Back to Journals » Journal of Pain Research » Volume 15
Efficacy of Moxibustion Smoke for Stage 1 Post-Stroke Shoulder-Hand Syndrome: Protocol for a Multi-Center, Single-Blind Randomized Sham-Controlled Trial
Authors Meng X , Wang L, Li C, Gao S, Yu H, Zhang L, Sun J
Received 4 December 2021
Accepted for publication 17 February 2022
Published 3 March 2022 Volume 2022:15 Pages 643—653
DOI https://doi.org/10.2147/JPR.S351576
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Houman Danesh
Xiaonan Meng,1 Liping Wang,1 Chunying Li,1 Sen Gao,2 Haikuo Yu,3 Lufen Zhang,4 Jie Sun5
1Acupuncture Department, Beijing Huguosi TCM Hospital, affiliated with Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 2Rehablitation Department, Beijing Huguosi TCM Hospital, affiliated with Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 3Rehabilitation Department, Xuanwu Hospital Capital Medical University, Beijing, People’s Republic of China; 4School of Acupuncture-Moxibustion and Tuina, Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 5Rehabilitation Department of Integrated Chinese and Western Medicine, Beijing Xiaotangshan Hospital, Beijing, People’s Republic of China
Correspondence: Jie Sun, Rehabilitation Department of Integrated Chinese and Western Medicine, Beijing Xiaotangshan Hospital, No. 390 Spring Road, Changping District, Beijing, 102211, People’s Republic of China, Email [email protected]
Objective: This study aims to evaluate the safety and efficacy of various levels of moxibustion smoke concentration (MSC), represented by particulate matter 10mm (PM10), on pain and motor dysfunction in patients with stage 1 post-stroke shoulder-hand syndrome (SHS).
Materials and Methods: In this multi-center, sham-controlled, single-blind, randomized controlled trial (RCT), a total of 140 eligible patients with stage 1 post-stroke SHS will be recruited from March 2022 to February 2023 and randomly allocated to five groups in a ratio of 1:1:1:1:1. Moxibustion, in addition to standard medical care, will be applied to subjects in all groups. No acupoints on the affected upper limb will be utilized. Moxibustion smoke therapy, with varying levels of MSC, will be applied to the five groups as follows: (A) sham control group, (B) zero MSC group, (C) low MSC group, (D) medium MSC group, and (E) high MSC group. Patients in each group will be treated for 20 minutes per session, with five sessions each week, over a course of six weeks, with a total follow-up interval of eight weeks. The primary outcome measure will be a visual analog scale (VAS) assessment of the intensity of regionalized pain in the affected upper limb. Secondary outcome measures will include scoring on the Fugl-Meyer Assessment of the Upper Extremity Scale (FMA-UE), the Modified Barthel Index (MBI) and the measurement of somatosensory evoked potential (SEP). All participants will be evaluated before treatment, during treatment (ie, at two weeks and four weeks), immediately after concluding treatment (ie, at six weeks) and at two weeks post-treatment (ie, at eight weeks). Intention-to-treat analysis will be applied.
Trial Registration Number: ChiCTR2100043076.
Keywords: moxibustion smoke concentration, shoulder-hand syndrome, stroke, protocol, randomized controlled trial
A Letter to the Editor has been published for this article.
A Response to Letter has been published for this article.
Introduction
Stroke, as one of the main causes of death and disability worldwide, is the largest contributor to the loss of neurological disability-adjusted life-years (DALYs),1 and thus, is the cause of tremendous economic and social burdens for humanity.2,3 Post-stroke shoulder-hand syndrome (SHS) is a form of type 1 complex regional pain syndrome (also known as reflex sympathetic dystrophy), and is a common complication of cerebrovascular injury, with an incidence of up to 70%.4 This syndrome was first described by Oscar Stejnbroker,5,6 and is characterized by three stages: acute (stage 1), dystrophic (stage 2) and atrophic (stage 3). Stage 1 SHS manifests with regional pain, edema and motor dysfunction on the affected side.7–9 It is of crucial importance to prevent the progression of post-stroke SHS from stage 1 to stage 3, as the final stage is characterized by irreversible amyotrophy, muscle contracture and joint deformity, often resulting in total incapacitation of joint motor function.10 However, a lack of clearly defined guidelines regarding the definitive, evidence-based treatment for this condition means that the gold standard of treatment for SHS is still yet to be determined.11–13
Moxibustion is an important component of treatment according to the principles of Traditional Chinese Medicine (TCM), and it involves the non-invasive stimulation of acupoints through burning moxa floss—a dried and aged preparation of the plant Folium Artemisiae argyi—on or near the skin. For over 2500 years in China, moxibustion has served as a fundamental form of disease treatment and health cultivation, and its anecdotally documented effectiveness over the millennia has earned it an esteemed reputation that has been on par with that of Acupuncture since ancient times.14
