Back to Journals » OncoTargets and Therapy » Volume 12
Efficacy of afatinib in a HER2 amplification-positive endometrioid adenocarcinoma patient– a case report
Authors Zhou L, Ren Y, Wang X, Miao D, Lizaso A , Li H, Han-Zhang H, Qian J, Yang H
Received 25 February 2019
Accepted for publication 20 June 2019
Published 4 July 2019 Volume 2019:12 Pages 5305—5309
DOI https://doi.org/10.2147/OTT.S206732
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Li Zhou,1,* Yifeng Ren,2,* Xia Wang,1 Dongliu Miao,3 Analyn Lizaso,4 Haiyan Li,4 Han Han-Zhang,4 Jun Qian,1 Hui Yang1
1Oncology Department, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou, Jiangsu, People’s Republic of China; 2Hepatobiliary and Pancreatic Surgery Department, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou, Jiangsu, People’s Republic of China; 3Interventional Department, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou, Jiangsu, People’s Republic of China; 4Burning Rock Biotech, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Abstract: Afatinib has improved the prognosis of epidermal growth factor receptor-positive advanced non-small cell lung cancer and has been explored in the treatment of human epidermal growth factor receptor 2 (HER2)-amplified breast cancer. However, its clinical efficacy in HER2-amplified endometrial cancer has not been reported. Herein, we present the clinical benefit of afatinib in a case of stage IIIC endometrioid adenocarcinoma refractory to multiple lines of chemotherapy and eventually developed pulmonary, abdominal and pelvic metastasis. Upon referral to our clinic, capture-based targeted sequencing was performed on both blood and tumor samples and revealed HER2 amplification. The patient was administered with afatinib and achieved partial response (PR) after two months of treatment, reflected by a significant reduction in pulmonary lesions and serum levels of tumor markers including carcinoembryonic antigen (CEA), cancer antigen (CA) 19-9, 125, 15-3 and cytokeratin 19 fragment antigen 21-1 (CY211). The patient passed away after 3 months of afatinib treatment due to suspected complications of severe intestinal obstruction. Our report demonstrates the efficacy of afatinib in a heavily pre-treated HER2-amplified endometrial cancer patient with multi-organ metastasis. This case also highlights the need to include comprehensive mutational profiling in the standard management of endometrial cancer patients for treatment guidance.
Keywords: afatinib, HER2-amplification, HER-2 positive, endometrial cancer, case report, lung metastasis
Introduction
Cancer of the corpus uteri, commonly referred to as endometrial cancer, is the second most common gynecological cancer in China.1 The most frequent clinical symptom of endometrial cancer is abnormal uterine bleeding, with about 75–90% of women diagnosed presenting with this symptom.2 The standard treatment approach for endometrial cancer consists of surgery followed by chemotherapy and/or radiotherapy.3,4
Endometrial carcinoma is further categorized into two distinct subtypes: types 1 and 2 based on the differences in histology, risk factors, clinical behaviors and molecular profile. Type 1 endometrial carcinoma, including endometrioid adenocarcinoma, generally express estrogen and progesterone receptors and is associated with a higher frequency of mutations in KRAS, PTEN and mismatch repair genes.5 In contrast, a majority of type 2 endometrial carcinomas have aneuploidy, TP53 mutations6 and HER2 amplification.7,8
Gene amplification and protein overexpression of ERBB2, commonly referred to as HER2, is often associated with more aggressive tumor biology, poor prognosis and has become an effective therapeutic target in a number of cancers, including breast,9 ovarian,10 and gastric.11 US Food and Drug Administration-approved agents targeting HER2 amplification include monoclonal antibodies such as trastuzumab and tyrosine kinase inhibitors (TKIs) such as lapatinib have significantly improved the prognosis of HER2-positive breast and gastric cancer,12,13 but was shown to be ineffective for HER2-positive advanced or recurrent endometrial carcinoma.14,15 Possible primary resistance mechanisms to HER2 inhibitors includes increased expression of truncated p95HER2 that lacks the trastuzumab binding site16 and increased mutation rate of PIK3CA in endometrial cancer.17,18 On the other hand, other TKIs, neratinib and afatinib, showed efficacy in HER2-amplified serous endometrial carcinoma in vitro and in vivo, highlighting the potential of such agents in clinical settings.19,20 Afatinib, a second generation TKI, irreversibly binds to and inhibits epidermal growth factor receptor (EGFR) and HER2.21 It has been approved for the treatment of EGFR-mutant advanced non-small cell lung cancer patients.22 Despite showing promising results in patients with HER2-amplified breast cancer in phase II clinical trials,23 afatinib did not provide any benefit in phase III trials.24 Thus far, no study has reported the clinical benefits of afatinib in HER2-amplified endometrial cancer. In this study, we report the efficacy of afatinib in a heavily pre-treated chemotherapy-refractory HER2-amplified endometrial cancer patient with multi-organ metastasis. In addition, this report also highlights the need for comprehensive mutational profiling of endometrial cancer patients to guide treatment decisions.
