Back to Journals » International Journal of General Medicine » Volume 17
Efficacy of Fire-Needle Therapy in Improving Neurological Function Following Cerebral Infarction and Its Effect on Intestinal Flora Metabolites
Authors Feng YJ, Wang BQ, Cao LL, Dong LY, Zhang CY, Hu DJ, Zhou Z, Cao JX
Received 15 November 2023
Accepted for publication 16 January 2024
Published 2 February 2024 Volume 2024:17 Pages 387—399
DOI https://doi.org/10.2147/IJGM.S450027
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Redoy Ranjan
Yi-Jun Feng,1,* Bing-Quan Wang,1,* Lu-Lu Cao,2 Li-Ying Dong,3 Chu-Yi Zhang,1 Dong-Jian Hu,1 Zhen Zhou,3 Jin-Xiu Cao4
1Tianjin University of Traditional Chinese Medicine, Tianjin, 301617, People’s Republic of China; 2Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, People’s Republic of China; 3Department of Encephalopathy and Acupuncture, Second Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, 300250, People’s Republic of China; 4Department of Geriatrics, Shanghai Eighth People’s Hospital, Shanghai, 200235, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhen Zhou, Department of Encephalopathy and Acupuncture, Second Teaching Hospital of Tianjin University of Traditional Chinese Medicine, No. 69 of Zengchan Road, Hebei District, Tianjin, 300250, People’s Republic of China, Tel +86-2260637106, Email [email protected] Jin-Xiu Cao, Department of Geriatrics, Shanghai Eighth People’s Hospital, No. 8 Caobao Road, Xuhui District, Shanghai, 200235, People’s Republic of China, Tel +86-13502089031, Email [email protected]
Objective: This study was to investigate the mechanism of action and clinical efficacy of fire-needle therapy in improving neurological function in patients with acute cerebral infarction (identified as a wind-phlegm-blood stasis syndrome in traditional Chinese medicine).
Methods: We included patients diagnosed with acute cerebral infarction (wind-phlegm-blood stasis syndrome) admitted to the Encephalopathy and Acupuncture Center of the Second Affiliated Hospital of Tianjin University of Chinese Medicine. We randomly allocated them into the treatment and control groups, with 45 cases in each group. Acupuncture treatments that focused on regulating the mind and dredging the collaterals were used in the control group, while the treatment group additionally received fire-needle therapy. Our indicators included the National Institutes of Health Stroke Scale (NIHSS) scores, the Fugl-Meyer Assessment (FMA) scale, peripheral blood tumor necrosis factor-α (TNF-α), interleukin-17 (IL-17), hypersensitivity C-reactive protein (hs-CRP), and intestinal metabolites short-chain fatty acids (SCFAs). We measured these indicators before treatment and 14 days after treatment.
Results: The post-treatment NIHSS scores of the two groups were significantly reduced (P < 0.05), and the treatment group showed a more significant decline in the score when compared to the control group (P < 0.05). The treatment group showing significant improvement in the domains of reflex activity, mobility, cooperative movement, and finger movement (P < 0.05). Both groups showed a significant decrease in the IL-17 and hs-CRP levels (P < 0.05), with the treatment group demonstrating a significant declining trend when compared to the control group (P < 0.05). The levels of acetic acid, propionic acid, butyric acid, and valeric acid all increased significantly in the two groups (P < 0.05), with acetic acid and butyric acid increasing significantly in the treatment group when compared to the control group (P < 0.05). Clinical efficacy rate: 78.6% of patients in the treatment group had an excellent rate, whereas it was 30.0% in the control group, and the difference was statistically significant (P < 0.001).
Conclusion: Fire-needle therapy was effective in upregulating the SCFA content in patients with acute cerebral infarction (wind-phlegm-blood stasis syndrome), inhibiting the level of the inflammatory response, and improving the recovery of neurological functions.
Clinical registration number: Registration website link: https://www.chictr.org.cn. Registration date: 2022/9/27. Registration number: ChiCTR2200064122.
Keywords: acute cerebral infarction, fire-needle therapy, short-chain fatty acids, wind-phlegm-blood stasis syndrome
Introduction
Acute cerebral infarction (ACI) is a neurological disorder caused by the narrowing or occlusion of cerebral blood vessels, resulting in hypoxic ischemic necrosis of brain tissue.1 After a cerebral infarction, multiple systems of the body impact the intestinal microorganisms;2 the stable flora structure is destroyed in a short time, and the degree of flora disorder is positively correlated with the severity of cerebral infarction.3 Dysbacteriosis further reduces the content of short-chain fatty acids (SCFAs), the main metabolite of the intestinal flora, and can have a negative impact on anti-inflammation.4 This can intensify local and systemic immune inflammatory responses and worsen nerve damage in cerebral infarction.
