Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 12
Efficacy of long-term noninvasive positive pressure ventilation in stable hypercapnic COPD patients with respiratory failure: a meta-analysis of randomized controlled trials
Authors Liao H , Pei W, Li H, Luo Y, Wang K, Li R , Xu L, Chen X
Received 5 August 2017
Accepted for publication 5 September 2017
Published 10 October 2017 Volume 2017:12 Pages 2977—2985
DOI https://doi.org/10.2147/COPD.S148422
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chunxue Bai
Video abstract presented by Hao Liao, Wendi Pei and Hongfu Li.
Views: 351
Hao Liao,* Wendi Pei,* Hongfu Li,* Yuwen Luo, Kai Wang, Rui Li, Limei Xu, Xin Chen
Department of Respiratory Medicine, Zhujiang Hospital, Southern Medical University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Introduction: The efficacy of long-term noninvasive positive pressure ventilation (NPPV) in stable hypercapnic COPD patients with respiratory failure remains unclear. The aim of this meta-analysis was to critically assess the efficacy of long-term NPPV on mortality, acute exacerbation, exercise capacity, symptoms and significant physiological parameters (lung function, respiratory muscle function and gas exchange).
Patients and methods: We performed an electronic literature search using the PubMed, Cochrane Library, Embase, OVID and Chinese Biomedical Literature Database in May 2017. Studies comparing treatment effects of NPPV with oxygen therapy in stable hypercapnic COPD patients with respiratory failure were conducted, and at least one of the following parameters were reviewed: frequency of acute exacerbation, mortality, lung function, respiratory muscle function, gas exchange, exercise capacity.
Results: Seven studies with 810 subjects were identified. The partial pressure of arterial carbon dioxide (PaCO2) significantly decreased in patients who received long-term NPPV (weighted mean difference [WMD] −3.73, 95% CI: −5.83 to −1.64, P=0.0005). No significant difference was found in mortality, partial pressure of arterial oxygen (PaO2), frequency of acute exacerbation, lung function, respiratory muscle function and exercise capacity. The subgroup analysis showed that NPPV significantly improved the survival of patients when it was targeted at greatly reducing hypercapnia (WMD 0.35, 95% CI: 0.19 to 0.64, P=0.0006).
Conclusion: The results indicate that long-term NPPV decreases the PaCO2 of stable hypercapnic COPD patients with respiratory failure and improves mortality with the aim of reducing PaCO2.
Keywords: COPD, meta-analysis, noninvasive positive pressure ventilation, mortality, gas change
Introduction
COPD is the fourth fatal cause worldwide;1 it has been predicted by the World Health Organization that COPD would become the third by 2030.2 Airflow limitation and alveolar hypoventilation can damage the arterial oxygen exchange function of COPD patients.3 As disease gets worse, hypoxemia and hypercapnia caused by progressing peripheral airway obstruction contribute to the decreasing pulmonary gas exchange ability, destruction of pulmonary parenchyma and abnormality of pulmonary vessels, which increases the frequency of acute exacerbation. Patients with chronic respiratory failure and hypercapnia have severe dyspnea, lower quality of life and even increased mortality.4
Long-term oxygen therapy (LTOT) has already been demonstrated to improve hypoxemia, dyspnea, life quality and survival.5 Noninvasive positive pressure ventilation (NPPV) could improve gas exchange function by increasing tidal volume and producing positive airway pressure;6 relax and relieve respiratory muscle fatigue7 and improve respiratory center’s sensitivity to CO2.8 Besides, NPPV could be associated with lower hospitalization rates.9
Despite the abovementioned positive elements, randomized controlled trials (RCTs) have not achieved an agreement about applying long-term NPPV to treating stable COPD patients with respiratory failure so far. In 2014, a meta-analysis by Struik et al10 failed to evaluate the mortality, the frequency of acute exacerbation and the hospitalization rate due to the lack of experiment support. In recent years, several new large RCTs have evaluated the potential efficacy of long-term NPPV on stable COPD patients with respiratory failure, especially in mortality and acute exacerbation.11,12
We conducted a meta-analysis for the update of current literature. The aim of this meta-analysis was to evaluate the effect of long-term NPPV on stable hypercapnic COPD patients with respiratory failure in mortality, acute exacerbation, exercise capacity, symptoms and significant physiological parameters (lung function, respiratory muscle function and gas exchange).
