Back to Journals » Cancer Management and Research » Volume 13
Efficacy of Chemotherapy versus Transcatheter Arterial Chemoembolization in Patients with Advanced Primary Hepatic Neuroendocrine Carcinoma and an Analysis of the Prognostic Factors: A Retrospective Study
Authors Li S , Niu M, Deng W , Li N , Wei C, Zhang C, Luo S
Received 9 October 2021
Accepted for publication 1 December 2021
Published 10 December 2021 Volume 2021:13 Pages 9085—9093
DOI https://doi.org/10.2147/CMAR.S343572
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Shuyi Li,1 Mengke Niu,2 Wenying Deng,1 Ning Li,1 Chen Wei,1 Chi Zhang,1 Suxia Luo1
1Department of Medical Oncology, The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Zhengzhou, 450008, Henan Province, People’s Republic of China; 2Department of Oncology, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, Hubei Province, People’s Republic of China
Correspondence: Suxia Luo
Department of Medical Oncology, The Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, No. 127 Dongming Road, Zhengzhou, 450008, Henan Province, People’s Republic of China
Tel +86 371 65587805
Email [email protected]
Background: Primary hepatic neuroendocrine carcinoma (PHNEC) is a rare liver tumor, and there is no clear therapeutic recommendation for patients with advanced PHNEC. This study aims to compare the efficacy of platinum-based chemotherapy (etoposide combined with cisplatin/carboplatin, EP/EC) and transcatheter arterial chemoembolization (TACE) in patients with advanced PHNEC, and to evaluate the relevant prognostic factors.
Patients and Methods: The clinical data of 41 patients with advanced PHNEC from June 2014 to October 2019 were retrospectively reviewed.
Results: At a median follow-up time of 13.9 months, the median overall survival (OS) was 14.8 months in the EP/EC group and 12.2 months in the TACE group (P = 0.040). The median progression-free survival (PFS) was 4.4 months and 2.7 months in the EP/EC group and the TACE group, respectively (P = 0.005). No significant differences in the overall response rate and disease control rate were observed between the EP/EC group and the TACE group (26.1% vs 11.1%, P = 0.429; 73.9% vs 44.4%, P = 0.055, respectively). A univariate analysis indicated that the Eastern Cooperative Oncology Group performance status (ECOG PS), Ki-67, tumor number, and treatment options were prognostic factors for OS. A multivariate analysis further showed that ECOG PS (P < 0.001), Ki-67 (P = 0.003), and treatment options (P = 0.022) were independent prognostic factors for OS.
Conclusion: Ki-67, ECOG PS, and treatment options were the independent prognostic factors for OS in patients with advanced PHNEC. EP/EC may be a better choice for patients with advanced PHNEC.
Keywords: chemotherapy, transcatheter arterial chemoembolization, hepatic neuroendocrine carcinoma, prognosis
Introduction
Neuroendocrine neoplasms (NENs) are a heterogeneous group of tumors that originate from neuroendocrine cells. Neuroendocrine carcinoma (NEC) is defined as poorly differentiated NEN, with a mitotic rate > 20 per 2 mm2 and/or Ki-67 > 20%.1 NEC typically occurs in the lungs and the gastrointestinal tract, and it often metastasizes to the liver. However, primary hepatic neuroendocrine carcinoma (PHNEC) is extremely rare, only accounting for 0.1% of NENs and 0.09–0.15% of malignant liver tumors.2–9 Fewer than 150 cases of PHNEC have been described in English-language articles,10 primarily in case reports.11 Patients with PHNEC lack the typical clinical symptoms, and the most common initial symptoms are abdominal pain, liver mass without significant clinical manifestations, and jaundice. A definitive diagnosis relies on histopathological and immunohistochemical examinations. Due to the rarity of PHNEC, the efficacy of different treatment options in patients with advanced PHNEC is not clear. In this study, we retrospectively analyzed the clinicopathological characteristics, therapeutic approaches, and prognostic factors of 41 patients with advanced PHNEC, aiming to identify efficient treatment options and prognostic factors of advanced PHNEC.