The clinical applications of moxibustion are wide-ranging, and include the facilitation of post-stroke Rehabilitation through its anti-inflammatory and analgesic effects.9,15 Currently, one of the most popular methods of applying moxibustion is a technique known as indirect moxibustion, which involves warming the skin and underlying tissues with a stick of loosely packed moxa floss, which is lit and held with its smoldering end suspended over various acupuncture points at a relatively close distance. Indirect moxibustion is felt to be a relatively gentle and safe procedure that can generate clinically relevant, physiological effects through a variety of factors, including radiant heat stimulation, infrared radiation and the chemical effects of volatile oils and vaporized substances that are generated through the combustion of moxa floss, which are then both inhaled and cutaneously absorbed as precipitates that have deposited on the skin overlying the treated acupoints.16
Most recent studies with moxibustion have tended to focus on examining the clinical effects of heat generated from the combustion of moxa floss.16–20 However, our hypothesis is that the chemicals present in the smoke that is generated from burning the dried leaves of Folium Artemisiae argyi could also represent a key factor underlying the mechanism of therapeutic effects that are observed with moxibustion. However, as air pollution has become a global concern that threatens human health worldwide, the safety of moxibustion smoke (MS) has drawn increasing scrutiny, with at times fierce debates about its safety.21
At the present time, most studies evaluating MS involve basic science examinations of its chemical components, or experiments with animals, and few clinical trials with human participants have been published.22–28 Some components of MS, such as carbon monoxide (CO) and nitrogen oxides (NOx), are toxic to human beings.29 While several animal experiments have demonstrated both clinical efficacy and the safety of MS when utilized at carefully prescribed dosages, few clinical trials have been conducted to evaluate the clinical efficacy and safety of various MS concentrations (MSCs) when applied to human participants.22,30 Nevertheless, the titration of MSC could be pivotal for establishing the therapeutic and safety windows of moxibustion. One important caveat is that the various grades and ages of moxa floss could greatly impact its thermal behavior and smoke composition. According to clinical experience accumulated by doctors over many centuries in China, it is felt that Folium Artemisiae argyi that has been aged for at least three years is the most suitable material for moxibustion.31 The mass concentration of particulate matter (PM) generated with an aerodynamic diameter of less than 10 mm (PM10) has been evaluated in some studies in order to quantify MSC and evaluate the degree of air pollution produced during moxibustion.29,32 According to one noteworthy study conducted in Beijing and Tianjin, China, the average PM10 produced in a typical moxibustion treatment in China, using standard materials in an enclosed room, was observed to be 3.54 mg/m3.33
Given the dearth of published studies that have rigorously evaluated the questions outlined above, the clinical trial that we propose is designed to evaluate both the clinical efficacy and safety of MS, applied at various levels of MSC as quantified by PM10, for treating patients suffering from stage 1 post-stroke SHS, with the goal of alleviating pain and motor dysfunction. Accordingly, given the lack of evidence-based practice guidelines for delineating safe levels of MSC for clinical use with human participants, we will be beginning by examining significantly lower levels of MSC than those that are utilized in a typical moxibustion treatment in modern China.33
Methods
Study Design
This sham-controlled, single-blind, randomized controlled trial (RCT) will be conducted at Beijing Huguosi TCM Hospital, affiliated with Beijing University of Chinese Medicine (BUCM), and Beijing Xiaotangshan Hospital, with the assistance of Xuanwu Hospital Capital Medical University. All of these Hospitals are located in Beijing, China. This trial was registered on February 4, 2021 in the Chinese Clinical Trial Registry (ChiCTR2100043076), with its protocol being based on the Standard Protocol Items: Recommendations for Intervention Trials (SPIRIT) 2013 that is provided in the Supplementary File.34 The trial will be conducted in accordance with the Declaration of Helsinki and will take place from March 1, 2022 to June 30, 2023. After a thorough discussion of risks and benefits with the opportunity to ask questions about the study, and the signing of a written informed consent document, 140 eligible SHS patients will be recruited and randomly allocated into five groups in a 1:1:1:1:1 ratio (Figure 1): (A) sham control group, (B) zero MSC group, (C) low MSC group, (D) medium MSC group and (E) high MSC group.
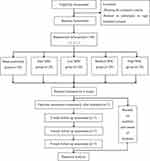 |
Figure 1 Study flow chart. Abbreviation: MSC, moxibustion smoke concentration. |
Participants
Diagnosis Criteria
Participants should meet all following criteria: (1) stroke diagnosed by cerebral computed tomography (CT) or magnetic resonance imaging (MRI); and (2) stage 1 post-stroke SHS, characterized by regional pain, edema and motor dysfunction.4,35
Inclusion Criteria
Participants must meet all of the following criteria: (1) onset of stage 1 post-stroke SHS symptoms within less than 90 days prior to beginning treatment in this trial, (2) a lifetime history of less than three strokes, (3) age 35–75 years, (4) clinically stable vital signs (ie, blood pressure of 100–140/60-90 mmHg, heart rate of 60–110 beats per minute, respiratory rate of 16–20 breaths per minute, pulse oximetry of 94–100% oxygen saturation on room air), and (5) full understanding of and agreement with the purpose of this study, as indicated through signing of the informed consent documents. There will be no enrollment restrictions based on gender or any other demographic factors, other than the criteria listed above.