Patients and methods
Literature search
We conducted a comprehensive search based on the Cochrane Handbook (version 5.1.0) by searching the PubMed, Embase, Cochrane Library, OVID and Chinese Biomedical Literature Database for RCTs in humans through May 2017. The following words were used as key words: chronic obstructive pulmonary disease OR COPD AND noninvasive positive pressure ventilation OR NPPV OR NIV OR NIPPV AND routine treatment OR long-term oxygen therapy OR LTOT. Additionally, we performed a review of the reference lists of recognized studies, and when necessary a supplementary search of relevant studies was conducted.
Selection of studies and data collection
Our findings included studies that met the following criteria: 1) RCT of adults (>18 years); 2) stable COPD patients diagnosed according to Global Initiative for Chronic Obstructive Lung Disease 2017: the diagnosis of COPD was based on appropriate symptoms (such as dyspnea, chronic cough and sputum production), significant exposures to noxious stimuli (such as tobacco and pollution) and the presence of a post-bronchodilator forced expiratory volume in 1 second to forced vital capacity ratio (FEV1/FVC) <0.70;13 3) associated with chronic type II respiratory failure (diagnostic criteria: partial pressure of arterial oxygen [PaO2] <60 mmHg and partial pressure of arterial carbon dioxide [PaCO2] >50 mmHg);14 4) NPPV was used at least 5 hours per day for at least 3 months in the intervention group, while control group received the same management except NPPV and 5) measurement of hospital admission, mortality, lung function, respiratory muscle function, gas exchange and exercise capacity.
A prespecified protocol was used to extract data from included studies by two reviewers independently. In this process, the following study characteristics were collected: study type, first author, location, publication date, study design, sample size and outcomes. Differences in opinion between the two reviewers were settled by discussion and team consensus.
Risk of bias assessment
A methodological quality and risk of bias assessment of included studies was performed by the two reviewers independently. An approach based on the Cochrane Risk of Bias Tool that evaluated studies as high, low or unclear risk was used.15 Divergences between the two reviewers in particular studies were settled by discussion.
Statistical analysis
We used Review Manager (version 5.3.5) to pool the results and assess the treatment efficacy. A random efficacy model was used in the case of significant heterogeneity between the studies. A relative risk (RR) with 95% confidence interval (95% CI) was computed for binary variables and for consecutive variables; a weighted mean difference (WMD) with 95% CI was calculated.
Both the Cochrane Q test and I2 statistic were used to assess heterogeneity across study outcomes.16 For Cochrane Q test, significant heterogeneity was considered with a P-value of <0.1. For I2 statistic, I2<25%, 25%≤I2<50% or I2≥50% corresponds to low, moderate or high significance of heterogeneity, respectively.17
Results
Study inclusion and characteristics
The process of study selection is shown in Figure 1. A total of 1,004 studies were retrieved through database search. Among them, 211 duplicates and 780 irrelevant studies were removed. After screening the full-text articles, six studies were excluded: five studies for using NPPV in a short time (time <4 hours per day or <3 months)18–22 and one study for enrolling patients who were not hypercapnic.23
  | Figure 1 Flowchart of the study selection process. |
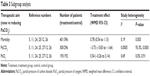
Finally, seven studies with 810 subjects were used for this meta-analysis,5,11,12,24–27 and the basic characteristics of included studies are listed in Table 1.
Risk of bias assessment
As shown in Figure 2, two studies were judged to be at low risk of bias,24,26 while the other five studies were unclear. Six studies used the random sequence generation to generate allocation sequences.5,11,12,24–26 Three studies used appropriate allocation and concealment methods,5,25,26 but the other four studies did not state specific methods. Because NPPV could not be blinded, the researchers and patients were aware of which kind of therapy was chosen, no studies performed blinding.
  | Figure 2 Risk of bia summary. |
Mortality
Six studies (797 subjects) reporting mortality5,11,12,24–26 were pooled and summarized in Figure 3. In patients treated with NPPV, mortality was not lowered significantly (95% CI: 0.54 to 1.13, P=0.19), and a moderate degree of heterogeneity was present (I2=49%).
  | Figure 3 Mortality forest plot. |
Acute exacerbation
Five studies evaluated the frequency of acute exacerbation including the hospitalization and/or intensive care unit (ICU) admission. Among them, four studies showed that there was no significant difference in hospitalization rate and/or ICU admission rate between experiment group and control group.5,11,24,25 However, Köhnlein et al12 found that NPPV reduced hospitalization significantly in the intervention group (average hospitalization frequency =2.2 times/person) compared with the control group (average hospitalization frequency =3.1 times/person) after a follow-up for 1 year. However, because the indices and methods for evaluating acute exacerbation were not the same and some literature did not show sufficient data, the data of acute exacerbation have not been combined and analyzed.