Patients and Methods
Patients
We retrospectively reviewed the clinical data of patients who had been diagnosed with PHNEC in the Affiliated Cancer Hospital of Zhengzhou University from June 2014 to October 2019. The primary inclusion criteria were as follows: (1) histologically confirmed NEC with a hepatic primary location and unresectable or recurrent disease; (2) platinum-based chemotherapy (etoposide combined with cisplatin/carboplatin, EP/EC) or transcatheter arterial chemoembolization (TACE) as the first-line treatment; (3) an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2; and (4) adequate organ function. Finally, 41 patients were included in the study. A response evaluation was performed according to the Response Evaluation Criteria in Solid Tumor (RECIST) version 1.1 based on a computed tomographic (CT) scan of the abdomen. This study was approved by the Research Ethics Committee of the Affiliated Cancer Hospital of Zhengzhou University. All patients were admitted with a signed pan-informed consent form, which included consent to review their medical records.
End Point
Progression-free survival (PFS) was measured from the treatment initiation to disease progression or death from any cause. Overall survival (OS) was measured from the treatment initiation to death from any cause. The overall response rate (ORR) was defined as the proportion of patients with a confirmed complete (CR) or partial response (PR), while the disease control rate (DCR) was defined as the proportion of patients with CR, PR, or stable disease (SD). The primary end point was OS, while the secondary end points included PFS, ORR, and DCR.
Statistical Analysis
All of the statistical analyses were performed using IBM SPSS Advanced Statistics software (version 21; SPSS Inc., Chicago, IL, USA). Intergroup comparisons were conducted using a χ2-test for the categorical variables. Survival rates were estimated using the Kaplan–Meier method and compared using the Log rank test. Prognostic factors were analyzed by searching all of the clinical variables in the univariate analysis. All of the variables with a P-value < 0.1 in the univariate analysis were entered into a multivariate Cox regression analysis. A two-sided P-value < 0.05 was considered to be significant, and 95% confidential intervals (CIs) were calculated.
Results
Patient Characteristics
Forty-one patients satisfying the eligibility criteria were included in the study. The study population was composed of 23 men (56.1%) and 18 women (43.9%). The median age of the patients was 61 years (range, 30–78 years). Abdominal pain was the most common clinical symptom (31.7% of the patients). Eleven patients (26.8%) had no obvious clinical symptoms. Other symptoms were abdominal distension (22.0%), jaundice (12.2%), and nausea or vomiting (7.3%). No hormone-related symptoms were observed. At baseline, 32 patients (78.0%) had an ECOG PS of 0 or 1, and nine patients (22.0%) had an ECOG PS of 2. Among the 41 patients, 23 patients (56.1%) received EP/EC treatment, while 18 patients (43.9%) received TACE treatment. In the EP/EC group, nine patients were treated with EC, and the remaining patients received EP chemotherapy. The median Ki-67 index was 70% (range, 30–90%). The positivity rates of chromogranin A (CgA) and synaptophysin (Syn) were 58.5% and 92.7%, respectively. The characteristics of the patients are presented in Table 1. No significant differences were observed between the two groups.
