Back to Journals » International Journal of General Medicine » Volume 16
Efficacy of JAK Inhibitors versus DMARDs in the Treatment of Polymyalgia Rheumatica in China
Authors Gu J, Yang M, Zhang B, Wang H
Received 26 March 2023
Accepted for publication 24 June 2023
Published 12 July 2023 Volume 2023:16 Pages 2981—2986
DOI https://doi.org/10.2147/IJGM.S414267
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Woon-Man Kung
Juanfang Gu,1,2 Mingfeng Yang,1,2 Bin Zhang,1,2 Hongzhi Wang1,2
1Department of Rheumatology and Immunology, The Affiliated Hospital of Jiaxing University (The First Hospital of Jiaxing), Jiaxing, Zhejiang, 314300, People’s Republic of China; 2Jiaxing Key Laboratory of Osteoporosis and Bone Metabolism, Jiaxing, Zhejiang, 314300, People’s Republic of China
Correspondence: Hongzhi Wang; Bin Zhang, Email [email protected]; [email protected]
Objective: This retrospective analysis was to assess the role of Janus kinases (JAK) inhibitors compared with conventional disease modifying anti-rheumatic drugs (DMRADs) in the treatment of polymyalgia rheumatica (PMR) with glucocorticoids (GCs) reduction.
Methods: Clinical information was collected from PMR patients in the JAK inhibitor group and the DMARDs group from January 2020 to August 2021 at Jiaxing first Hospital. Serum C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), hemoglobin (Hb), albumin and dose of GCs before and after treatment were compared between two groups.
Results: Thirty female patients with PMR were included into this study. The dose of GCs in the JAK inhibitor group was significantly lower than in the DMARDs group at baseline and at 3 and 6 months after treatment. There were no significant differences in various laboratory parameters (including CRP, ESR, Hb and albumin) between two groups (P > 0.05) except that Hb in the DMARDs group was significantly higher than in the JAK inhibitor group at 3 and 6 months after treatment (P< 0.05). One patient in the JAK inhibitor group developed herpes zoster, and received tofacitinib treatment after herpes zoster was relieved.
Conclusion: Our study indicates that JAK inhibitors in the treatment of PMR are as effective as DMRADs and are also helpful for the reduction of GCs dose.
Keywords: JAK inhibitors, polymyalgia rheumatica, glucocorticoids, disease modifying anti-rheumatic drug
Introduction
Polymyalgia rheumatica (PMR) is a chronic inflammatory disease of unknown etiology 1, exclusively affecting people over 50 years of age. PMR affects about 2.4% of women and 1.7% of men, its incidence ranks only second to rheumatoid arthritis and it has been one of the most common inflammatory rheumatic diseases in the older adults.1 PMR is characterized by scapular and pelvic girdle pain, morning stiffness, and elevated acute phase reactants, and is sensitive to glucocorticoids (GCs) therapy.2
Currently, GCs are still considered the cornerstone in treatment for PMR.3 A systematic review and meta-analysis showed that the proportion of PMR patients receiving GCs for 1, 2 and 5 years was 77% (95% CI 71–83%), 51% (95% CI 41–61%) and 25% (95% CI 15–36%), respectively.4 In addition, an observational study revealed that it was difficult to completely discontinue GCs therapy in women with PMR.5 However, the long-term use of GCs raises great concern, especially in older patients, because it is closely associated with recognized adverse effects. Therefore, increasing guidelines have recommended the disease-modifying antirheumatic drugs (DMARDs) in combination with GCs for the disease control and synergistic GCs reduction. Methotrexate (MTX) is recommended more frequently in available guidelines, but tumor necrosis factor (TNF) inhibitors are not recommended for PMR, and there are no recommendations for other biologics.6,7 Several studies have shown that tocilizumab, an anti-IL-6 receptor monoclonal antibody, is effective and safe in the treatment of PMR.8–11 To date, the use of sarilumab has been approved in the US. However, the therapeutic efficacy of Janus kinase (JAK) inhibitors, which inhibit the IL-6 signaling pathway, in PMR is unclear. In the present study, PMR patients treated with JAK inhibitors at our institution were retrospectively analyzed and patients receiving conventional DMARDs treatment during the same period served as controls. This study was to evaluate the role of JAK inhibitors in the treatment of PMR with synergistic GCs reduction, which may provide an alternative treatment for PMR.
