-Elsayed A. Lumbar level peripheral nerve stimulation for low back pain. Ochsner J. 2022;22:265–272.
23. Abd‐Elsayed A. Wireless peripheral nerve stimulation for treatment of peripheral neuralgias. Neuromodulation. 2020;23(6):827–830.
24. Abd-Elsayed A, D’Souza RS. Peripheral nerve stimulation: the evolution in pain medicine. Biomedicines. 2021;10(1):18.
25. Helm S, Shirsat N, Calodney A, et al. Peripheral nerve stimulation for chronic pain: a systematic review of effectiveness and safety. Pain Ther. 2021;10(2):985–1002.
26. Busch C, Smith O, Weaver T, Vallabh J, Abd-Elsayed A. Peripheral nerve stimulation for lower extremity pain. Biomedicines. 2022;10(7):1666.
27. Khan H, Pilitsis JG, Prusik J, Smith H, McCallum SE. Pain remission at one-year follow-up with spinal cord stimulation. Neuromodulation. 2018;21(1):101–105.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