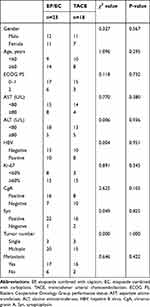 |
Table 1 Baseline Patients’ Characteristics |
Response Evaluation and Survival
In our study, all of the patients were included in the response evaluation. No significant differences in the ORR and DCR were observed between the EP/EC group and the TACE group (ORR: 26.1% vs 11.1%, P = 0.429; DCR: 73.9% vs 44.4%, P =0.055). At the time of analysis, two patients were alive with a median follow-up time of 13.9 months (range, 3.4–21.2 months). According to the results of our study, the medians of the PFS and OS of the EP/EC group were 4.4 months (95% CI, 2.6–5.4 months) and 14.8 months (95% CI, 12.9–15.3 months), respectively. The medians of the PFS and OS of the TACE group were 2.7 months (95% CI, 1.9–3.5 months) and 12.2 months (95% CI, 7.6–14.3 months), respectively. A significant survival benefit was seen in the EP/EC group compared with the TACE group (PFS: P = 0.005, Figure 1A; OS: P = 0.040, Figure 1B). The medians of the PFS of patients who received EP and EC chemotherapy were 4.0 months (95% CI, 2.4–5.7 months) and 3.3 months (95% CI, 3.0–3.6 months), respectively (P = 0.755, Supplementary Figure S1A). The median OS of patients who received EP and EC chemotherapy was 14.0 months (95% CI, 11.6–16.4 months) and 13.1 months (95% CI, 5.8–20.4 months), respectively (P = 0.892, Supplementary Figure S1B). Patients with ECOG PS 0–1 had a significantly longer PFS (4.2 vs 1.9 months, P < 0.001) (Figure 1C) and OS (13.7 vs 8.3 months, P = 0.002) (Figure 1D) than patients with ECOG PS 2. Similarly, patients with a single tumor of the liver had a significantly longer OS compared with those with multiple tumors of the liver (14.7 vs 12.1 months, P = 0.030) (Figure 1E). Patients with Ki-67 < 60% had a significantly longer OS than those with higher Ki-67 levels (15.7 vs 11.3 months, P = 0.003) (Figure 1F). Patients with Ki-67 ≥ 55% who received EP/EC had a significantly longer PFS compared with those who received TACE (5.0 vs 2.8 months, P = 0.001) (Supplementary Figure S2). A univariate analysis identified the ECOG PS (hazard ratio [HR] = 7.699; 95% CI, 2.836–20.899; P < 0.001) and treatment options (HR = 2.593; 95% CI, 1.294–5.196; P = 0.007) as prognostic factors for PFS. The ECOG PS (HR = 3.197; 95% CI, 1.462–6.992; P = 0.004), Ki-67 (HR = 3.358; 95% CI, 1.465–7.697; P = 0.004), tumor number (HR = 3.107; 95% CI, 1.062–9.087; P = 0.038), and treatment options (HR = 1.943; 95% CI, 1.017–3.713; P = 0.044) were the prognostic factors for OS. A multivariate analysis identified the ECOG PS (HR = 9.823; 95% CI, 3.453–27.946; P < 0.001) and treatment options (HR = 3.046; 95% CI, 1.520–6.105; P = 0.002) as the independent prognostic factors for PFS. The ECOG PS (HR = 6.104; 95% CI, 2.419–15.400; P < 0.001), Ki-67 (HR = 3.890; 95% CI, 1.581–9.573; P = 0.003), and treatment options (HR = 2.262; 95% CI, 1.128–4.538; P = 0.022) were significant prognostic factors for OS. The results of the univariate and multivariate regression analyses of the prognostic factors for survival are shown in Tables 2 and 3.
 |
Table 2 Univariate and Multivariate Analyses of Clinicopathologic Factors and Treatment Options for Progression-Free Survival of Patients with Primary Hepatic Neuroendocrine Carcinoma |
 |
Table 3 Univariate and Multivariate Analyses of Clinicopathologic Factors and Treatment Options for Overall Survival of Patients with Primary Hepatic Neuroendocrine Carcinoma |
Adverse Events
The major adverse events according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0 are summarized in Table 4. Regarding hematological adverse events, grade 3/4 adverse events occurred more frequently in the EP/EC group than in the TACE group (leukopenia, 17.4% vs 11.1%; neutropenia, 13.0% vs 5.6%; anemia, 13.0% vs 11.1%; and thrombocytopenia, 13.0% vs 5.6%; respectively). As for non-hematological adverse events, grade 3/4 adverse events were observed more frequently in the EP/EC group than in the TACE group (nausea, 26.1% vs 16.7%; vomiting, 21.7% vs 16.7%; anorexia, 13.0% vs 0, respectively), whereas fatigue, post-embolization syndrome, and bleeding were more common in the TACE group. During the treatment period, no treatment-related death was observed in either of group.