Exclusion Criteria
Participants will be excluded if any of the following criteria are met: (1) diagnosis of any cerebral or cerebrovascular diseases other than stroke, such as cerebral aneurysm, arteriovenous malformation, CNS vasculitis, cavenous malformation, venous sinus thrombosis, amyloid angiopathy, cerebral carcinoma, neurodegenerative diseases, CNS infection, dysautonomia, and primary epilepsy, or any instance of hemodynamic instability, (2) a lifetime history of three or more strokes, (3) any serious psychiatric disorders that may or may not have been caused by stroke, (4) thalamic allodynia or hyperesthesia leading to an abnormally distorted pain threshold, (5) dislocation or subluxation of the shoulder joint or any regional soft tissue injuries sustained prior to stroke, (6) active pulmonary infection, MS intolerance, or any past medical history of respiratory diseases, such as chronic obstructive pulmonary disease (COPD), pulmonary interstitial fibrosis (PIF) or allergic asthma, (7) refusal to sign informed consent documents.
Recruitment
Participants will be recruited through advertisement of trial recruitment on the official websites, WeChat social media accounts and bulletin boards of the Acupuncture and Rehabilitation Departments of Beijing Huguosi TCM Hospital and Beijing Xiaotangshan Hospital. Contact information, including e-mail addresses, phone numbers and WeChat accounts of the investigators will be made available to all potential participants. Those who meet all of the inclusion criteria and agree to sign the informed consent documents will be officially enrolled in this study (Table 1).
 |
Table 1 Study Schedule for Timepoints |
Procedures of Randomization and Allocation
A randomization sequence based on a random number table will be generated by an independent researcher and sealed in an opaque envelope. Eligible participants will be randomly allocated into groups A, B, C, D or E, in a ratio of 1:1:1:1:1. After baseline assessment and signing informed consent documents, the envelopes containing the randomization numbers for each participant will be unsealed by research assistants, and participants will be informed of their individual randomization numbers. The investigators providing experimental treatment will be informed of the group information through the WeChat social media platform. In order to prevent the intentional or unintentional crossover of participants among the groups, a backup copy of the randomization sequence will be stored by the Clinical Research Monitoring Department (CRMD) of Beijing Huguosi TCM Hospital.
Blinding
Given that this study will be an interventional RCT, investigators providing clinical intervention must be informed of nature of the allocation in order to perform the appropriate moxibustion smoke procedure. However, all participants will remain blinded to the nature of their treatment allocation throughout the course of the study. The Happy-all Moxibustion Device (manufactured by the Chongqing Happy-all Medical Device Co., Ltd) will be utilized in this trial. Nineteen ventilation holes are present at the base of this device, which allows the skin and its underlying acupoints to be bathed in MS and heat as the base of the apparatus rests on the body. For the sham control group, an aluminum foil and high silicon glass fiber insulation layer will be placed inside of the device, in order to fully occlude the MS ventilation holes to prevent smoke contact with the skin, and to limit the transmission of radiant heat (Figure 2B). Additionally, instead of conventional moxa, smokeless charcoal moxa will be placed inside of the device and kept hidden from view, so that these sham devices will appear externally identical to the verum devices applied in the treatment groups (Figure 2A). The rehabilitation physicians evaluating treatment outcomes, and the statisticians analyzing the outcomes, will also be blinded to the allocation.
 |
Figure 2 The exterior view (A) and the interior view (B) of moxibustion devices. |
Interventions
Throughout the study, we will closely monitor participants’ vital signs and cardiovascular risk factors, while continuing their medications and standard medical treatment for related conditions such as hypertension, hyperlipidemia, diabetes mellitus, atrial fibrillation and hyperhomocysteinemia. In order to avoid the confounding the effects of acupuncture with moxibustion and MS, acupuncture treatment will not be administered in this study.
All participants will be hospitalized on the inpatient ward, and will each be treated, one at a time, in a room with dimensions of 5.1 m (Length) × 3.5 m (Width) × 3.1 m (Height). The average indoor temperature and humidity will be continuously monitored using the thermometer and hygrometer functions of a weather meter (Kestrel NK3000, USA) and maintained within a temperature range of 24 ± 2°C and a relative humidity range of 50 ± 4%, respectively. The treatment facilities have had no interior remodeling performed over the past four years, which should minimize the presence of any additional volatile chemical substances in the air. Only one physician or nurse will be present in the room with each participant throughout the course of moxibustion. After completing each intervention, the participant and healthcare provider will vacate the room immediately, and all remaining MS will be removed from the ambient air through ventilation. All moxa floss utilized in this study will have been aged for five years.