Cerebral infarction is categorized as a “stroke disease” in traditional Chinese medicine. Previous syndrome studies have found that wind-phlegm-blood stasis syndrome is the most common type of ACI.5 Fire-needle therapy has been shown to have remarkable efficacy in the treatment of cerebral infarction and related diseases in clinical practice. This treatment modality belongs to the category of “Wen Tong Method” in the “San Tong Method” advocated by Professor He Puren, the first master of traditional Chinese medicine in China. Previous studies have found that fire-needle therapy is effective in regulating intestinal flora, modulating the inflammatory response,6 promoting nerve repair, and regulating cellular apoptosis.7 Therefore, in this study, based on earlier research, we examined the treatment efficacy of fire-needle therapy on neurological function, inflammatory response, and intestinal flora metabolites following cerebral infarction so as to provide evidence-based support for promoting the clinical use of this therapy.
Materials and Methods
General Information
We selected patients who were hospitalized in the Encephalopathy and Acupuncture Center of the Second Affiliated Hospital of Tianjin University of Chinese Medicine between March 2022 and January 2023 and diagnosed with ACI (wind-phlegm-blood stasis syndrome) as the research participants. We randomly allotted a total of 82 patients into the treatment and control groups using the random number method. The treatment group consisted of 42 patients, including 27 males and 15 females, with an average age of (59.60±7.79) years and an average disease course of (2.31±1.00) years. There were 40 patients in the control group, including 24 males and 16 females, with an average age of (60.25±7.75) years and an average disease course of (2.25±1.01) years. The flowchart of patient inclusion, see Figure 1
 |
Figure 1 The flowchart of patient inclusion. |
There was no significant difference between the two groups in the general sociodemographic profile of patients (P > 0.05). This study was reviewed and approved by the Ethics Committee of the Second Affiliated Hospital of Tianjin University of Chinese Medicine, and the ethics number was EC.AT/03.19–02/09.0. Additionally, this study was registered and reviewed in the China Clinical Trial Registry with the number ChiCTR2200064122. (Registration website link: https://www.chictr.org.cn, Registration date: 2022/9/27).
Diagnostic Criteria
Diagnostic Criteria as per Western Medicine
We referred to the following diagnostic criteria for ACI as per the “Chinese Guidelines for the Diagnosis and Treatment of Acute Ischemic Stroke 2018”8 published by the Cerebrovascular Disease Group of the Neurology Branch of the Chinese Medical Association.
- Acute onset in a resting state;
- The condition peaks between several hours and several days, and some patients show progressive aggravation of symptoms;
- Clinical symptoms or signs are mostly manifested as focal neurological deficits, including: numbness and weakness of one side of the face or limbs; language dysfunction, etc.; a small number of patients have comprehensive neurological deficits;
- Imaging clearly identifies the lesions that are responsible; in the absence of imaging to confirm the diagnosis, clinical symptoms and signs should have lasted for more than 24 hours;
- Brain CT or MRI results exclude non-vascular disease causes and cerebral hemorrhage.
Differentiation Criteria of Traditional Chinese Medicine
We referred to the “Compilation and Methodological Discussion of the Diagnostic Scale of Ischemic Stroke Syndrome Elements”9 for the criteria for the wind-phlegm-blood stasis syndrome.
Main symptoms include hemiplegia, deviation of the mouth and tongue, dysphasia or aphasia, and hypoesthesia or anesthesia.
Secondary symptoms include dizziness, excessive and sticky phlegm, a bleak tongue, a thin white or white greasy coating on the tongue, and a thready and slippery pulse.
Inclusion Criteria
- Patients who fulfilled the above diagnostic criteria;
- Patients aged between 40 and 75 years old, irrespective of gender;
- The onset time was within 14 days;
- Patients with grade II–IV muscle strength;
- The neurological deficit score is 5–15 points;
- Patients with stable vital signs, clear consciousness, and no serious complications.
