Back to Journals » Journal of Pain Research » Volume 15
Efficacy of Intrathoracic Intercostal Nerve Block on Postoperative Acute and Chronic Pains of Patients Undergoing Video-Assisted Thoracoscopic Surgery
Authors Zhao X, Li X, Wang Y, Xiao W, Zhang B, Meng X, Sun X
Received 1 April 2022
Accepted for publication 31 July 2022
Published 6 August 2022 Volume 2022:15 Pages 2273—2281
DOI https://doi.org/10.2147/JPR.S369042
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jinlei Li
Xiaoning Zhao, Xiaoqian Li, Ying Wang, Weijie Xiao, Baihui Zhang, Xin Meng, Xijia Sun
The Department of Anesthesiology, The First Affiliated Hospital of China Medical University, Shenyang, People’s Republic of China
Correspondence: Xijia Sun, The Department of Anesthesiology, The First Affiliated Hospital of China Medical University, Shenyang, People’s Republic of China, Tel +86 15840015620, Email [email protected]
Background: Patients undergoing video-assisted thoracoscopic surgery (VATS) frequently suffered postoperative acute and chronic pains. In recent years, intrathoracic intercostal nerve block (INB) is regularly used thanks to its safety and accuracy, especially under the circumstance of lacking ultrasound or in face of the contraindications of the thoracic paravertebral block (TPVB). However, clinical evidence of comparing TPVB and INB for pain management after VATS has been limited and the observation of the chronic pain has been less than clear.
Methods: A total of 180 patients undergoing VATS were randomly divided into three groups: A single multi-point paravertebral nerve block (Group P), intrathoracic intercostal nerve block (Group I), and general anesthesia without any regional block (Group C). Postoperative acute pain was scored at rest and coughing by the Visual Analog Scale (VAS) for recording 24h, 48h and 72h after VATS. All patients were interviewed 1, 3 and 6 months after the surgery to investigate both the incidence and intensity of chronic pains.
Results: There were significantly less incidence and intensity of acute pain in Group P and Group I, compared to those in Group C. The patients in Group I showed the least incidence and intensity of chronic pain after 3 months compared with those in Group P and Group C. There are 89 of 98 patients suffering pains after 1 month, which grew into chronic pains after 3 months and 78 of them still suffered chronic pains even after 6 months.
Conclusion: The intrathoracic INB offers excellent relief from acute and chronic pains, which does as effectively as TPVB. Besides, one-month postoperative pain could increase the risk of a chronic one.
Keywords: video-assisted thoracoscopic surgery, acute pain, chronic pain, intrathoracic intercostal nerve block, paravertebral nerve block
Introduction
The video-assisted thoracoscopic surgery (VATS) is a less-invasive treatment for lung cancer, but many patients still suffer moderate and severe postoperative pain from it.1,2 As the most representative region block, thoracic paravertebral block (TPVB) has been confirmed in the clinic; however, its limitations include the dependency on ultrasound and the not-target spaces spread of local anesthetic.2,3 In recent years, the intrathoracic intercostal nerve block (INB) is frequently applied for its safety and accuracy in pain relief, especially when lacking ultrasound or in face of the contraindications of TPVB.4 It has been confirmed to have increased patients’ satisfaction with postoperative analgesia after VATS.4,5 However, clinical evidence comparing TPVB and INB for pain management after VATS has been limited, while the INB efficacy revealed in some experiments has been controversial.6,7
Furthermore, a considerable number of previous studies had already confirmed the acute pain to be a predictor of the chronic one. Chronic Post-Surgical Pain (CPSP) is defined as the one persisting more than 3 months after the surgery which severely impairs patients’ long-term outcomes and qualities of life.5,8 However, little is known regarding the effects of different analgesic methods on long-term chronic pain. In terms of its Numerical Rating Scale (NRS) scores, few reports directly compared the efficacies of TPVB and INB. The incidence of chronic pain and its relationship with the early stage of pain are also unclear.
Therefore, we conducted this study to confirm the efficacy of intrathoracic INB on postoperative acute and chronic pains of patients undergoing VATS to improve their long-term outcomes.