Patients and Methods
The clinical information was collected from 18 PMR patients who were treated with JAK inhibitors in the Department of Rheumatology, the first Hospital of Jiaxing City from January 2020 to August 2021. Data from three patients were not included in the final analysis, including two patients with missing data on relevant laboratory tests and one 91-year-old patient with recurrent infection. In addition, clinical information was also collected from 24 PMR patients treated with conventional DMARDs who were matched by age and gender during the same period. Nine PMR patients treated with tripterygium wilfordii were excluded, and the remaining 15 patients were treated with MTX (Figure 1). All the patients were diagnosed with PMR in accordance with the 2012 EULAR/ACR classification criteria for PMR.12 Patients treated with JAK inhibitors served as the treatment group, and those treated with conventional DMARDs served as the control group. All patients were isolated PMR patients, except for one case in the treatment group with giant cell arteritis (GCA). Clinical data were collected at the beginning of steroid sparing agent treatment, including general characteristics (age, gender, disease duration), laboratory findings (erythrocyte sedimentation rate [ESR], C-reactive protein [CRP], etc.) before and after treatment, and treatment status.
 |
Figure 1 Flow chart of patients’ inclusion. |
Statistical Analysis
Statistical analysis was performed using SPSS version 20.0, and quantitative data are expressed as mean ± standard deviation (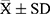 ). The t-test was used for comparison of data that satisfied normal distribution and Chi-square, and Welch’s t-test was used for those that satisfied normal distribution but not chi-square. The Mann–Whitney U-test was used for the comparisons of data without normal distribution. Paired t-test was applied for the comparisons of data before and after treatment. A value of P < 0.05 was considered statistically significant.
). The t-test was used for comparison of data that satisfied normal distribution and Chi-square, and Welch’s t-test was used for those that satisfied normal distribution but not chi-square. The Mann–Whitney U-test was used for the comparisons of data without normal distribution. Paired t-test was applied for the comparisons of data before and after treatment. A value of P < 0.05 was considered statistically significant.
Results
Baseline Characteristics
A total of 30 female PMR patients were included in the treatment and control groups. The mean age was 65.1 ± 8.24 years and the course of disease was 13.94 ± 16.68 months. There were no significant differences in the age, course of disease, CRP level, ESR level, hemoglobin (Hb) level, and albumin level between two groups at baseline (Table 1).
 |
Table 1 Baseline Characteristics of PMR Patients in Both Groups |
Treatment Status
Two patients in the treatment group were previously treated with conventional DMARDs in combination with GCs, and JAK inhibitors were added due to the recurrence of PMR during GCs reduction. Of 15 patients in the treatment group, 11 (73.3%) were treated with GCs combination, 4 (26.7%) with NSAIDs, 14 (93.3%) with tofacitinib (5 mg po bid, Pfizer) and MTX, and 1 (6.67%) with tofacitinib (5 mg po bid, Pfizer). All the patients in the control group were treated with GCs combined with MTX.
Follow Up
The ESR level in the treatment and control groups was significantly lower at 1, 3 and 6 months after treatment as compared to that before treatment (P<0.05). The CRP level in the treatment group decreased significantly at 1, 3 and 6 months after treatment as compared to that before treatment (P < 0.05). The CRP level in the control group decreased significantly (P < 0.05) at 1, 3 and 6 months after treatment as compared to that before treatment. The serum Hb level in the treatment group remained unchanged after treatment (P > 0.05), while Hb level in the control group rebounded at 1, 3 and 6 months after treatment as compared to that before treatment (P<0.05). Albumin level in the treatment group rebounded significantly at 1, 3 and 6 months after treatment as compared to that before treatment (P < 0.05). Meanwhile, the albumin level in the control group increased at 1, 3 and 6 months after treatment as compared to that before treatment (P < 0.05). There were no significant differences in the laboratory parameters between two groups at each time point (P > 0.05), except that the Hb level in the control group was significantly higher than that in the treatment group at 3 and 6 months after treatment (P<0.05) (Table 2).