Exclusion Criteria
- Patients with other co-existing diseases, such as tumors, brain trauma, psychiatric disorders, or serious complications of the metabolic system;
- Patients with vital organ failure, severe cardiovascular disease, liver and kidney insufficiency, respiratory disease, hematological system disease, endocrine system disease, poor disease control, and in critical condition;
- Patients with severe cerebral infarction or relapse of multiple diseases;
- Lactating or pregnant women;
- Patients who received anti-inflammatory, intravenous thrombolysis, alteration of intestinal flora, laxative, and other treatments in the past one month;
- Patients who had participated in other clinical trials in the past 90 days.
Exclusion Criteria
- Patients who did not receive acupuncture treatment during the specified time of the study as required, because of which we were not able to determine the treatment efficacy;
- Patients who used other treatments other than those administered in the trial during the treatment process, because of which we could not determine the treatment efficacy;
- Patients with serious changes in the condition or allergic reactions during the treatment process, resulting in discontinuation of the treatment;
- Patients who did not consent to fire-needle and filiform-needle therapy during the treatment.
Grouping and Allocation Methods
Excel software and the random number method were used for random grouping, and we utilized the envelope method for allocation concealment.
Treatment
Control Group
Patients in the control group received treatment with basic medication, acupuncture treatments for regulating the mind and dredging the collaterals, as well as corresponding symptomatic treatment.
(1) The basic medication treatment was as follows: (1) intravenous infusion: butylphthalide sodium chloride injection (specification 100 mL: 25 mg, drug approval number: H20400041, manufacturer: China Shijiazhuang Pharmaceutical Group Enbipu Pharmaceutical Co., Ltd.), 25 mg per time, twice a day, for 14 days. The timings for the intravenous drip were 9:00 a.m. and 15:00 p.m.;10,11 (2) antiplatelet therapy: after admission, patients were administered aspirin 100 mg/tablet orally at 7:00 a.m. every morning, once a day, for 14 days. If the patient had bleeding or the systolic blood pressure was ≥ 180 mmHg, the drug was immediately switched to clopidogrel bisulfate 75 mg, once a day; (3) lipid-lowering drugs: atorvastatin calcium tablets 100 mg/tablet were administered orally before bedtime, once a day,12,13 for 14 days.
(2) Acupuncture treatments for regulating the mind and dredging the collaterals.
The selection of acupuncture points for the acupuncture treatments for regulating the mind and dredging the collaterals is detailed in Table 1.
 |
Table 1 Acupuncture Points Selected for the Acupuncture Treatments for Regulating the Mind and Dredging the Collaterals |
The following procedure was used: Huatuo brand disposable sterile acupuncture needles of sizes 1.5-inches, 2-inches, and 2.5-inches were used (Suzhou Medical Supplies Factory Co., Ltd., specification 0.3×40 mm, 0.3×50 mm, 0.3×60 mm, product model H, product standard GB2024-94). All head needles were inserted using the horizontal needle-insertion method, with a depth of 25–40 mm. The needle insertion was from Baihui to Qianding for the top middle line; from Chengling to Zhengying for the top side line; from Baihui for the top oblique line 1; and from Chengling for the top oblique line 2, both inserted from the back to the outward front, with an angle of 45° between the needle and the top middle line.
Patients were asked to move the limb on the affected side as much as possible during the procedure. The head needles used on the affected side and the corresponding parts on the opposite side could be switched alternately. All body needles were inserted using the straight needle-insertion method, except the Fengchi, where the needle was inserted towards the tip of the nose. Except for the Siqiang and Yongquan, where the needle-insertion depth was 15–20 mm, the remaining body needle insertion depths were 25–40 mm. Twirling and lifting-thrusting needling manipulations were used for both the head and body needles, with a twisting frequency of about 150 r/min for each acupuncture point, an insertion depth of about 15 mm for lifting-thrusting, and a lifting-thrusting frequency of 60 times/min. The duration of needle manipulation was about 30 seconds for each acupuncture point; the needle was left in place for 30 minutes, once a day. The acupuncture treatment was administered from the 2nd day after admission for a total of 14 days, and no acupuncture treatment was given on the 7th, 8th, or 14th days after admission.
Treatment Group
Patients in the treatment group received fire-needle therapy on the basis of the treatment of the control group.
The following acupuncture points were selected for the fire-needle therapy: Ren channels: Zhongwan, Xiawan, and Guanyuan. Governor meridian: Dazhui, Zhiyang, Mingmen, and Yaoyangguan.