As noted above, in the sham control group, four Happy-all Moxibustion Devices will be prepared with an insulation layer that fully occludes the nineteen smoke ventilation holes at the base of the device. One stick of smokeless charcoal moxa will be lit and placed inside of each device, and then the four devices will be affixed using adhesive to four sham points, each being one cun (acupuncture measurement unit) away from each verum acupoint listed in Table 2. In contrast, in each of the zero MSC, low MSC, medium MSC and high MSC groups, four unmodified Happy-all Moxibustion Devices will be utilized, with all of the nineteen ventilation holes left open at the base, to allow for unobstructed air and smoke exchange with the underlying skin. These four devices will be affixed to the four verum acupoints listed in Table 2, with a variety of conventional and smokeless charcoal moxa combinations as listed below.
 |
Table 2 The Acupuncture Points and Locations |
In the zero MSC group, all four devices will each contain one lit stick of smokeless charcoal moxa. In the low MSC group, only one device will contain one lit stick of conventional moxa floss, whereas the other three devices will each contain one lit stick of smokeless charcoal moxa. In the medium MSC group, two devices will each contain one lit stick of conventional moxa, and two devices will each contain one lit stick of smokeless charcoal moxa. In the high MSC group, all four devices will each contain one lit stick of conventional moxa. Each moxibustion treatment will last for 20 minutes per session, with 5 weekly sessions being conducted over the course of 6 weeks.
Air ventilation will be controlled by leaving only one window slightly ajar, to ensure that the PM10 concentration in the room remains stable throughout the intervention. During moxibustion for all five groups, the PM10 concentrations of MS will be sampled and recorded using a portable microcomputer dust monitor (Model No. LD-5C [B], made by Beijing Lvlin Innovation Digital Technology Co., Ltd) that will be positioned 10 cm away from the participant’s nose, where any smoke present will also be directly inhaled by each participant. According to our pre-test results, the average PM10 concentrations for MSC in five groups should be projected to be (A) 0.03 ± 0.01 mg/m3, (B) 0.04 ± 0.01 mg/m3, (C) 0.92 ± 0.34 mg/m3, (D) 1.65 ± 0.57 mg/m3 and (E) 3.04 ± 0.74 mg/m3, respectively.
Outcome Measurements
Primary Outcome
The VAS score for pain intensity will be designated as the primary outcome, since the pain is the most common and distressing symptom of SHS, and since it contributes to further exacerbation of regional edema and motor dysfunction.4,36,37 A 100-mm horizontal line will be used for the VAS measurement, with its left and right extremes delineating “0” (no pain) and “10” (unbearable pain). Participants will mark their scores along the straight line using a pen, based on their subjective experience of pain.
Secondary Outcome
The Fugl-Meyer Assessment of the Upper Extremity Scale (FMA-UE) will be utilized to quantify motor and non-motor function on the affected side.38 This scoring system consists of 33 items, with 0–2 points counted for each item, resulting in a total score of 66 points.
The Modified Barthel Index (MBI), a functional measurement for stroke patients, will be applied to evaluate ability to independently perform activities of daily living (ADL).39–41 In contrast to the original Barthel Index (BI) rating system, the modified index consists of a five-point scale, with a total score of 100 points.
Somatosensory evoked potentials (SSEPs), utilized as an indicator of functional recovery of post-stroke patients,42,43 will be measured by an electromyograph system (Model: Viking Quest, Nicolet, US). Participants will be asked to rest supine in bed, with an ambient room temperature of 23–25°C, and with a skin temperature at least 32°C. An electrode will be affixed over the contralateral somatosensory area known as Cc’ (2 cm behind C3 or C4), and the reference electrode will be placed at Fz, according to the International 10–20 System for scalp electrode placement.44,45 The median nerve at the affected wrist will be stimulated with constant current square wave pulses of 0.1–0.2 ms duration, at a frequency of 2–3 Hz, in order to induce a visible thumb twitch. The overall band pass width will be 20–3000 Hz, with a 50 ms analysis timeframe, two hundred responses, and two repetitions for each participant. The potential and N20 amplitude will be recorded.
Safety Monitoring
During the course of the clinical trial, any adverse events (AEs) that could possibly be related to the moxibustion therapy, such as allergies, burns, infections, cough, asthma or nausea, will be carefully monitored, recorded and categorized into three levels: mild (ie, tolerable and temporary symptoms), moderate (ie, unpleasant symptoms affecting the participant’s daily quality of life, but with no impact on the clinical trial’s proceedings) and severe (ie, hemodynamic instability or other complications that compel the participant to withdraw from the study and receive emergent higher level medical treatment).20 Standard, evidence-based medical care will be immediately provided to address any AEs that do occur, and the total occurrence ratio of AEs will be analyzed and reported at the conclusion of the trial.
Attrition
Any discontinuation of participation occurring during the course of this clinical trial will be closely monitored and recorded using case report forms (CRFs), including the date(s) and reason(s) for withdrawal.
Sample Size Calculation
As noted above, the VAS for pain intensity will serve as the primary outcome measure of post-stroke SHS treatment in this clinical trial.46–48 To date, there do not yet exist any previously published clinical trials that have examined the effects of moxibustion applied at tiered MSC levels for treating post-stroke SHS. Based on our pre-test analysis, following six weeks of moxibustion treatment, the VAS reduction in pain (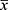 ±s) across five groups (A-E) was 1.89 ± 0.91, 2.01 ± 0.97, 2.23 ± 0.99, 3.01 ± 1.23, and 2.97 ± 1.14, respectively. The PASS (Power Analysis and Sample Size) software program, version 15.0, was utilized for sample size calculation. According one-way analysis of variance (ANOVA), with a significance level of α = 0.05, β = 0.20 with a power of 80% (1 – β), planning for equal numbers of participants among all groups, and assuming a 10% dropout rate, the minimum sample size was calculated to be 20 participants in each of the five groups. The final sample size was then expanded to a total of n = 140 participants, or 28 participants in each group.