Method
Patients Selection
The study was conducted under the principles of the Declaration of Helsinki. It was also approved by the Ethics Committee of Medical Science Research of the First Affiliated Hospital of China Medical University (Approval number: 2021–40-2) with written informed consents obtained from all participating subjects. The trial was registered before patient enrollment in the Chinese Clinical Trial Registry at www.chictr.org.cn (ChiCTR2100046730, principal investigator, Zhao Xiaoning, Date of registration: 2021/5/27). Subjects eligible for the study were 180 patients with lung cancer undergoing VATS in June 2021, aged 18–65 years with the American Society of Anesthesiologists (ASA)’s identified physical status of I–II. The exclusion criteria consisted of preoperative chronic medication with opioids, a history of the previous thoracotomy, severe cardiovascular/hepatic/renal disorders, intraoperative conversion from minimally invasive surgery into open thoracotomy, re-operations due to various complications with accidental transfers to the intensive care unit (ICU) within three days, and breast or spine surgery performed during the observation period.9 Patients were randomly allocated into 3 groups via a computer-generated list of random numbers by an assistant who was not involved in this research at an 1:1:1 allocation ratio. The paravertebral group (Group P) received a single multi-point thoracic paravertebral block. The intercostal group (Group I) received an intrathoracic intercostal nerve block. The control group (Group C) was without any regional block. The intravenous Patient-Controlled Analgesia (PCA) was used in all three groups, which would be removed 72h after surgery.
Routine Anesthesia Method
All the patients were fasting for 8 hours and prohibited from drinking for 4 hours. When they were transferred into the operating room where the multi-functional monitor electrocardiograms for heart rate, blood pressure, pulse oximetry, and bispectral index monitoring (BIS) were performed. Peripheral intravenous and radial artery catheters were both placed. General anesthesia was induced with propofol (4μg·mL−1plasma target-controlled infusion), sufentanil (0.4μg·kg−1), cis-atracurium (0.2mg·kg−1). Double-lumen tracheal intubation was performed after 3 minutes of mask ventilation. The lung was ventilated with 100% oxygen, the tidal volume was 6mL·kg−1, the ventilatory frequency was 12 bpm, respectively. Anesthesia was maintained with propofol and remifentanil to keep BIS values in the range from 40 to 60. Mean Arterial Pressure (MAP) was controlled between −20% and +20% of the baseline value. Tropisetron (5mg) was injected before the end of surgery.10 After being extubated, the patients were admitted to the Post-Anesthesia Care Unit (PACU).
Group P received a 2-space TPVB under ultrasonic guidance in the lateral position after induction. Adjust the position and depth of the probe to search for the superior costotransverse, spinous process and pleura. An in-plane sagittal block was performed and ropivacaine (0.5%) was administered at paravertebral spaces between T4-T5 and T6-T7 vertebrae with a bolus of a 10 mL in each interspace region after confirming pleura being decreased by fluid pressure.
Group I received a multi-point intrathoracic Intercostal Nerve Block (INB) performed by the surgeon under direct vision into the proximal intercostal space at the level from T4 to T7 before the closure of the chest. A 3mL bolus of 1% ropivacaine was injected at every target spot with the visualized slight distention of the pleura after performing a negative aspiration.
Group C was performed without any regional block.
After the surgery, alcohol cotton swabs were applied in Group P and Group I to test the effect on the plane of the sensory block.
All patients were connected with PCA after the surgery. The pump was programmed as follows: Volume of 100mL contained 40mg oxycodone, 4mL patient-controlled bolus, 30-minute lockout period, and 1mL background infusion.
After surgery, the score criteria were explained as follows: 0 points, no pain; 10 points, severe pain. Patients were introduced to the use of the pumps and were trained to press for an additional bolus in case the 10 cm Visual Analog Scale (VAS) for postoperative acute pain exceeded 3 points. All pumps were maintained until the evening of the fourth postoperative day.
Observation and Indicators Recording
- ) Postoperative acute pain was recorded for each patient at rest and coughing by the VAS then scored 24h (P1), 48h (P2) and 72h (P3) after the surgery.
- ) All patients were interviewed 1(M1), 3 (M2) and 6(M3) months after the surgery by telephone for investigating both the incidence and intensity of their chronic pains, and if necessary, encouraged to elaborate individual symptoms.
- ) Changes in hemodynamics including those in heart rate and blood pressure at various time points: Baseline (T0), 5 minutes after induction (T1), 5 min after skin incision (T2), 1 h after the beginning of the surgery (T3), upon extubation (T4), at PACU (T5).