Gas exchange
All seven studies reported gas exchange data,5,11,12,24–27 and in patients treated with NPPV, PaCO2 significantly decreased (WMD −3.73, 95% CI: −5.83 to −1.64, P=0.0005) (Figure 4). NPPV could not improve PaO2 statistically (WMD 0.30, 95% CI: −1.78 to 2.37, P=0.78) (Figure 5).
  | Figure 4 Partial pressure of carbon dioxide in arterial blood forest plot. |
  | Figure 5 Partial pressure of oxygen in arterial blood forest plot. |
Lung function and respiratory muscle strength
This research evaluated lung functional indexes of FEV1, FEV1 total predicted value (FEV1% pred), FVC and maximal inspiratory pressure (PImax). Combined analysis showed that NPPV could not improve lung function and strength of respiratory muscle (Table 2).
Exercise capacity
Although 6-minute walk distance (6MWD) was used in four studies to evaluate the exercise capacity, data could not be combined due to the different data types used in these studies. One study showed a significant improvement in the NPPV group; the minimal clinical improvement in 6MWD of 54 m was reached in 21 (36.8%) patients in the NPPV group versus 10 (17.2%) patients in the control group (P=0.02).26 However, the other three studies showed no significant improvement in the NPPV group.12,24,27
Dyspnea
Due to the different measurement scales used in different studies, data could not be combined. Casanova et al25 showed a reduction in dyspnea at 3 months on two dyspnea scales (modified medical research council dyspnea scale [mMRC], P=0.035 and Borg scale, P=0.039), and this improvement was maintained at 6 months on the Borg scale (P=0.033) in the NPPV group. Clini et al24 reported that the improvement of dyspnea was more significant after 2 years (P=0.013) than the first year (P=0.048) in the NPPV group. The studies by Struik et al11 and Zhou et al26 showed no significant difference in dyspnea.
Subgroup analysis
According to the subgroup analysis of different NPPV techniques, the results showed that there was no decrease in mortality when NPPV did not aim at improving PaCO2 (RR =0.92, 95% CI: 0.74 to 1.15, P=0.47) (Table 3). However, within the subset of 310 patients in the studies by Köhnlein et al12 and Zhou et al,26 patients who received NPPV mainly aimed at decreasing PaCO2 demonstrated significant improvement in mortality compared with those who underwent standard oxygen therapy only (RR =0.35, 95% CI: 0.19 to 0.64, P=0.0006) (Figure 3).
There was substantial heterogeneity in PaCO2 (I2=79%). We found that the heterogeneity of PaCO2 was significantly decreased in both groups (I2=25%/0%) and significant heterogeneity existed between two groups (I2=93.3%). No significant improvement was found in PaO2 in both groups (P=0.79/P=0.71).
Discussion
This meta-analysis of seven RCTs including 810 patients with stable hypercapnic COPD and respiratory failure comparing the efficacy of long-term NPPV and LTOT showed that long-term NPPV significantly decreased the PaCO2 of COPD patients with chronic type II respiratory failure; however, no significant difference was found in mortality, frequency of acute exacerbation, PaO2, lung function, respiratory muscle function and exercise capacity.
The subgroup analysis showed that different ventilation parameters had an impact on the effect of NPPV. The efficacy in improving mortality was not obvious with parameters that were only set to improve respiratory muscle function and reduce dyspnea symptoms. However, the subgroup analysis of combined data from the two studies revealed that patients in the NPPV group mainly aimed at reducing PaCO2 had a lower risk of mortality than those in the control group.12,26
Several studies have shown that chronic hypercapnia was an important symbol of poor prognosis in COPD patients.28,29 Long-term survival analysis showed that mortality would increase by 30%–40% in 2 years if patients have both chronic respiratory failure and hypercapnia.30 According to a long-term observation on stable COPD patients carried out by Leger et al,31 stable COPD patients with long-term survival had a significantly lower PaCO2 than the early death.