We referred to the “Acupuncture Technical Operation Specification: Part 12 Fire needle”14 for the specific procedure of performing the fire-needle therapy. The patient was placed in a comfortable position, and the acupuncture area was fully exposed. The operator, holding a hemostatic forceps in the left hand, used a 95% alcohol cotton ball to ignite the fire, moved the flame to a position directly above the acupuncture point, and using the thumb, index, and middle fingers of the right hand to control the fire needle handle, placed the needle on the flame envelope and burned it from the root to the tip until it was red and shiny white. The needle was then quickly inserted into the acupuncture point and immediately pulled out, with a depth between 0.2–0.5 inches for various parts.
Frequency of treatment: Fire-needle therapy was administered on the 2nd, 4th, 6th, 9th, 11th, and 13th days after admission.
Needle specifications were as follows: medium and fine fire needles with the specification of 0.4 mm × 25 mm were used; the manufacture of these needles was overseen at the Beijing He Clinic, and the needles were fabricated using a tungsten-based high-density cemented carbide material.
Observation Indicators
(1) Primary observation indicators:
The National Institutes of Health Stroke Scale (NIHSS)15 was administered before treatment and 14 days after treatment.
(2) Secondary observation indicators:
(1) The Fugl-Meyer Assessment Scale15 was administered before treatment and 14 days after treatment.
(2) Hematological indicators: hypersensitivity C-reactive protein (hs-CRP), tumor necrosis factor (TNF-α), and interleukin-17 (IL-17).
About 3 mL of fasting venous blood was collected in a coagulant blood collection tube at 6 a.m. after admission and on the 14th day after treatment. The serum was separated within 30 minutes at 3000 r/min for 5 minutes. The supernatant was collected and frozen in an ultra-low-temperature freezer at −80°C for further analysis. We used enzyme-linked immunoassay (ELISA) kits purchased from Elabscience to determine the levels of hs-CRP, TNF-α, and IL-17. We followed the instructions specified by the manufacturer of the kit, and the study was conducted in the laboratory of our hospital.16
(3) Detection of intestinal SCFAs
The first stool sample was collected within 36 hours of the occurrence of cerebral infarction, and the last sample was collected on the 14th day after admission, preferably in the morning. Stool samples were immediately aliquoted and labeled, stored in sterile tubes, and frozen in a −80°C freezer until further analysis.
Safety Observation Indicators
Grade 1: Safe, with no adverse events.
Grade 2: Relatively safe, with mild adverse events that did not require treatment, and the therapy could be continued.
Grade 3: Safety concerns, with moderate adverse events, and the therapy could be continued after treating the adverse events.
Grade 4: Discontinuation from the study due to adverse events.
Efficacy Evaluation Criteria
We established the following criteria for evaluation of treatment efficacy based on the “Evaluation Standards for the Degree of Clinical Neurological Deficit in Stroke Patients (1995)”17 and relevant literature.18
- Cured: elimination of hemiplegia and language retardation; muscle strength recovers to grade IV–V; self-care improves; the patient can walk independently; and the decrease in neurological deficit as per the NIHSS score is 91%–100%;
- Excellent response: speech becomes clear; the muscle strength of the hemiplegic limb recovers to grade II or above; self-care is adequate in some daily activities; and the decrease in neurological deficit as per the NIHSS score is 46%–90%;
- Progress: the hemiplegia and aphasia have improved, but the patient is unable to take care of himself; and the decrease in neurological deficit as per the NIHSS score is 18%–45%;
- No change: no significant improvement in symptoms and signs; and the decrease in neurological deficit as per the NIHSS score is less than 17% or the deficit increases;
- Deterioration: the neurological deficit as per the NIHSS score increases by more than 18%.
Blinding
In this study, we used third-party assessors to evaluate the neurological function of patients with ACI before and after treatment to ensure blinding of the efficacy evaluation. Neither the data analyst nor the efficacy assessor were involved in the trial data collection process, and the trial protocol implementer did not participate in the data analysis.19
Statistical Analysis
SPSS 19.0 software was used for statistics. The significance threshold of the two-tailed test was set at α=0.05. The t-test was used when the data conformed to the normal distribution and variance homogeneity, and the nonparametric test was used if the data did not conform to the normal distribution. Measurement data or data that did not conform to a normal distribution were described using mean ± standard deviation (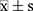 ), and counting data were described using frequency (n) and percentage (%).
), and counting data were described using frequency (n) and percentage (%).