 |
Table 2 Laboratory Findings Before and After Treatment in Two Groups |
The GCs dose was significantly reduced in both groups at 3 and 6 months after treatment as compared to that before treatment (P < 0.01). Moreover, the GCs dose in the treatment group significantly reduced at 3 and 6 months after treatment as compared to that in the control group (P < 0.05) (Table 3).
 |
Table 3 Average Daily Dose of GCs Before and After Treatment |
Adverse Effects
One of 18 patients in the treatment group (a 91-year-old patient) had recurrent infections during the JAK inhibitor treatment; another patient developed herpes zoster after 3-month tofacitinib treatment, but tofacitinib treatment was initiated again after the remission of herpes zoster with antiviral and other symptomatic treatment. No significant adverse effects were observed in the control group.
Discussion
PMR is also characterized by anemia and decreased serum albumin, and the rapid initiation of GCs therapy is also a feature of the disease.2,13 The pathogenesis of PMR is still poorly understood. Studies have shown elevated serum IL-6 level in patients with active and recurrent PMR, and IL-6 is considered to be involved in the pathogenesis of PMR.14,15
GCs are still the cornerstone in the treatment of PMR. Although GCs are effective, the associated adverse effects cannot be ignored, and the incidence of adverse effects are as high as 65% in PMR patients receiving GCs therapy.16 In the 2015 EULAR/ACR recommendations for the management of PMR, early introduction of MTX is conditionally recommended on top of GCs for disease control and synergistic GCs tapering, especially in patients at high risk of relapse or requiring extended GCs therapy.6 However, MTX has some adverse effects, such as bone marrow suppression and hepatic impairment, and some patients are intolerant to the upper gastrointestinal symptoms caused by MTX. Moreover, other DMRADs are not further recommended for patients who are intolerant to MTX.
JAKs are a class of multi-structured non-receptor tyrosine kinases with important roles in cellular signaling.17 In humans, the JAK family consists of JAK1, JAK2, JAK3 and TYK2, and all cytokine receptors are associated with one or more JAKs to facilitate signaling. Activation and phosphorylation of JAKs may induce the recruitment, dimerization and nuclear translocation of signal transducer and activator of transcription (STAT) and the resulting transcriptional response, which are essential for the downstream regulation of inflammatory mediators (eg IL-6).17 JAK inhibitors function by inhibiting the JAK-STAT signaling pathway and are used to treat a variety of rheumatic diseases, including rheumatoid arthritis, psoriasis, systemic lupus erythematosus, inflammatory bowel disease, etc.17 Preclinical studies have shown that tofacitinib can block T-cell accumulation in the vascular wall and inhibit gamma-interferon production and signaling in an animal model of GCA.18,19 However, the role of JAK inhibitors in the PMR treatment remains unknown.