In addition, respiratory muscle dysfunction is an important feature of chronic respiratory failure.32 It was said that hypercapnia would decline the strength and endurance of diaphragm.33 At the same time, an unsatisfactory position in length–tension curve caused by chronic hyperventilation would affect the contraction of diaphragm, which would lead to respiratory muscle fatigue and respiratory pump failure. Therefore, some scholars believed that NPPV could benefit the patients with stable hypercapnic COPD by reducing the burden of respiratory muscle, relieving the fatigue and improving the gas exchange.34,35 However, most of the previous RCTs could not reduce PaCO2 significantly probably due to the low intermittent positive airway pressure (IPAP) of 10–20 cmH2O in NPPV. Current studies showed that IPAP in NPPV reaching 30 cmH2O could obviously improve the level of arterial blood gas in patients with stable hypercapnic COPD.6,35,36 A long-term study carried out by Windisch et al6 showed that NPPV targeted at greatly reducing hypercapnia (average IPAP =28 cmH2O) could significantly improve the levels of arterial blood gas and lung function of COPD patients with chronic respiratory failure and reduce the mortality to 86% in 2 years. Consistent with the abovementioned opinion, we found that high IPAP settings benefited patients more. In the subgroup analysis, one group’s primary end point was to reduce PaCO2 with an average IPAP of 19.7 cmH2O, and the mortality decreased significantly; the other group’s primary end point was not to reduce PaCO2 with an average IPAP of 13.62 cmH2O, and no significant improvement was found in mortality.
It was reported that hypercapnia was a risk factor to increase the frequency of acute exacerbation of COPD patients.37 A 6-month follow-up of 1,016 in-patients with both COPD and hypercapnia was carried out by Connors et al,38 which revealed that half of them needed rehospitalization and 7% of patients even needed it over three times. Köhnlein et al12 supported the abovementioned opinion by comparing the NPPV with oxygen therapy on COPD patients with hypercapnia, in which the aim of NPPV was to reduce PaCO2 to 20% of the basic value or 48.1 mmHg, and it revealed that NPPV could significantly lower the frequency of hospitalization compared with standard oxygen therapy.
Dyspnea and decreasing ventilation function limited the exercise capacity of patients with COPD. However, relevant studies showed that injury of lung function was the explanation for not only decreasing exercise capacity but also peripheral and respiratory muscle dysfunction. Gosselink et al39 tested the respiratory and peripheral muscle force of 40 COPD patients and found that COPD patients have reduced respiratory muscle strength (mean 64% of control subjects’ value) and peripheral muscle strength (mean 75% of control subjects’ value) compared to normal subjects. By searching the literature, we noticed that hypercapnia was an important contributing cause of muscle dysfunction.40 The study of Zhou et al,26 which aimed to reduce PaCO2, showed that NPPV was helpful in improving exercise capacity, yet we failed to combine the data due to different data types. Therefore, more high-quality studies are needed to confirm the effect of NPPV in improving exercise capacity of COPD patients.
As different syndrome evaluating scales were used in inclusive trials, including the Borg scale,25 the Baseline and Transitional Dyspnea Index5 and the MRC scale,11,24,25 we were unable to comment on the symptom of dyspnea.
A meta-analysis by Struik et al10 in 2014 found no evidence to support the application of routine NPPV in stable COPD patients, yet the subgroup analysis showed a significant decrease of PaCO2 in patients with higher IPAP levels (IPAP ≥18 cmH2O) as well as in hypercapnic patients (baseline PaCO2 ≥55 mmHg). Comparing with this meta-analysis, we analyzed the data of updated studies, aiming to assess the efficacy of long-term NPPV on stable hypercapnic COPD patients. We noticed that three recent studies11,12,25 changed the results greatly and provided our research more sense. In addition, it was identified that all of the three studies set ventilatory pressures at a high level, of which the IPAP/expiratory positive airway pressures (EPAPs) were set at 21.6/4.8,11 19.2/4.812 and 17.8/4.2 cmH2O,25 which might significantly improve the level of arterial blood gas and lung function in COPD patients. Importantly, the study by Zhou et al was the first reported randomized clinical trial to use NPPV ventilators equipped with built-in software to monitor the ventilator parameters in home setting in stable hypercapnic COPD patients, which found that using NPPV ventilator equipped with built-in software decreased the rate of abnormalities. In other words, using NPPV ventilator equipped with built-in software facilitated the improvement of compliance and quality control of NPPV use so as to improve the accuracy of data.