±s) across five groups (A-E) was 1.89 ± 0.91, 2.01 ± 0.97, 2.23 ± 0.99, 3.01 ± 1.23, and 2.97 ± 1.14, respectively. The PASS (Power Analysis and Sample Size) software program, version 15.0, was utilized for sample size calculation. According one-way analysis of variance (ANOVA), with a significance level of α = 0.05, β = 0.20 with a power of 80% (1 – β), planning for equal numbers of participants among all groups, and assuming a 10% dropout rate, the minimum sample size was calculated to be 20 participants in each of the five groups. The final sample size was then expanded to a total of n = 140 participants, or 28 participants in each group.
Statistical Analysis
Statistical calculations will be performed using the SPSS software program (Windows version 21.0) with intention-to-treat analysis. All baseline characteristics, such as gender, will be analyzed by chi-squared or Fisher’s exact test. Given that patients suffering from post-stroke SHS may exhibit intra-group fluctuations in outcomes as a result of differences in variables such as age, time of symptom onset, courses of illness and associated complications, the participants’ initial continuous variable index value will be set as a covariate. Continuous variables, reported as mean ± standard deviation (SD) for each group, will be calculated using analysis of covariance (ANCOVA). The confidence interval (CI) will be set at 95%, with a significance level of 5% (p < 0.05).
Quality Control
The final version of this clinical trial protocol was developed in consultation with specialists in the related fields of acupuncture, neurology, rehabilitation medicine and statistics. All researchers participating in this study will undergo requisite training regarding study protocol, recruitment, moxibustion procedures, data collection and assessment of clinical efficacy. All recorded clinical data will be closely monitored by CRMD of Beijing Huguosi TCM Hospital, which is an independent entity that thus does not involve any conflicts of interest. All clinical documents, including baseline data, intervention methods, outcome assessments, follow-up assessments, AEs and participant withdrawals will be recorded using CRFs. Any subsequent changes to the original data documented on the paper CRFs must be justified in writing, approved by the CRMD and documented as authorized data revisions. Only senior physicians of Chinese Medicine with at least five years of clinical experience in practicing acupuncture and moxibustion will be permitted to perform the moxibustion interventions, and only senior rehabilitation physicians with at least five years of clinical experience in rehabilitation medicine will be permitted to perform outcome measurements.
Discussion
Post-stroke SHS, with its high incidence and disability rates, is a significant threat to the health, wellbeing and quality of life of patients who have suffered a stroke.12 Research has demonstrated clinical effectiveness of acupuncture and moxibustion as potential treatments for SHS.9,49 However, MS itself has not yet been studied in depth as one of the potential underlying factors that may drive the clinical effects of moxibustion therapy.
The safety of MS is one major area of concern, and involves factors such as the level of MSC and duration of exposure. The chemical composition of MS may vary at different levels of MSC, and with concerns about environmental pollution rising in recent years, the total volatile organic compounds (TVOC) and PM10 levels in MS, as well as the presence of specific molecules such as CO2, CO, formaldehyde (CH2O), have come under increasing levels of scrutiny.20,50 On the other hand, molecular analysis of the effects of moxibustion, particularly in animal studies, has also been increasingly demonstrating its beneficial mechanisms of action from a biochemical standpoint. One study with APP/PS1 double transgenic mice expressing a chimeric mouse/human amyloid precursor protein that had induced symptoms of Alzheimer’s disease (AD) showed that, at a concentration of 10–15 mg/m3, MS appeared to improve the cognitive and behavioral function of the afflicted mice through modulating the tricarboxylic acid cycle and fatty acid metabolism.22 Another murine study that evaluated different levels of MSC also showed positive effects of MS on monoamine neurotransmitters function at a low MSC of 5–15 mg/m3, a medium MSC of 25–35 mg/m3, and a high MSC of 85–95 mg/m3.25 A study that was conducted to determine the toxic threshold of MS found that medium (168.76 mg/m3) and high (384.67 mg/m3) levels of MSC adversely affected murine pulmonary function as measured via spirometry, which remained unaffected at low levels of MSC (27.45 mg/m3), a concentration level that is already 775% higher than the average MSC of 3.54mg/m3 that has been recorded during typical moxibustion treatments for human patients.33 A study of patients and staff working in Chinese medicine clinics in Taiwan found that ongoing exposure to airborne components of moxibustion smoke in treatment rooms and waiting areas may slightly increase the risk of adverse health issues, although a critical question that remains is how measures such as robust ventilation and the regular use of air purification devices in these spaces, as well as N95 or other medical grade masks worn by staff and patients, might help eliminate the tiny amount of risk that was noted in this study.51 On the other hand, one trial examining moxibustion as treatment for osteoarthritis of the knee observed no significant differences in outcomes between conventional moxibustion and moxibustion applied with an air purification device placed near the moxibustion tool.52 However, lack of clarity in terms of the placement of the air purification and particulate measurement devices, makes it difficult to interpret how much clinically relevant moxibustion smoke was actually present in the room, able to contact and precipitate on the patients’ skin, and available to be inhaled by the participants, so a number of questions regarding the clinically effective and safe amounts of MSC remain at this time.