In a recent study of Zhang et al, 14 patients with highly active PMR (PMR Activity Scale [PMR-AS] >17) were included, and tofacitinib at 10 mg/day was administrated concomitantly with prednisone at 15 mg/day at baseline.20 All the patients achieved low disease activity (LDA) (PMR-AS <7) at week 24 with a median VAS-pain of 5 (0–17.5) with prednisone at 2.2±1.1 mg/day. They speculated that tofacitinib could be used for the treatment of PMR because it may maintain LDA, improve quality of life and reduce the need for GCs. Similarly, our results showed that the CRP, ESR, Hb and albumin levels were comparable between treatment group and control group, except for the Hb level at 3 and 6 months after treatment, which was lower than in the control group. In contrast, the dose of GCs used in the treatment group was significantly lower than in the control group, suggesting that JAK inhibitors have similar efficacy to conventional DMRADs with less GCs use and can be used as GCs reducers in the PMR patients. The treatment group had a mild rebound in Hb level after 1-month treatment as comparable to the control group. The decrease in the treatment group was lower than in the control group at 3 and 6 months after treatment, which may be related to the use of JAK inhibitors. The hematopoietic growth factor erythropoietin (EPO) mediates erythropoiesis via the JAK2-STAT signaling pathway.21 The decrease in Hb level has been observed in several studies on the treatment of rheumatoid arthritis with baricitinib and tofacitinib, particularly high-dose tofacitinib.22
So far, the safety profile of JAK inhibitors is similar to that of other biologic agents used to treat autoimmune diseases. In contrast, the risk of infection (herpes zoster) is slightly increased and generally limited to a single dermatome.23 One patient in the treatment group developed herpes zoster in our study, but tofacitinib treatment was initiated again after remission following symptomatic treatment. Another patient treated with JAK inhibitor developed recurrent infections, but the advanced age and inherent high risk of infection in this patient suggest that the use of JAK inhibitors in patients of advanced age should be cautious.
There were several limitations in this study. This was a retrospective study with small sample size, which may bias our findings. In addition, all the patients included were females. The degree of activity of PMR was assessed, and thus the disease status might be different among patients. The duration of follow-up was short, and therefore the recurrence rate and comorbidities were not determined. Thus, we could not confirm the clinical efficacy of JAK inhibitors. More randomized, double-blind, controlled studies with large sample size are warranted to elucidate the efficacy and safety of JAK inhibitors in the treatment of PMR. The cost-effect of treatment with JAK inhibitors should be further assessed if clinical efficacy of these drugs has been confirmed.
In conclusion, the efficacy of JAK inhibitors in PMR is comparable to that of conventional DMRADs with less GCs use and may help to reduce GCs dose, thus effectively avoiding or mitigating GCs-related adverse effects. More studies with elegant design are needed to confirm our findings.
Conclusions
JAK inhibitors have comparable efficacy to conventional DMRADs with less GCs use in the treatment of PMR, and may also reduce GCs use in these patients. In the future, more randomized, controlled, double-blind, prospective studies are required to confirm the efficacy and safety of JAK inhibitors in the treatment of PMR.
Data Sharing Statement
The datasets generated for this study are available on request to the corresponding author Hongzhi Wang.
Ethical Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Jiaxing first Hospital (LS2022-KY-013). This is a retrospective analysis, and the data are anonymous, so the informed consent was waived in this study and patient data confidentiality was kept.
Funding
This work was supported by the Natural Science Foundation of Zhejiang (No. LQ19H010001) and 2023 Jiaxing Key Discipline of Medicine, Rheumatology and Autoimmunology (Supporting Subject) (No. 2023-ZC-016).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Crowson CS, Matteson EL, Myasoedova E, et al. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 2011;63(3):633–639. doi:10.1002/art.30155