Our findings supported that NPPV decreased PaCO2, and when primary end point was aimed at greatly reducing hypercapnia, NPPV improved the survival of patients. However, consistent with Struik et al, no significance was found in PaO2, frequency of acute exacerbation, lung function, respiratory muscle function and exercise capacity.
There were several limitations in this meta-analysis: 1) the quality of trials was inconsistent, for example, two studies were judged to be at low risk of bias, whereas more than two uncertain or improper methodologies were found in one study; 2) the differences in the types of data and evaluation methods that were reported limited the application of meta-analysis in some indices, such as exercise capacity, acute exacerbation and dyspnea, which may be of interest. Future studies that include the abovementioned outcomes will enable conclusions to be drawn. However, further studies are desperately required to identify the improvements by NPPV on stable hypercapnic COPD patients.
Conclusion
This meta-analysis suggests that NPPV decreases the PaCO2 of stable hypercapnic COPD patients with respiratory failure and improves mortality with the aim of reducing PaCO2.
Acknowledgment
This work was supported by Open Project of State Key Laboratory of Respiratory Disease (SKLRD2016OP019), the Medical Scientific Research Foundation of Guangdong Province (A2016399), the Clinical Research training program of Southern Medical University (LC2016PY032), and the Guangdong Provincial College Students’ Innovation and Entrepreneurship Training Program (201612121054).
Author contributions
All authors contributed towards data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Ojo O, Lagan AL, Rajendran V, et al. Pathological changes in the COPD lung mesenchyme – novel lessons learned from in vitro and in vivo studies. Pulm Pharmacol Ther. 2014;29(2):121–128. | ||
Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379(9823):1341–1351. | ||
Tan WC, Mahayiddin AA, Charoenratanakul S, et al. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (UPDATE 2014) Global Initiative for Chronic Obstructive Lung Disease 2014 GOLD. Respirology. 2005;10(1):9–17. | ||
Slenter RH, Sprooten RT, Kotz D, Wesseling G, Wouters EF, Rohde GG. Predictors of 1-year mortality at hospital admission for acute exacerbations of chronic obstructive pulmonary disease. Respiration. 2013;85(1):15–26. | ||
McEvoy RD, Pierce RJ, Hillman D, et al. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax. 2009;64(7):561–566. | ||
Windisch W, Kosti S, Dreher M, Virchow JC Jr, Sorichter S. Outcome of patients with stable COPD receiving controlled noninvasive positive pressure ventilation aimed at a maximal reduction of PaCo2. Chest. 2005;128(2):657–662. | ||
Anonymous. Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation – a consensus conference report. Chest. 1999;116(2):521–534. | ||
Nickol AH, Nicholas H, Hopkinson NS, et al. Mechanisms of improvement of respiratory failure in patients with COPD treated with NIV. Int J Chron Obstruct Pulmon Dis. 2008;3(3):453–462. | ||
Tuggey JM, Plant PK, Elliott MW. Domiciliary non-invasive ventilation for recurrent acidotic exacerbations of COPD: an economic analysis. Thorax. 2003;58(10):867–871. | ||
Struik FM, Lacasse Y, Goldstein RS, Kerstjens HA, Wijkstra PJ. Nocturnal noninvasive positive pressure ventilation in stable COPD: a systematic review and individual patient data meta-analysis. Respir Med. 2014;108(2):329–337. | ||
Struik FM, Sprooten RT, Kerstjens HA, et al. Nocturnal non-invasive ventilation in COPD patients with prolonged hypercapnia after ventilatory support for acute respiratory failure: a randomised, controlled, parallel-group study. Thorax. 2014;69(9):826–834. | ||
Köhnlein T, Windisch W, Kohler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014;2(9):698–705. | ||
Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease 2017 report. Am J Resp Crit Care. 2017;195(5):557–582. | ||
Oana S, Mukherji J. Acute and chronic respiratory failure. Handb Clin Neurol. 2014;119:273–288. | ||
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. | ||
Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | ||