 |
Table 4 Treatment-Related Adverse Events |
Discussion
While the liver is a frequent site of gastrointestinal and lung NEC metastases, PHNEC is extremely rare, only accounting for 0.3–4.0% of all NECs.12,13 Currently, surgical resection is the most effective therapy for PHNEC.3,14,15 TACE, platinum-based chemotherapy (EP/EC), radiofrequency ablation (RFA), and liver transplantation have been used to treat PHNEC in previous cases.3,6,11,16–18 However, a systematic analysis of the treatment outcomes and prognostic factors in patients with PHNEC has not been conducted. In our study, 23 patients with advanced PHNEC received EP/EC, and 18 patients received TACE treatment. The European Society for Medical Oncology (ESMO) guideline recommends EP or EC as first-line chemotherapy for advanced NEC.19 Sorbye et al found no significant differences in outcomes when comparing EP with EC treatments in patients with advanced gastrointestinal NEC.20 Similarly, our study showed that survival did not differ between patients treated with EP and EC chemotherapy. ORR in the EP/EC group and the TACE group were 26.1% and 11.1% (P = 0.429), and DCR in the EP/EC group and the TACE group were 73.9% and 44.4% (P = 0.055).
Patients with advanced gastroenteropancreatic NEC and those with PHNEC16,18,21–23 have a poor prognosis. In our study, the median PFS of the EP/EC group and the TACE group was 4.4 months vs 2.7 months, respectively (P = 0.005), and the median OS was 14.8 months vs 12.2 months, respectively (P = 0.040). Furthermore, patients with Ki-67 ≥ 55% who received EP/EC had a significantly longer PFS than those who received TACE (5.0 vs 2.8 months, P = 0.001). This result is consistent with the observation of Sorbye et al that NENs with Ki-67 ≥ 55% display generally display a better response to platinum-based chemotherapy.20 Iwasa et al included 21 patients with advanced NEC of the hepatobiliary tract and pancreas who received EP as the first-line chemotherapy. Their results showed that the median PFS was 1.8 months and the median OS was 5.8 months.16 Similarly, Park et al reported that the median OS of four patients with advanced PHNEC who received chemotherapy was 11.3 months.11 These results are consistent with our study. In addition, our data indicated that EP/EC was more effective than TACE in prolonging PFS and OS.
Our study identified ECOG PS, Ki-67, and treatment options as the independent prognostic factors for OS in univariate and multivariate regression analyses. Patients with ECOG PS 0–1 had significantly longer median PFS and OS than those with ECOG PS 2. Similarly, several studies reported poorer survival in NEC patients with ECOG PS ≥ 2 than in those with ECOG PS 0–1.20,24–26 Ki-67 is a cellular marker of proliferation.27 In our study, the OS of patients with Ki-67 ≥ 60% was significantly shorter than that in patients with Ki-67 < 60% (11.3 vs 15.7 months, P = 0.003). This result is consistent with several studies that have shown that a high expression of Ki-67 is associated with a worse prognosis in NEC.20,22,23,28,29 According to our study, EP/EC contributed to a significant survival benefit compared with TACE in patients with advanced PHNEC. In contrast, Sorbye et al and Yamaguchi et al reported that survival did not differ between gastroenteropancreatic NEC patients treated with different therapeutic options.20,30 TACE is a specific local treatment applied only to liver tumors, and this is a different treatment modality from systemic chemotherapy. Our study was the first to evaluate the efficacy of systematic chemotherapy with EP/EC and local treatment with TACE in patients with PHNEC. These two previous studies only compared the efficacy of different platinum-based chemotherapy regimens. This may have led to disparate conclusions and deserves to be confirmed in future clinical trials. Similarly, our study showed that survival did not differ between patients treated with EP and EC chemotherapy.