- ) The pressing times and durations from the end of the surgery to the first pressing time of PCA.
- ) The side effects of these three groups included postoperative nausea and vomiting within three days.
- ) The intraoperative consumption of opioids and vasoactive drugs. All the data were recorded by an investigator who was blind to the group allocation.
Statistical Analysis
Sample size calculations were performed via an online power’s sample size calculator based on our previous pilot study. It showed different acute pains’ VAS scores for patients in the paravertebral and intercostal groups (2.5 ±1.1 and 2.0 ± 1.4 respectively) compared with that in the control group (3.2 ± 1.7) at 24h after the surgery. A total of 135 patients (45 per group) were needed at a power of 85% and a two-sided type-I error of 0.05. Considering countervail potential dropouts and losses for follow-up, eventually, 180 patients (60 per group) were enrolled in the trial. Data analysis was primarily performed via SPSS, version 23.0 (SPSS Inc. Chicago, IL, USA) and GraphPad Prism version 8.0 (GraphPad Software, Inc., La Jolla, CA, USA). These data were assessed for normality using the Kolmogorov–Smirnov test. Quantitative variables were reported as mean±SD/median ± interquartile spacing. Normally distributed quantitative variables were calculated through one-way ANOVA. Abnormally distributed quantitative variables were compared with each other using the Mann–Whitney U-test. Categorical data were reported as numbers (percentages) and were analyzed by chi-square or Fisher’s exact test as appropriate. Differences were considered statistically significant at p < 0.05.
Results
At last, 180 patients were randomly allocated into three groups according to their anesthesia methods, 1 of them was excluded due to intraoperative conversion into thoracotomy, 3 of them were accidentally transferred to the intensive care unit (ICU) within three days, 1 of them was excluded due to blocking failure. The remaining 175 patients were considered for analysis. Further 26 patients were withdrawn, 11 of whom were excluded due to re-operation on the chest, breast or spine within half a year, and 15 of whom were lost to follow-up. Finally, a total of 149 patients completed the entire project: Group P (50 cases), Group I (50 cases), Group C (49 cases), as shown in Figure 1. There was no statistically significant difference in general information like age, sex, Systolic Baseline Pressure (SBP), Diastolic Baseline Pressure (DBP), Heart Rate (HR), height, weight and ASA grade among these 3 groups (all P>0.05) (Table 1). With the prolonging time, Figure 2 shows significantly declining trends of SBP, DBP, and HR compared with the baseline among these three groups. Further comparisons between these groups revealed that at T2, the SBP and DBP of Group I and Group C were both significantly higher than those of Group P (P<0.05). At T5, the SBP and DBP of Group P and Group I were both significantly lower than those of Group C (P<0.05).
 |
Table 1 Demographic Data of Three Groups |
 |
Figure 1 Flowchart of the study. Abbreviations: TPVB, paravertebral nerve block; INB, intercostal nerve block; ICU, intensive care unit. |
Acute pains’ VAS scores at rest and coughing were exhibited significantly and gradually decreasing with prolonging time (Figure 3). The patients in Group C showed the highest intensity of pain at rest and coughing, respectively, compared with those of the other groups. There was no significant difference in resting VAS between Group P and Group I at each time point, but the VAS at coughing in Group P was significantly higher than those in Group I at P2 and P3 (P < 0.05). The patients of Group C shared more perioperative opioid consumption than Group P (P < 0.05). The patients of Group C presented the most analgesic pump-pressing times compared with Groups P and I. The duration from the end of the surgery to the first pressing time of PCA in group C was significantly shorter compared with those in the other groups. A considerable number of patients treated with PCA had suffered from nausea and vomiting, while the ones in Group C suffered more than those in the other two groups. There was no significant difference in the pump pressing times, durations from the end of the surgery to the first pressing time of PCA, or the incidences of nausea and vomiting within three days between Group P and Group I, respectively, as shown in Table 2.