Results
Observation Indicators
Primary Observation Indicators
As can be seen in Table 2, there was no statistically significant difference (P > 0.05) between the two groups at baseline, showing that the two groups were consistent and comparable. After treatment, the NIHSS scores of the two groups significantly decreased (P < 0.05), with the treatment group showing a more significant decrease as compared to the control group (P < 0.05).
 |
Table 2 NIHSS Score Scale |
Secondary Observation Indicators
(1) Fugl-Meyer Assessment score
As can be observed in Table 3, there was no statistically significant difference (P > 0.05) between the two groups at baseline for any of the items on the Fugl-Meyer Assessment. After treatment, the scores of all items in the FMA significantly increased in both groups (P < 0.05), and the increase in the treatment group in the areas of reflex activity, mobility, cooperative movement, and finger movement was better than that of the control group (P < 0.05).
 |
Table 3 Fugl-Meyer Assessment Score Scale |
(2) Hematology-related indicators
1) Tumor necrosis factor (TNF-α)
It can be seen from Table 4 that there was no significant difference in the TNF-α levels between the two groups before treatment (P > 0.05), indicating that the baselines of the two groups were consistent and comparable. After treatment, the TNF-α levels of the two groups were significantly reduced (P < 0.05), and there was no significant difference between the groups (P > 0.05).
 |
Table 4 Levels of TNF-α |
2) Hypersensitivity C-reactive protein (hs-CRP)
From Table 5, we see that there was no significant difference in the levels of hs-CRP between the two groups before treatment (P > 0.05), indicating that the two groups were consistent and comparable at baseline. After treatment, the hs-CRP levels were significantly reduced in both groups (P < 0.05), with the decrease in the treatment group significantly better than that in the control group (P < 0.05).
 |
Table 5 Levels of Hs-CRP |
3) Interleukin-17 (IL-17)
It can be seen from Table 6 that the pre-treatment baseline difference in IL-17 levels was not statistically significant between the two groups (P > 0.05). After treatment, the IL-17 levels of the two groups significantly decreased (P < 0.05), and the decrease in the treatment group was significantly better than that of the control group (P < 0.05).
 |
Table 6 Levels of IL-17 |
(3) SCFAs
The results of our intra-group comparison showed that the contents of acetic acid, propionic acid, butyric acid, and valeric acid in the treatment group significantly increased after treatment as compared with the pre-treatment levels (P < 0.05). The comparison between groups showed that the increase in levels of acetic acid and butyric acid in the treatment group was significantly better than that of the control group (P < 0.05). No statistically significant difference was found in the contents of the remaining SCFAs in either intra- or between-group comparisons (Table 7).
 |
Table 7 Changes in SCFAs Content (μg/mL) |
Clinical Efficacy Evaluation Indicators
It can be seen from Table 8 that 78.6% of patients in the treatment group showed an excellent response to treatment, and the progress rate was 21.4%, while in the control group, 30.0% showed an excellent response to treatment, and the progress rate was 70.0%. The excellent rate of response to treatment in the treatment group was higher than that of the control group, and the difference between the two groups was statistically significant as determined by the rank sum test of ranked data (P < 0.001), indicating that the clinical efficacy was better in the treatment group than in the control group.
 |
Table 8 Clinical Efficacy in the Two Groups |
Safety Evaluation
No patient in either group had any adverse events such as allergies, fever, or infection during the treatment, and there were no abnormalities in the blood routine examination, liver and kidney function, or any of the four coagulation tests in either group post-treatment, indicating that the treatment methods used in this study were safe and feasible.
Discussion
ACI is accompanied by intensified central and peripheral inflammatory responses and commonly induces multisystem infection.20 In recent years, a large number of scholars have studied the relationship between cerebral infarctions and intestinal flora to better understand the role and related mechanisms of intestinal flora in the process of recovery of neurological deficits following cerebral infarctions. Under normal physiological conditions, the balanced distribution of intestinal flora helps the body maintain normal metabolic levels and provides a basis for physiological functions such as host immunity, digestion and absorption, and nutrient synthesis.21–23
Numerous studies have demonstrated that the type, abundance, and colonization of intestinal flora change abnormally after a cerebral infarction. Yamashiro et al24 used quantitative PCR to analyze the dynamic changes in intestinal flora in patients with cerebral infarctions and found that the proportion of pathogenic bacteria increased significantly. Disruption of the intestinal flora causes γδT cells to secrete a large number of IL-17 inflammatory factors, resulting in exacerbation of the local and systemic inflammatory responses and further aggravating cerebral ischemic injury.25 Studies have confirmed that IL-17 can increase the infiltration of inflammatory cells at the cerebral infarction site, stimulate the expression of various pro-inflammatory factors such as TNF-α, IL-1β, IL-6, etc,26 aggravate the inflammatory response, and affect the repair process of the central nervous system.