There are several limitations to our study. First, potential selection bias and unmeasured confounders may exist. Second, the sample size was relatively small. The treatment of patients with advanced PHNEC remains a challenge. Novel data on PHNEC are critically needed. The efficacy of EP/ EC vs TACE, and prognostic factors for patients with advanced PHNEC need to be tested in larger cohorts of patients.
In conclusion, Ki-67, ECOG PS, and treatment options are independent prognostic factors for OS in patients with advanced PHNEC. Patients with Ki-67 < 60% and ECOG PS 0–1 have a longer OS. Furthermore, EP/ EC may be a better choice for patients with advanced PHNEC, but further clinical studies are required to validate this conclusion.
Ethics Statement
This retrospective study was approved by the Research Ethics Committee of Affiliated Cancer Hospital of Zhengzhou University. We promise that all the data of the participants was maintained with confidentiality. The study protocol abided by the principles of the Declaration of Helsinki.
Acknowledgments
This work was supported by the National Natural Science Fund Youth Fund of China (8200304), and the Mechanism of TAMs Mediating Immunosuppression Microenvironment of Gastric Cancer via exosomal LncRNA H19/STAT3/PD-L1 Pathway (Science and Technology of Henan Province, 202102310111).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–188. doi:10.1111/his.13975
2. Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–1342. doi:10.1001/jamaoncol.2017.0589
3. Huang YQ, Xu F, Yang JM, Huang B. Primary hepatic neuroendocrine carcinoma: clinical analysis of 11 cases. Hepatobiliary Pancreat Dis Int. 2010;9(1):44–48.
4. Maggard MA, O’Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg. 2004;240(1):117–122. doi:10.1097/01.sla.0000129342.67174.67
5. Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi:10.1200/JCO.2007.15.4377
6. Wang HH, Liu ZC, Zhang G, et al. Clinical characteristics and outcome of primary hepatic neuroendocrine tumors after comprehensive therapy. World J Gastrointest Oncol. 2020;12(9):1031–1043. doi:10.4251/wjgo.v12.i9.1031
7. Li YF, Zhang QQ, Wang WL. Clinicopathological characteristics and survival outcomes of primary hepatic neuroendocrine tumor: a Surveillance, Epidemiology, and End Results (SEER) Population-Based Study. Med Sci Monit. 2020;26:e923375. doi:10.12659/MSM.923375
8. Song JE, Kim BS, Lee CH. Primary hepatic neuroendocrine tumor: a case report and literature review. World J Clin Cases. 2016;4(8):243–247. doi:10.12998/wjcc.v4.i8.243
9. Camargo ÉS, Viveiros Mde M, Corrêa Neto IJ, Robles L, Rezende MB. Primary hepatic carcinoid tumor: case report and literature review. Einstein. 2014;12(4):505–508. doi:10.1590/S1679-45082014RC2745
10. Fenoglio LM, Severini S, Ferrigno D, et al. Primary hepatic carcinoid: a case report and literature review. World J Gastroenterol. 2009;15(19):2418–2422. doi:10.3748/wjg.15.2418
11. Park CH, Chung JW, Jang SJ, et al. Clinical features and outcomes of primary hepatic neuroendocrine carcinomas. J Gastroenterol Hepatol. 2012;27(8):1306–1311. doi:10.1111/j.1440-1746.2012.07117.x
12. Parkash O, Ayub A, Naeem B, et al. Primary hepatic carcinoid tumor with poor outcome. J Coll Physicians Surg Pak. 2016;26(3):227–229.