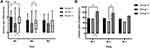
Group I and Group P both showed significantly lower incidences of chronic pain and NRS scores compared with Group C at M1. Group I exhibited the lowest incidence of chronic pain at M2, which had no significant difference among these three groups at M3 (Figure 4). The various symptoms of chronic pain were described as occasional pain (34.2%), scar numbness (22.1%), rest pain (21%), incision pain (9.3%), back pain (8%), incision surroundings numbness (7.4%), shoulder pain (2.7%), respectively. The chronic pain was mainly triggered by pressing the painful area (8.8%), coughing (4.8%), moving heavy objects (11.4%), cold or rainy weather (3.4%), bad emotion (5%), without any stimulus (33%) and described dull pain (34.2%), twinge (3.3%), severe pain (11.4%), squeezing (11%), whiny pain (20.8%).
Discussions
With the popularity of ultrasound, the regional block has been frequently used.5 The most representative one is TPVB, which has been confirmed in the clinic, but its success rate would decrease greatly in the absence of ultrasound. Moreover, the paravertebral space has many potential ones (epidural space or contra-lateral paravertebral space), which makes ropivacaine easy to spread to other non-target spaces.11 It also increases the risk of a non-purpose epidural block, leading to total spinal anesthesia, as well as hypotension and other reactions. Therefore, to make the injection more secure and more accurate, intrathoracic INB gradually emerged to be performed by the surgeon under direct vision into the intercostal space before the closure of the chest.6,12,13 Compared with TPVB, intrathoracic INB used the needle to accurately reach the intercostal region and guaranteed all drug deliveries into the target locations. It has been reported that INB could cause acute pains after VATS.7,10,14 However, clinical evidence comparing TPVB and INB for pain management is limited, while the efficacies revealed in some experiments are controversial.15 Perttunen6 reported no significant difference in acute pains between INB and TPVB, which was not consistent with the other study results that INB was inferior to TPVB.7 In our study, we found that TPVB and intrathoracic INB could both improve postoperative acute pain.7,10,16,17 Further comparisons revealed an equivalent analgesic effect during the following 24h between two of them. Moreover, we discovered that TPVB and INB could both reduce the incidence of nausea and vomiting within three days after the surgery, considering that less postoperative analgesic was administered because of sufficient relief from acute pain.
Not only compared the analgesic effect for acute pain between INB and TPVB but also we studied their influences on chronic pain. There is no convincing evidence that regional block significantly influences the development of chronic pain. In terms of evaluating chronic pain with NRS scores, few studies have directly compared TPVB and INB. Inspired, we confirmed that TPVB and INB can both reduce the incidence and intensity of 1-month postoperative pain, while INB could still reduce those of 3-month postoperative pain. In our study, 58% of patients in the TPVB group, 46% of patients in the INB group and 76% of patients in the control group were all found having suffered chronic pains even 3 months after the surgery, which was consistent with the previous result that 55.2% of patients in TPVB were still reporting pains after 3 months.18 It could be explained that the sound analgesic effect reduced the central sensitization process caused by acute pain.18 However, there was no difference at 6 months after the surgery among these three groups. It was noteworthy that the previous studies only reported the incidence of chronic pain, of which the description had not been involved. In our study, we performed statistical analysis on chronic pain symptoms, triggers, and natures according to the patients’ descriptions. The major symptoms of chronic pain were occasional pain (34.2%), scar numbness (22.1%) and rest pain (21%), those without any triggering stimulus (33%) or those by moving heavy objects (11.4%) and by pressing the painful area (8.8%). The nature of pain was mainly described as dull pain (34.2%) and whiny pain (20.8%).
In addition, we found that moderate and severe chronic pains appeared to be also common in patients after VATS. Our study revealed that 56% of all patients suffered from chronic pains 6 months after VATS, and 44% of them had moderate and severe pains; this was consistent with the previous study's conclusion that the average severity of chronic pain was mild.18 Because some patients suffered from chronic pain of neuropathic type caused by surgery-induced intercostal nerve damage, which also presented more severe pain and wider pain range. In our study, we found that 34.5% of patients with chronic pain (19.4% of all the patients) showed a neuropathic characteristic with the ID-Pain method.19,20 Similarly, a comparable prevalence (25%) had been reported by Mongardon N.20 Moreover, we learned that 47.1% of patients who suffered moderate and severe pain 1 month after the surgery had neuropathological agony, whereas only 10.6% of patients with mild pain had that. Meanwhile, among 29 patients who had neuropathological pain, 28 still suffered 6-month postoperative agony. Therefore, we inferred that moderate and severe agonies 1 month after VATS could be a predictor of neuropathological pain which was not easy to alleviate once it was established, this has not been reported in previous studies. We further investigated the relationship between 1-month of postoperative pain and chronic pain. We screened 98 patients with 1-month postoperative pain and found that 90.8% (n = 89) of them developed chronic pain after 3 months, 80.6% (n = 79) still suffered it even after 6 months. Previous studies had already confirmed the prediction of acute pain for chronic pain, we confirmed that 1-month postoperative agony also imposed a significant impact on the incidence of chronic pain.