Patients with cerebral infarction have a significant imbalance in the intestinal microbiota accompanied by a deficiency of SCFAs. Studies on the mechanism of the inflammatory response in the body have revealed that intestinal flora affect the inflammatory response of the central nervous system, the repair of the intestinal mucosal barrier, and the phenotypic differentiation of T lymphocytes following cerebral infarction by regulating the content of SCFAs.27 SCFAs can penetrate the blood-brain barrier (BBB) by regulating the status of microglia, neurotrophic factor,28 and BBB,29 and intervening in the progression of neuroinflammation,30–32 thus offering significant neuronal protection.33 In addition, SCFAs exert anti-inflammatory effects in regions other than the center by regulating the differentiation of T cells.34 It can be conjectured that regulating the intestinal flora disorder following cerebral infarction and increasing the expression of SCFAs can be conducive to reducing the immune inflammatory response, thereby improving neurological function.35
In traditional Chinese medicine, ACI belongs to the category of “stroke disease”. From the perspective of understanding ACI in traditional Chinese medicine, the location of the brain in the upper part of the body, where the cerebral channels crisscross, is a place where qi and blood are vigorous; the disorder of qi and blood and the imbalance of yin and yang are its basic pathogenesis. The stagnation of qi activity causes turbid phlegm to be generated from the inside, and blood stasis forms with chaotic blood circulation. Phlegm and stasis block the meridians or blind the clear orifices, thus inducing disease.
Professor He Puren, the first master of traditional Chinese medicine in China, established the acupuncture treatment system of “He’s Acupuncture San Tong Method” based on his core idea that “diseases are mostly induced by qi stagnation and should be treated with the San Tong method”.36 Among the modes of treatment, fire-needle therapy belongs to the “Wen Tong Method”; here, the needle can dispel cold and congestion from the body and stimulate the channels and meridians with the power of fire and warmth. It has the function of promoting qi and activating blood, as well as warming and smoothing the channels and meridians.36 It is used clinically in the treatment of cerebral infarction and its associated sequelae because of its potential to regulate muscular strength, improve motor and cognitive function after cerebral infarction, and enhance the patient’s daily living abilities.37–39
The results of this randomized clinical trial showed that after 14 days of treatment, the neurological deficits of the two groups improved, and the improvement in the treatment group was better than that of the control group. The Fugl-Meyer Assessment score showed an increasing trend after treatment, and in the treatment group, the improvement in terms of limb reflex activity, mobility, cooperative movement, and finger movement was better than that of the control group. A comparison of the hematological indicators (TNF-α, hs-CRP, and IL-17) using the ELISA detection method revealed that the levels of inflammatory indicators showed a downward trend in both groups, and the decrease in the treatment group was more significant than the control group. The comparison of intestinal SCFAs showed that the proportions of acetic acid, propionic acid, butyric acid, and valeric acid showed an increasing trend post-treatment, and the increase in levels of acetic acid and butyric acid in the treatment group was more significant compared to the control group.
These results are consistent with the hypotheses of previous studies, confirming that fire-needle therapy can promote neurological recovery in patients with ACI, and the efficacy was better than acupuncture alone. It provides some evidence to verify the hypothesis that
fire-needle therapy improves neurological deficits after cerebral infarction, regulates intestinal flora, increases the expression of SCFAs, and inhibits the inflammation response of central and peripheral tissues.
However, further experimental verification is needed, specifically with regard to the structure of intestinal flora, intestinal barrier function, and microglial polarization.
Conclusion
In this trial, we found that fire-needle therapy was effective in upregulating the content of SCFAs in patients with ACI (wind-phlegm-blood stasis syndrome), inhibiting the level of inflammatory response, and promoting the recovery of neurological function.
Abbreviations
NIHSS, National Institute of Health stroke scale; ACI, acute cerebral infarction; SCFAs, Short-chain fatty acids; TNF, tumor necrosis factor; IL, Interleukin; BBB, blood-brain barrier; PCR, Polymerase Chain Reaction; ELISA, Enzyme-linked immunosorbent assay; hs-CRP, High-sensitivity c-reactive protein.