2. Gonzalez-Gay MA, Matteson EL, Castaneda S. Polymyalgia rheumatica. Lancet. 2017;390(10103):1700–1712. doi:10.1016/S0140-6736(17)31825-1
3. Camellino D, Giusti A, Girasole G, Bianchi G, Dejaco C. Pathogenesis, diagnosis and management of polymyalgia rheumatica. Drugs Aging. 2019;36(11):1015–1026. doi:10.1007/s40266-019-00705-5
4. Floris A, Piga M, Chessa E, et al. Long-term glucocorticoid treatment and high relapse rate remain unresolved issues in the real-life management of polymyalgia rheumatica: a systematic literature review and meta-analysis. Clin Rheumatol. 2021;41(1):19–31. doi:10.1007/s10067-021-05819-z
5. Aoki D, Kajiwara N, Irishio K, et al. Withdrawal of glucocorticoid therapy is difficult in women with polymyalgia rheumatica: an observational study. Int J Gen Med. 2021;14:6417–6422. doi:10.2147/IJGM.S322111
6. Dejaco C, Singh YP, Perel P, et al.; European League Against R and American College of R. 2015 Recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis. 2015;74(10):1799–1807. doi:10.1136/annrheumdis-2015-207492
7. Ughi N, Sebastiani GD, Gerli R, et al. The Italian Society of Rheumatology clinical practice guidelines for the management of polymyalgia rheumatica. Reumatismo. 2020;72(1):1–15. doi:10.4081/reumatismo.2020.1268
8. Devauchelle-Pensec V, Berthelot JM, Cornec D, et al. Efficacy of first-line tocilizumab therapy in early polymyalgia rheumatica: a prospective longitudinal study. Ann Rheum Dis. 2016;75(8):1506–1510. doi:10.1136/annrheumdis-2015-208742
9. Lally L, Forbess L, Hatzis C, Spiera R. Brief report: a prospective open-label Phase IIa trial of tocilizumab in the treatment of polymyalgia rheumatica. Arthritis Rheumatol. 2016;68(10):2550–2554. doi:10.1002/art.39740
10. Akiyama M, Kaneko Y, Takeuchi T. Tocilizumab in isolated polymyalgia rheumatica: a systematic literature review. Semin Arthritis Rheum. 2020;50(3):521–525. doi:10.1016/j.semarthrit.2019.12.005
11. Chino K, Kondo T, Sakai R, et al. Tocilizumab monotherapy for polymyalgia rheumatica: a prospective, single-center, open-label study. Int J Rheum Dis. 2019;22(12):2151–2157. doi:10.1111/1756-185X.13723
12. Dasgupta B, Cimmino MA, Maradit-Kremers H, et al. 2012 provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis. 2012;71(4):484–492. doi:10.1136/annrheumdis-2011-200329
13. Horai Y, Otsuka M, Kawahara C, et al. Clinical analysis of gender and pre-existing diabetes mellitus in patients with polymyalgia rheumatica: a retrospective study in a Japanese population. Mod Rheumatol. 2023;33(1):182–186. doi:10.1093/mr/roac012
14. Carvajal Alegria G, Boukhlal S, Cornec D, Devauchelle-Pensec V. The pathophysiology of polymyalgia rheumatica, small pieces of a big puzzle. Autoimmun Rev. 2020;19(11):102670. doi:10.1016/j.autrev.2020.102670
15. Castaneda S, Garcia-Castaneda N, Prieto-Pena D, et al. Treatment of polymyalgia rheumatica. Biochem Pharmacol. 2019;165:221–229. doi:10.1016/j.bcp.2019.03.027
16. Camellino D, Matteson EL, Buttgereit F, Dejaco C. Monitoring and long-term management of giant cell arteritis and polymyalgia rheumatica. Nat Rev Rheumatol. 2020;16(9):481–495. doi:10.1038/s41584-020-0458-5
17. McLornan DP, Pope JE, Gotlib J, Harrison CN. Current and future status of JAK inhibitors. Lancet. 2021;398(10302):803–816. doi:10.1016/S0140-6736(21)00438-4
18. Gonzalez-Gay MA, Pina T, Prieto-Pena D, Calderon-Goercke M, Gualillo O, Castaneda S. Treatment of giant cell arteritis. Biochem Pharmacol. 2019;165:230–239. doi:10.1016/j.bcp.2019.04.027
19. Gazitt T, Zisman D, Gardner G. Polymyalgia rheumatica: a common disease in seniors. Curr Rheumatol Rep. 2020;22(8):40. doi:10.1007/s11926-020-00919-2
20. Zhang L, Li J, Yin H, et al. Efficacy and safety of tofacitinib in patients with polymyalgia rheumatica: a Phase 2 study. Ann Rheum Dis. 2023;82(5):722–724. doi:10.1136/ard-2022-223562
21. Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017;13(4):234–243. doi:10.1038/nrrheum.2017.23
22. Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O’Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;16(12):843–862. doi:10.1038/nrd.2017.201
23. Curtis JR, Xie F, Yun H, Bernatsky S, Winthrop KL. Real-world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75(10):1843–1847. doi:10.1136/annrheumdis-2016-209131
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