Although excellent reliefs from acute and chronic pains had been confirmed in this study, the adoption of INB was not sufficient. It still had some limitations including the unpredictability of systemic absorption of the local anesthetic and bleeding at the puncture sites, which should be taken into account. Meanwhile, the performance of the injection was at the end of the surgery, as there was no analgesic effect during the operation except opioids. Therefore, it failed to reduce the consumption of intraoperative opioids and maintained stable hemodynamics. In our study, none of the INB group suffered from any serious complications like infection, hematoma and nerve injury. So, we can draw the conclusion that INB was safe for clinical practices.21
Limitation
Nevertheless, there remained some limitations in this study: Firstly, it was not a double-blind RCT. Secondly, the score of pain depended mainly on the subjective and integrated feelings of patients, although it remained a routine method of assessment. Thirdly, the sample size was not sufficiently large to offer more appropriate powers for drawing a convincing conclusion. Forth, our observation period could be extended to one year, which might make a more accurate conclusion.
Conclusion
Intrathoracic INB not only provided excellent relief from acute pain but lowered the incidence of chronic pain within 3 months, which was more secure, convenient and effective than TPVB. It could be widely recommended in the clinic, especially when lacking ultrasound or encountering the contraindications of a paravertebral block. Besides, the incidence of chronic pain was high and 1-month postoperative pain could be an important predictor of further chronic pain, while moderate-to-severe pains 1 month after the surgery were also seen as a signal of neuropathologic agony, which was not easy to recover from once established.
Data Sharing Statement
The clinical data used to support the findings of this study are available from the corresponding authors upon reasonable request.
Acknowledgment
We thank all the individuals who contributed to this study. Xiaoning Zhao MD from the Department of Anesthesiology, The First Affiliated Hospital of China Medical University, Shenyang, China, is the first author.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Moon S, Lee J, Kim H, Kim J, Kim J, Kim S. Comparison of the intraoperative analgesic efficacy between ultrasound-guided deep and superficial serratus anterior plane block during video-assisted thoracoscopic lobectomy: a prospective randomized clinical trial. Medicine. 2020;99(47):e23214. doi:10.1097/MD.0000000000023214
2. Moorthy A, Eochagain AN, Dempsey E, Buggy D. Ultrasound-guided erector spinae plane catheter versus video-assisted paravertebral catheter placement in minimally invasive thoracic surgery: comparing continuous infusion analgesic techniques on early quality of recovery, respiratory function and chronic persistent surgical pain: study protocol for a double-blinded randomised controlled trial. Trials. 2021;22(1):965. doi:10.1186/s13063-021-05863-9
3. Wang L, Wang Y, Zhang X, Zhu X, Wang G. Serratus anterior plane block or thoracic paravertebral block for postoperative pain treatment after uniportal video-assisted thoracoscopic surgery: a retrospective propensity-matched study. J Pain Res. 2019;12:2231–2238. doi:10.2147/JPR.S209012
4. Hsu DS, Ely S, Alcasid NJ, et al. Reduced opioid utilization and post-operative pain in Asian vs. Caucasian populations after video-assisted thoracoscopic surgery lobectomy with liposomal bupivacaine-based intercostal nerve blockade. Ann Palliat Med. 2022;11:1635–1643. doi:10.21037/apm-21-2269
5. Piccioni F, Segat M, Falini S, et al. Enhanced recovery pathways in thoracic surgery from Italian VATS Group: perioperative analgesia protocols. J Thorac Dis. 2018;10(Suppl 4):S555–s63. doi:10.21037/jtd.2017.12.86