Given the lack of a definitive standard for the evidence-based safety range of MSC at this time, our clinical trial will cautiously employ a tiered system of lower MSC levels: 0.03 ± 0.01 mg/m3 (sham control group), 0.04 ± 0.01 mg/m3 (zero MSC), 0.92 ± 0.34 mg/m3 (low MSC) and 1.65 ± 0.57 mg/m3 (medium MSC) and 3.04 ± 0.74 mg/m3 (high MSC), with the highest MSC group being set at a concentration that is 17.7% lower than the average MSC of 3.54mg/m3 that has been noted during a typical moxibustion treatment session, as mentioned above.33
Regarding the factor of time, the age of moxa floss may also greatly impact the variety and ratio of chemical components that are produced in its smoke. Moxa floss that has been aged for at least three years is preferred because crossing this time threshold appears to produce the most favorable thermal profile. In this clinical trial, moxa floss that has been aged for five years will be utilized, in order to ensure an even higher standard of quality and safety.31 Few studies have been published that address the ideal duration of exposure to MS. One study found that exposure to moxibustion at a MSC level of 2.5 ± 0.5 mg/m3 twice in one week, for 25 minutes per session, induced no significant adverse effects on heart rate or heart rate variability in young, healthy adults.53 A cross-sectional survey of 803 acupuncturists in China who regularly practice moxibustion treatment, using the American Thoracic Society Division of Lung Disease questionnaire (ATS-DLD-78-A), showed that long-term, regular exposure to MS did not appear to have a significant causal relationship with respiratory diseases and symptoms in these practitioners.54 Therefore, in this clinical trial, we have decided to set the moxibustion treatment duration at 20 minutes, with a treatment frequency of 5 sessions per week, over the course of 6 weeks. And given that the PM10 concentration of moxibustion smoke may vary at different distances from the locus of combustion, the portable microcomputer dust monitor will be placed 10 cm away from each participant’s nose, in order to more accurately assess the PM10 concentration that is actually inhaled by each individual.
A large number of prospective clinical trials are needed to definitively elucidate the safe and clinically effective concentration ranges of MS, as well as the underlying mechanisms of action for the therapeutic effects of moxibustion and MS. This trial will aim to advance the field through providing clinical evidence regarding the possible effects of MS at different levels of MSC as used in treating patients suffering from post-stroke SHS. Therefore, it is our hope that this study will not only serve as a foundation for clarifying the safety and clinical efficacy of moxibustion when used for treating this particular patient population, but that it will also yield groundbreaking results regarding MSC that will have broadly generalizable implications for setting safety guidelines for the use of moxibustion in the service of the rest of humankind as well.
Trial Status
Participant enrollment has been postponed from July 2021 to March 2022, as a result of restrictions and regulations related to the ongoing COVID-19 pandemic. Initial enrollment is expected to begin on March 1, 2022.
Study Limitations
Out of an abundance of caution, and given the sparseness of definitive evidence regarding the therapeutic effects and safety of MS at different levels of MSC, this study will initially utilize only three tiered levels of MSC, that are all markedly lower than the average MSC level that has previously been reported in standard moxibustion treatments with human patients in China. Additionally, this clinical trial will be carried out in only two hospitals with a very specific patient population and a limited sample size, which may affect the applicability of its clinical results to the general human population worldwide. Additionally, long-term data on the clinical efficacy and safety of moxibustion and MS for post-stroke SHS will not be reported beyond the eight-week timeframe that has been designated for this study.
Data Sharing Statement
All raw data will be made available upon reasonable request.
Ethics and Dissemination
This clinical trial is registered in the Chinese Clinical Trial Registry and approved by the Medical Ethics Committees of Beijing Huguosi TCM Hospital, affiliated with BUCM (No. 2021-01) and Beijing Xiaotangshan Hospital (No. 2021-44). Participants will be fully informed of the trial details, with the opportunity to ask questions prior to signing the informed consent forms. All participants will have the right to freely withdraw from the study for any reason, at any time, without limitations. The results of the clinical trial will be presented for publication in a peer-reviewed, open-access medical journal.
Acknowledgments
The authors thank David J. Seto, M.D. for his suggestions, additions and extensive revisions of the English manuscript.
Author Contributions
All authors made significant contributions to the work reported, including in the areas of conception, study design, execution, data acquisition, analysis and interpretation, drafting, revising and critically reviewing the article, or in all of these areas. All authors approved the final version of this article and the journal to which it was submitted for publication, and agree to be held accountable for all aspects related to this work of scholarship.