Data Sharing Statement
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Second Teaching Hospital ofTianjin University of Traditional Chinese Medicine (EC.AT/03.19-02/09.0). A written informed consent was obtained from all participants.
Consent for Publication
Consent for publication was obtained from every individual whose data are included in this manuscript.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Funding
This study was supported by a grant from Tianjin Research Innovation Project for Postgraduate Students(2022SKY234).
Disclosure
The authors report no conflicts of interest for this work.
References
1. Chen HZ. Interpretation of Chinese and western medicine diagnosis and treatment guidelines for acute ischemic cerebrovascular disease. Clin J Trad Chin Med. 2013;25(11):949–954.
2. Blasco MP, Chauhan A, Honarpisheh P, et al. Age-dependent involvement of gut mast cells and histamine in post-stroke inflammation. J Neuroinflammation. 2020;17(1):160. doi:10.1186/s12974-020-01833-1
3. Liu Y, Luo S, Kou L, et al. Ischemic stroke damages the intestinal mucosa and induces alteration of the intestinal lymphocytes and CCL19 mRNA in rats. Neurosci Lett. 2017;658:165–170. doi:10.1016/j.neulet.2017.08.061
4. Ye D, Hu Y, Zhu UN, et al. Exploratory investigation of intestinal structure and function after stroke in mice. Mediators Inflammation. 2021;2021:1315797. doi:10.1155/2021/1315797
5. Huang YH. A study on the syndrome of acute stroke. Tianjin Colle Trad Chin Med. 2005;2:3.
6. Du X, Wen XH, Liu DS, et al. Preliminary study on the therapeutic effect and mechanism of fire needle therapy. J Clin Acupun Moxib. 2018;34(09):1–4.
7. Zhang T, Du X, Wang LP, et al. Observation on the therapeutic effect of He’s three way method on improving daily living ability of patients with cerebral infarction during recovery period. Jilin J Chin Med. 2018;38(3):297–300.
8. Peng B, Wu B. Chinese guidelines for the diagnosis and treatment of acute ischemic stroke 2018. Chin J Neurol. 2018;51(9):666–682.
9. Huang L. Research on traditional Chinese medicine for treating premature viscera and meridian identification. GuangZhou Univ Trad Chin Med. 2021;4:9.
10. Lu XL, Luo D, Yao XL, et al. dl-3n-Butylphthalide promotes angiogenesis via the extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase/Akt-endothelial nitric oxide synthase signaling pathways. J Cardiovasc Pharmacol. 2012;59(4):352–362. doi:10.1097/FJC.0b013e3182443e74
11. Xiong Z, Lu W, Zhu L, et al. Dl-3-n-butylphthalide treatment enhances hemodynamics and ameliorates memory deficits in rats with chronic cerebral hypoperfusion. Front Aging Neurosci. 2017;9:238. doi:10.3389/fnagi.2017.00238
12. Al-Khaled M, Matthis C, Eggers J. Statin treatment in patients with acute ischemic stroke. Int J Stroke. 2014;9(5):597–601. doi:10.1111/ijs.12256
13. Hong KS, Lee JS. Statins in acute ischemic stroke: a systematic review. J Stroke. 2015;17(3):282–301. doi:10.5853/jos.2015.17.3.282
14. National standard name: Standardized manipulations of acupuncture and moxibustion - Part 12: Fire acupuncture Standard number: GB/T 21709.12-2009.
15. Xie QW, Xiao JM, Deng XY, et al. A preliminary study on the establishment of Index set of core outcomes of ischemic stroke treated with traditional Chinese medicine. J Traditional Chin Med. 2022;63(03):220–228. doi:10.13288/j.11-2166/r.2022.03.005
16. Gao Q, Han ZY, Tian DF, et al. Xinglou Chengqi Decoction improves neurological function in experimental stroke mice as evidenced by gut microbiota analysis and network pharmacology. Chin J Nat Med. 2021;19(12):881–899. doi:10.1016/S1875-5364(21)60079-1
17. The Fourth National Academic Conference on Cerebrovascular Disease. Scoring standards for clinical neurological function deficit in stroke patients. Chin J Neurol. 1996;29(6):381.
18. Xu RQ. Clinical study on acupuncture and moxibustion treatment of cerebral infarction from heart and gallbladder. Guangzhou Univ Chin Med. 2013;4:7.