6. Perttunen K, Tasmuth T, Kalso E. Chronic pain after thoracic surgery: a follow-up study. Acta anaesthesiologica Scandinavica. 1999;43(5):563–567. doi:10.1034/j.1399-6576.1999.430513.x
7. Guerra-Londono CE, Privorotskiy A, Cozowicz C, et al. Assessment of intercostal nerve block analgesia for thoracic surgery: a systematic review and meta-analysis. JAMA network open. 2021;4(11):e2133394. doi:10.1001/jamanetworkopen.2021.33394
8. Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996;12(1):50–55. doi:10.1097/00002508-199603000-00009
9. Mao Y, Zuo Y, Mei B, et al. Efficacy of perineural dexamethasone with ropivacaine in thoracic paravertebral block for postoperative analgesia in elective thoracotomy: a randomized, double-blind, placebo-controlled trial. J Pain Res. 2018;11:1811–1819. doi:10.2147/JPR.S164225
10. Yeung JH, Gates S, Naidu BV, Wilson MJ, Gao Smith F. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. 2016;2(2):Cd009121. doi:10.1002/14651858.CD009121.pub2
11. Purcell-Jones G, Pither CE, Justins DM. Paravertebral somatic nerve block: a clinical, radiographic, and computed tomographic study in chronic pain patients. Anesth Analg. 1989;68(1):32–39. doi:10.1213/00000539-198901000-00008
12. Xu Y, Li XK, Zhou H, et al. Paravertebral block with modified catheter under surgeon’s direct vision after video-assisted thoracoscopic lobectomy. J Thorac Dis. 2020;12(8):4115–4125. doi:10.21037/jtd-20-1068B
13. Zhang X, Shu L, Lin C, et al. Comparison between intraoperative two-space injection thoracic paravertebral block and wound infiltration as a component of multimodal analgesia for postoperative pain management after video-assisted thoracoscopic lobectomy: a randomized controlled trial. J Cardiothorac Vasc Anesth. 2015;29(6):1550–1556. doi:10.1053/j.jvca.2015.06.013
14. Xu J, Pu M, Xu X, Xiang J, Rong X. The postoperative analgesic effect of intercostal nerve block and intravenous patient-controlled analgesia on patients undergoing lung cancer surgery. Am J Transl Res. 2021;13(8):9790–9795.
15. Kadomatsu Y, Mori S, Ueno H, Uchiyama M, Wakai K. Comparison of the analgesic effects of modified continuous intercostal block and paravertebral block under surgeon’s direct vision after video-assisted thoracic surgery: a randomized clinical trial. Gen Thorac Cardiovasc Surg. 2018;66(7):425–431. doi:10.1007/s11748-018-0936-8
16. Kosiński S, Fryźlewicz E, Wiłkojć M, Ćmiel A, Zieliński M. Comparison of continuous epidural block and continuous paravertebral block in postoperative analgaesia after video-assisted thoracoscopic surgery lobectomy: a randomised, non-inferiority trial. Anaesthesiol Intensive Ther. 2016;48(5):280–287. doi:10.5603/AIT.2016.0059
17. Naja MZ, Ziade MF, El Rajab M, El Tayara K, Lönnqvist PA. Varying anatomical injection points within the thoracic paravertebral space: effect on spread of solution and nerve blockade. Anaesthesia. 2004;59(5):459–463. doi:10.1111/j.1365-2044.2004.03705.x
18. Li XL, Zhang J, Wan L, Wang J. Efficacy of single-shot thoracic paravertebral block combined with intravenous analgesia versus continuous thoracic epidural analgesia for chronic pain after thoracotomy. Pain Physician. 2021;24(6):E753–e9.
19. Xin L, Hou N, Zhang Z, Feng Y. The effect of preoperative ultrasound-guided erector spinae plane block on chronic postsurgical pain after breast cancer surgery: a propensity score-matched cohort study. Pain Ther. 2021;11:93–106. doi:10.1007/s40122-021-00339-9
20. Mongardon N, Pinton-Gonnet C, Szekely B, Michel-Cherqui M, Dreyfus JF, Fischler M. Assessment of chronic pain after thoracotomy: a 1-year prevalence study. Clin J Pain. 2011;27(8):677–681. doi:10.1097/AJP.0b013e31821981a3
21. De Kock M. Expanding our horizons: transition of acute postoperative pain to persistent pain and establishment of chronic postsurgical pain services. Anesthesiology. 2009;111(3):461–463. doi:10.1097/ALN.0b013e3181afde28
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.