Funding
This study was funded by the 2021 Beijing Xicheng District Talents Funding Project (No. 2021-XCRC) and the Beijing Medical Science and Technology Development Fund Project (No. QN2018-23).
Disclosure
All authors declare no conflicts of interest for this work.
References
1. Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38(2):208–211.
2. Global, regional, and national mortality among young people aged. 10-24 years, 1950-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2021;398(10311):1593–1618.
3. Gershwin ME. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurology. 2021.
4. Pertoldi S, Di Benedetto P. Shoulder-hand syndrome after stroke. A complex regional pain syndrome. Eura Medicophys. 2005;41(4):283–292.
5. Thompson M. Shoulder-hand syndrome. Proc R Soc Med. 1961;54(8):679–681.
6. Steinbrocker O. The shoulder-hand syndrome: present perspective. Arch Phys Med Rehabil. 1968;49(7):388–395.
7. Borchers AT, Gershwin ME. Complex regional pain syndrome: a comprehensive and critical review. Autoimmun Rev. 2014;13(3):242–265.
8. Hannan MA, Sabeka MM, Miah MBA. Shoulder hand syndrome in hemispheric stroke. J Neurol Sci. 2013;2:333.
9. Lee MS, Shin BC, Kim JI, Han CH, Ernst E. Moxibustion for stroke rehabilitation: systematic review. Stroke. 2010;41(4):817–820.
10. Yamanaka H, Yamanaka H. [Relationship between cutaneous temperature and hand edema and allodynia after stroke–the etiology of shoulder-hand syndrome]. Rinsho Shinkeigaku. 2015;55(1):1–7. Japanese.
11. Kondo I, Hosokawa K, Soma M, Iwata M, Maltais D. Protocol to prevent shoulder-hand syndrome after stroke. Arch Phys Med Rehabil. 2001;82(11):1619–1623.
12. Berthelot JM. Current management of reflex sympathetic dystrophy syndrome (complex regional pain syndrome type I). Joint Bone Spine. 2006;73(5):495–499.
13. Pandian J, Sebastian I. Integrated approach to stroke burden: are we doing enough? Lancet Neurology. 2021.
14. Huang C, Liang J, Han L, Liu J, Yu M, Zhao B. Moxibustion in Early Chinese Medicine and Its Relation to the Origin of Meridians: a Study on the Unearthed Literatures. Evid Based Complemen Alternative Med. 2017;2017:8242136.
15. Liu Y, Chen W, Tan Y, et al. Analysis of the Registration Information on Interventions of Acupuncture and Moxibustion Trials in the International Clinical Trials Registry Platform. Evid Based Complemen Alternative Med. 2018;2018:1054629.
16. Sun C, Li Y, Kuang J, Liang X, Wu J, Ji C. The thermal performance of biological tissue under moxibustion therapy. J Therm Biol. 2019;83:103–111.
17. Zhao L, Cheng K, Wang L, et al. Effectiveness of moxibustion treatment as adjunctive therapy in osteoarthritis of the knee: a randomized, double-blinded, placebo-controlled clinical trial. Arthritis Res Ther. 2014;16(3):R133.
18. Gadau M, Yeung W, Liu H, et al. Acupuncture and moxibustion for lateral elbow pain: a systematic review of randomized controlled trials. BMC Complement Altern Med. 2014;14:136.
19. Deng H, Shen X. The mechanism of moxibustion: ancient theory and modern research. Evid Based Complement Alternat Med. 2013;2013:379291.
20. Xu J, Deng H, Shen X. Safety of moxibustion: a systematic review of case reports. Evid Based Complement Alternat Med. 2014;2014:783704.
21. Lim M, Huang J, Zhao B. Standardisation of moxibustion: challenges and future development. Acupuncture Med. 2015;33(2):142–147.
22. Ha L, Yu M, Yan Z, Rui Z, Zhao B. Effects of Moxibustion and Moxa Smoke on Behavior Changes and Energy Metabolism in APP/PS1 Mice. Evid Based Complement Alternat Med. 2019;2019:9419567.
23. He R, Han L, Liu P, et al. Lung Function Decline after 24 Weeks of Moxa Smoke Exposure in Rats. Evid Based Complement Alternat Med. 2019;2019:9236742.
24. Huang J, Lim MY, Zhao B, Shao L, Lao L. PM2.5 and ash residue from combustion of moxa floss. Acupunct Med. 2016;34(2):101–106.
25. Xu H, Zhao B, Cui Y, et al. Effects of Moxa Smoke on Monoamine Neurotransmitters in SAMP8 Mice. Evid Based Complement Alternat Med. 2013;2013:178067.
26. Kwon OS, Cho SJ, Choi K-H, et al. Safety Recommendations for Moxa Use Based on the Concentration of Noxious Substances Produced during Commercial Indirect Moxibustion. Acupuncture Med. 2017;35(2):93–99.
27. Ouyang X, Duan H, Jin Q, et al. Moxibustion may delay the aging process of Wistar rats by regulating intestinal microbiota. Biomed Pharmacother. 2021;2:112147.