13. Chen RW, Qiu MJ, Chen Y, et al. Analysis of the clinicopathological features and prognostic factors of primary hepatic neuroendocrine tumors. Oncol Lett. 2018;15(6):8604–8610. doi:10.3892/ol.2018.8413
14. Qiu MJ, Chen YB, Bi NR, Yang SL, He XX, Xiong ZF. Comparative clinical analysis of gastroenteropancreatic neuroendocrine carcinomas with liver metastasis and primary hepatic neuroendocrine carcinomas. Dis Markers. 2018;2018:9191639. doi:10.1155/2018/9191639
15. Jiang S, Wu H, Fu R, et al. The outcome of primary hepatic carcinoid tumor: a Retrospective Study based on propensity score matched survival analysis. Front Oncol. 2021;11:609397. doi:10.3389/fonc.2021.609397
16. Iwasa S, Morizane C, Okusaka T, et al. Cisplatin and etoposide as first-line chemotherapy for poorly differentiated neuroendocrine carcinoma of the hepatobiliary tract and pancreas. Jpn J Clin Oncol. 2010;40(4):313–318. doi:10.1093/jjco/hyp173
17. Okumura Y, Kohashi K, Wang H, et al. Combined primary hepatic neuroendocrine carcinoma and hepatocellular carcinoma with aggressive biological behavior (adverse clinical course): a case report. Pathol Res Pract. 2017;213(10):1322–1326. doi:10.1016/j.prp.2017.06.001
18. Li MX, Li QY, Xiao M, et al. Survival comparison between primary hepatic neuroendocrine neoplasms and primary pancreatic neuroendocrine neoplasms and the analysis on prognosis-related factors. Hepatobiliary Pancreat Dis Int. 2019;18(6):538–545. doi:10.1016/j.hbpd.2019.03.009
19. Pavel M, Öberg K, Falconi M, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(7):844–860. doi:10.1016/j.annonc.2020.03.304
20. Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24(1):152–160. doi:10.1093/annonc/mds276
21. Smith JD, Reidy DL, Goodman KA, Shia J, Nash GM. A retrospective review of 126 high-grade neuroendocrine carcinomas of the colon and rectum. Ann Surg Oncol. 2014;21(9):2956–2962. doi:10.1245/s10434-014-3725-3
22. Bongiovanni A, Riva N, Ricci M, et al. First-line chemotherapy in patients with metastatic gastroenteropancreatic neuroendocrine carcinoma. Onco Targets Ther. 2015;8:3613–3619. doi:10.2147/OTT.S91971
23. Sorbye H, Strosberg J, Baudin E, Klimstra DS, Yao JC. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer. 2014;120(18):2814–2823. doi:10.1002/cncr.28721
24. Freis P, Graillot E, Rousset P, et al. Prognostic factors in neuroendocrine carcinoma: biological markers are more useful than histomorphological markers. Sci Rep. 2017;7:40609. doi:10.1038/srep40609
25. Walter T, Tougeron D, Baudin E, et al. Poorly differentiated gastro-entero-pancreatic neuroendocrine carcinomas: are they really heterogeneous? Insights from the FFCD-GTE national cohort. Eur J Cancer. 2017;79:158–165. doi:10.1016/j.ejca.2017.04.009
26. Shiba S, Morizane C, Hiraoka N, et al. Pancreatic neuroendocrine tumors: a single-center 20-year experience with 100 patients. Pancreatology. 2016;16(1):99–105. doi:10.1016/j.pan.2015.11.001
27. Sobecki M, Mrouj K, Colinge J, et al. Cell-cycle regulation accounts for variability in Ki-67 expression levels. Cancer Res. 2017;77(10):2722–2734. doi:10.1158/0008-5472.CAN-16-0707
28. Welin S, Sorbye H, Sebjornsen S, Knappskog S, Busch C, Oberg K. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer. 2011;117(20):4617–4622. doi:10.1002/cncr.26124
29. Olsen IH, Sørensen JB, Federspiel B, et al. Temozolomide as second or third line treatment of patients with neuroendocrine carcinomas. Sci World J. 2012;2012:170496. doi:10.1100/2012/170496
30. Yamaguchi T, Machida N, Morizane C, et al. Multicenter retrospective analysis of systemic chemotherapy for advanced neuroendocrine carcinoma of the digestive system. Cancer Sci. 2014;105(9):1176–1181. doi:10.1111/cas.12473
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