Su CL, Chiang LL, Chiang TY, Yu CT, Kuo HP, Lin HC. Domiciliary positive expiratory pressure improves pulmonary function and exercise capacity in patients with chronic obstructive pulmonary disease. J Formos Med Assoc. 2007;106(3):204–211. | ||
Gurgun A, Tuncel S, Karapolat H, Uluer H. The effect of adding noninvasive ventilation to supplemental oxygen during exercise training in severe COPD: a randomized controlled study. Chest. 2015;148(4):909A. | ||
Díaz O, Bégin P, Torrealba B, Jover E, Lisboa C. Effects of noninvasive ventilation on lung hyperinflation in stable hypercapnic COPD. Eur Respir J. 2002;20(6):1490–1498. | ||
Diaz O, Bégin P, Andresen M, et al. Physiological and clinical effects of diurnal noninvasive ventilation in hypercapnic COPD. Eur Respir J. 2005;26(6):1016–1023. | ||
van’t Hul A, Gosselink R, Hollander P, Postmus P, Kwakkel G. Training with inspiratory pressure support in patients with severe COPD. Eur Respir J. 2006;27(1):65–72. | ||
Sin DD, Wong E, Mayers I, et al. Effects of nocturnal noninvasive mechanical ventilation on heart rate variability of patients with advanced COPD. Chest. 2007;131(1):156–163. | ||
Clini E, Sturani C, Rossi A, et al. The Italian multicentre study on noninvasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J. 2002;20(3):529–538. | ||
Casanova C, Celli BR, Tost L, et al. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest. 2000;118(6):1582–1590. | ||
Zhou L, Li X, Guan L, et al. Home noninvasive positive pressure ventilation with built-in software in stable hypercapnic COPD: a short-term prospective, multicenter, randomized, controlled trial. Int J Chron Obstruct Pulmon Dis. 2017;12:1–8. | ||
Gay PC, Hubmayr RD, Stroetz RW. Efficacy of nocturnal nasal ventilation in stable, severe chronic obstructive pulmonary disease during a 3-month controlled trial. Mayo Clin Proc. 1996;71(6):533–542. | ||
Matkovic Z, Huerta A, Soler N, et al. Predictors of adverse outcome in patients hospitalised for exacerbation of chronic obstructive pulmonary disease. Respiration. 2012;84(1):17–26. | ||
Yang H, Xiang P, Zhang E, et al. Is hypercapnia associated with poor prognosis in chronic obstructive pulmonary disease? A long-term follow-up cohort study. BMJ Open. 2015;5(12):e008909. | ||
Gore JM, Brophy CJ, Greenstone MA. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax. 2000;55(12):1000–1006. | ||
Leger P, Bedicam JM, Cornette A, et al. Nasal intermittent positive pressure ventilation. Long-term follow-up in patients with severe chronic respiratory insufficiency. Chest. 1994;105(1):100–105. | ||
Klimathianaki M, Vaporidi K, Georgopoulos D. Respiratory muscle dysfunction in COPD: from muscles to cell. Curr Drug Targets. 2011;12(4):478–488. | ||
Juan G, Calverley P, Talamo C, Schnader J, Roussos C. Effect of carbon dioxide on diaphragmatic function in human beings. N Engl J Med. 1984;310(14):874–879. | ||
Wedzicha JA, Muir J. Noninvasive ventilation in chronic obstructive pulmonary disease, bronchiectasis and cystic fibrosis. Eur Respir J. 2002;20(3):777–784. | ||
Schucher B, Zerbst J, Baumann HJ. Die nichtinvasive Beatmung bei Patienten mit stabiler, schwergradiger chronisch-obstruktiver Bronchitis und Lungenemphysem (COPD). [Noninvasive mechanical ventilation in patients with stable severe COPD]. Pneumologie. 2004;58(6):428–434. German. | ||
Windisch W, Vogel M, Sorichter S, et al. Normocapnia during nIPPV in chronic hypercapnic COPD reduces subsequent spontaneous PaCO2. Respir Med. 2002;96(8):572–579. | ||
Almagro P, Barreiro B, Ochoa De Echagüen A, et al. Risk factors for hospital readmission in patients with chronic obstructive pulmonary disease. Respiration. 2006;73(3):311–317. | ||
Connors AF Jr, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease the SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med. 1996;154(4 pt 1):959–967. | ||
Gosselink R, Troosters T, Decramer M. Distribution of muscle weakness in patients with stable chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2000;20(6):353–360. | ||
Decramer M, De Benedetto F, Del Ponte A, Marinari S. Systemic effects of COPD. Resp Med. 2005;99(12):S3–S10. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.