28. Yang J, Zheng X, Jin R, et al. Effect of moxa smoke produced during combustion of Aiye (Folium Artemisiae Argyi) on behavioral changes in mice inhaling the smoke. J Traditional Chin Med. 2016;36(6):805–811.
29. Mo F, Chi C, Guo M, Chu X, Li Y, Shen X. Characteristics of selected indoor air pollutants from moxibustion. J Hazard Mater. 2014;270:53–60.
30. Han L, Liu C, Sun W, et al. Twelve-week study of moxa smoke: occupational exposure in female rats. J Traditional Chin Med. 2019;39(2):207–212.
31. Lim M, Zhang X, Huang J, et al. Study of Thermal Behavior of Moxa Floss Using Thermogravimetric and Pyrolysis-GC/MS Analyses. Evid Based Complemen Alternative Med. 2021;2021:6298565.
32. Kan H, London S, Chen G, et al. Differentiating the effects of fine and coarse particles on daily mortality in Shanghai, China. Environ Int. 2007;33(3):376–384.
33. Huang C, Zhao B, Liu P, et al. Mass concentration and morphological characteristics of PM10 in moxibustion rooms in Beijing and Tianjin during summer. China J of Trad Chin Med & Pharma. 2012;27(12):3104–3108. Chinese
34. Chan A, Tetzlaff J, Gøtzsche P, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586.
35. Frank A. The latest national clinical guideline for stroke. Clin Med. 2017;17(5):478.
36. Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019;160(1):19–27.
37. Peng L, Zhang C, Zhou L, Zuo HX, He XK, Niu YM. Traditional manual acupuncture combined with rehabilitation therapy for shoulder hand syndrome after stroke within the Chinese healthcare system: a systematic review and meta-analysis. Clin Rehabil. 2018;32(4):429–439.
38. Gladstone DJ, Danells CJ, Black SE. The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16(3):232–240.
39. Mahoney FI, Barthel DW. FUNCTIONAL EVALUATION: THE BARTHEL INDEX. Md State Med J. 1965;14:61–65.
40. Shah S, Vanclay F, Cooper B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42(8):703–709.
41. Ohura T, Hase K, Nakajima Y, Nakayama T. Validity and reliability of a performance evaluation tool based on the modified Barthel Index for stroke patients. BMC Med Res Methodol. 2017;17(1):131.
42. Al-Rawi MA, Hamdan FB, Abdul-Muttalib AK. Somatosensory evoked potentials as a predictor for functional recovery of the upper limb in patients with stroke. J Stroke Cerebrovasc Dis. 2009;18(4):262–268.
43. Han EY, Jung HY, Kim MO. Absent median somatosensory evoked potential is a predictor of type I complex regional pain syndrome after stroke. Disabil Rehabil. 2014;36(13):1080–1084.
44. Jurcak V, Tsuzuki D, Dan I. 10/20, 10/10, and 10/5 systems revisited: their validity as relative head-surface-based positioning systems. NeuroImage. 2007;34(4):1600–1611.
45. Klem G, Lüders H, Jasper H, Elger C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:3–6.
46. Liu S, Zhang CS, Cai Y, et al. Acupuncture for Post-stroke Shoulder-Hand Syndrome: a Systematic Review and Meta-Analysis. Front Neurol. 2019;10:433.
47. Ju ZY, Wang K, Cui HS, et al. Acupuncture for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;12:CD012057.
48. Zhao H, Nie W, Sun Y, et al. Warm Needling Therapy and Acupuncture at Meridian-Sinew Sites Based on the Meridian-Sinew Theory: hemiplegic Shoulder Pain. Evid Based Complement Alternat Med. 2015;2015:694973.
49. Chau JPC, Lo SHS, Yu X, et al. Effects of Acupuncture on the Recovery Outcomes of Stroke Survivors with Shoulder Pain: a Systematic Review. Front Neurol. 2018;9:30.
50. Lu CY, Kang SY, Liu SH, Mai CW, Tseng CH. Controlling Indoor Air Pollution from Moxibustion. Int J Environ Res Public Health. 2016;13(6):34.
51. Hsu Y, Chao H, Shih S. Human exposure to airborne aldehydes in Chinese medicine clinics during moxibustion therapy and its impact on risks to health. J Environ Sci Health a Tox Hazard Subst Environ Eng. 2015;50(3):260–271.
52. Luo L, Liao M, Peng J, et al. Comparison of the Efficacy between Conventional Moxibustion and Smoke-Free Moxibustion on Knee Osteoarthritis: a Randomized Controlled Trial. Evid Based Complemen Alternative Med. 2019;2019:1291947.
53. Cui Y, Zhao B, Huang Y, et al. Effects of Moxa (Folium Artemisiae argyi) Smoke Exposure on Heart Rate and Heart Rate Variability in Healthy Young Adults: a Randomized, Controlled Human Study. Evid Based Complement Alternat Med. 2013;2013:510318.
54. Yu C, Zhang N, Zhu W, et al. Does Moxa Smoke Have Significant Effect on the Acupuncturist’s Respiratory System? A Population-Based Study. Evid Based Complement Alternat Med. 2019;2019:4873235.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