19. Huo XH. Clinical study of moxibustion combined with rehabilitation training on spasticity of stroke hemiplegia. Beijing Univ Chin Med. 2014;8:7.
20. Cuenca-Lópe MD, Brea D, Segura T, et al. La inflamación como agente terapéutico en el infarto cerebral:respuesta inflamatoria celulary mediadores inflamatorios[Inflammation as a therapeutic agent in cerebral infarction:cellular inflammatory response and inflammatory mediators]. Rev Neurol. 2010;50(6):349–359.
21. Chen Y, Liang J, Ouyang F, et al. Persistence of gut microbiota dysbiosis and chronic systemic inflammation after cerebral infarction in cynomolgus monkeys. Front Neurol. 2019;10:661. doi:10.3389/fneur.2019.00661
22. O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7(7):688–693. doi:10.1038/sj.embor.7400731
23. Pickard JM, Zeng MY, Caruso R, et al. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017;279(1):70–89. doi:10.1111/imr.12567
24. Yamashiro Y. Gut microbiota in health and disease. Ann Nutr Metab. 2017;71(3–4):242–246. doi:10.1159/000481627
25. Wang H, Zhong D, Chen H, et al. NLRP3 inflammasome activates interleukin-23/interleukin-17 axis during ischaemia-reperfusion injury in cerebral ischaemia in mice. Life Sci. 2019;227:101–113. doi:10.1016/j.lfs.2019.04.031
26. Shichita T, Sugiyama Y, Oobosh H, et al. Pivotal role of cerebral interleukin-17-producinggammadeltaT cells in the delayed phase of ischemic brain injury. Nature Med. 2009;15(8):946–950. doi:10.1038/nm.1999
27. Oyama N, Winek K, Backer-Koduah P, et al. Exploratory investigation of intestinal function and bacterial translocation after focal cerebral ischemia in the mouse. Front Neurol. 2018;9:937. doi:10.3389/fneur.2018.00937
28. Sadler R, Cramer JV, Heindl S, et al. Short-chain fatty acids improve poststroke recovery via immunological mechanisms. J Neurosci. 2020;40(5):1162–1173. doi:10.1523/JNEUROSCI.1359-19.2019
29. Den Besten G, Van Eunen K, Groen AK, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. JLipid Res. 2013;54(9):2325–2340. doi:10.1194/jlr.R036012
30. Wang HB, Wang PY, Wang X, et al. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. 2012;57(12):3126–3135. doi:10.1007/s10620-012-2259-4
31. Louis P, Hold GL, Flight HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10):661–672. doi:10.1038/nrmicro3344
32. Liu C, Li J, Zhang Y, et al. Influence of glucose fermentation on CO(2)assimilation to acetate in homoacetogen Blautia coccoides GA-1. J Ind Microbiol Biotechnol. 2015;42(9):1217–1224. doi:10.1007/s10295-015-1646-1
33. Hou TS, Han XX, Yang Y, et al. Protective effect of electroacupuncture on intestinal microecology in rats with Ulcerative colitis. Acupuncture Res. 2014;39(01):27–34.
34. Agirman G, Yu KB, Hsiao EY. Signaling inflammation across the gut-brain axis. Science. 2021;374(6571):1087–1092. doi:10.1126/science.abi6087
35. Progatzky F, Shapiro M, Chng SH, et al. Regulation of intestinal immunity and tissue repair by enteric glia. Nature. 2021;599(7883):125–130. doi:10.1038/s41586-021-04006-z
36. Li Y, Zhou Z, He XJ. Illustrated Fire Needle Therapy.
37. Yu YL. Clinical observation on the treatment of vascular cognitive impairment with fire acupuncture. Heilongjiang Univ Chin Med. 2022. doi:10.27127/d.cnki.ghlzu.2022.000582
38. Zheng XY, Zhang YH, Song WT, Chang D, Liu JX. Research progress on the pharmacological mechanisms of Chinese medicines that tonify Qi and activate blood against cerebral ischemia/ reperfusion injury. World J Tradit Chin Med. 2022;8(2):225–235. doi:10.4103/wjtcm.wjtcm_21_21
39. Du X, Zhang F, Guo J, et al. Clinical study on He’s fire needle therapy for spastic hemiplegia after cerebral infarction. Acupu Moxibu Clin J. 2021;37(06):41–45.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