Case report
Prior to referral to our clinic, a 49-year old female patient with no history of chronic disease was examined in a gynecology clinic in September 2015 due to complaint of abnormal vaginal bleeding. Cervical thinprep cytology test (TCT) showed atypical glandular cells. Subsequently, the patient underwent laparoscopic radical hysterectomy, bilateral salpingo-oophorectomy and pelvic lymphadenectomy. Pathologic examination of surgical tissues revealed moderately differentiated endometrioid adenocarcinoma, involving the lower uterine segment invading the muscular layer and left side of the uterus. Results from immunohistologic analysis were positive for ER, PR, P16, CerbB-2 (3+), Ki67 (70%) and negative for P53, vimentin, and CEA. The patient was diagnosed with T3bN1M0 (stage IIIC) endometrioid adenocarcinoma according to the 2009 TNM/FIGO classification. From the time of diagnosis to May 2016, the treatment regimen of the patient included 2 cycles of adjuvant chemotherapy with liposomal paclitaxel and carboplatin, 4 cycles of chemotherapy with synchronous radiotherapy and 4 cycles of chemotherapy with liposomal paclitaxel and carboplatin. Despite having received multiple lines of chemotherapy and radiotherapy, the patient developed pulmonary metastasis in early June 2016. After 3 cycles of liposomal doxorubicin and nedaplatin chemotherapy, treatment response of the pulmonary lesions were assessed with thoracic computed tomography (CT) which revealed disease progression based on RECIST 1.1 criteria.25 The treatment regimen was then switched to gemcitabine and cisplatin; however, the patient remained refractory to treatment and developed metastasis to the lungs, abdomen and pelvis in early September 2017. A ureteral stent was implanted on her right ureter due to hydronephrosis following ureteroscopy. The patient also underwent pelvic brachytherapy for the pelvic metastases in late September. In late November, the patient presented with incomplete intestinal obstruction. The patient underwent stereotactic gamma knife radiosurgery for the lung metastases in December 2017.
Upon referral to our clinic in January 2018, the patient, refractory to multiple lines of chemotherapy and radiotherapy, with partial intestinal obstruction, scored 3 upon assessment of her ECOG performance status (PS) and had unknown genetic status. To understand the mutation profile of the patient, both plasma and preserved endometrial tissue sample from the previous hysterectomy were subjected to targeted sequencing using a panel consisting of 295 cancer-related genes. Mutational analysis revealed HER2 (ERBB2) amplification with a copy number of 4.22 and 22.19 in the plasma and tissue samples, respectively. In addition, ARID1A and TP53 mutations were also detected from both plasma and tissue samples. CTNNB1, SPEN, ACVR1B mutations and CCNE1 amplification were only observed in the plasma sample. Due to no information on the standard of care available and a PS of 3, we have decided to administer afatinib to the patient. After one month of treatment with afatinib at a dose of 40 mg once daily, the dose was adjusted to 30 mg once daily due to severe diarrhea. No other adverse effect was observed. After 2 months of afatinib treatment, the patient achieved partial response (PR) reflected by the reduction of pulmonary lesions according to RECIST (Figure 1). In addition, serum levels of CEA, CA19-9, CA125, CA15-3 and CY211 gradually decreased in response to the treatment, with reductions between 62–79% after 2 months. On the other hand, neuron-specific enolase (NSE), a specific tumor marker for small cell lung cancer,26 remained constant (Figure 2). However, abdominal CT could not accurately assess pelvic lesions due to severe intestinal adhesions. Partial response was maintained from January 24 until her death on April 18, 2018. Disease progression was not observed until her death. The cause of her death was suspected to be complications from intestinal obstruction, including diffuse intravascular coagulation and gastrointestinal bleeding following sepsis.
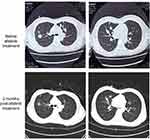 |
Figure 1 Thoracic computed tomography images illustrating the size reduction of the pulmonary lesions after 2 months of afatinib treatment (lower panels) as compared to baseline (top panels). |
Discussion
HER2 amplification is often associated with more aggressive tumor behavior and poor prognosis in a number of cancers, including endometrial cancer. HER2 inhibitors such as trastuzumab and lapatinib have improved the prognosis of HER2-amplified breast and gastric cancer patients.12,13 Unfortunately, both trastuzumab and lapatinib were proven to be ineffective in HER2-amplified endometrial cancer in 2 phase II clinical trials.14,15 Possible primary resistance mechanisms to HER2 inhibitors include increased expression of truncated p95HER2 that lacks the trastuzumab binding site,16 increased signaling thru PI3K pathway17,18,27,28 and MET activation.29,30 Hence, the current standard of treatment for recurrent/metastatic endometrial cancer remains to be chemotherapy. Herein, we report a case demonstrating the clinical benefit of afatinib in a heavily pre-treated, HER2-amplified stage IIIC endometrioid adenocarcinoma patient who was refractory to multiple lines of chemotherapy and developed pulmonary, abdominal and pelvic metastasis accompanied by intestinal obstruction. The patient achieved PR after 2 months of treatment, possibly due to the absence of concurrent oncogenic mutations in PIK3CA and other genes involved in PI3K pathway, which has been implicated as one of the primary HER2 inhibitor resistance mechanisms.17,31 The patient remained responsive to afatinib treatment until her death with a progression-free survival of 3 months. Schwab et al have demonstrated the efficacy of afatinib against HER2-amplified endometrial cancer in uterine serous carcinoma (USC) cell lines and mice harboring xenografts of HER2-amplified USC.20 Our case demonstrates the efficacy of afatinib in a HER2-amplified endometrial cancer patient, extending the encouraging pre-clinical findings to bedside. We are anticipating the findings from the phase 2 clinical trial on the benefit of afatinib in HER2-amplified stage I-IV persistent or recurrent endometrial patients (NCT02491099). In addition, this case also highlights the importance of comprehensive mutational profiling. Apart from the assessment of Lynch syndrome and genetic testing for mutations in mismatch repair genes,32 mutational profiling is also necessary for treatment guidance. Hence, we endorse the incorporation of comprehensive mutational profiling in the standard management of endometrial cancer patients.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of The Affiliated Suzhou Hospital of Nanjing Medical University and/or National Research Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standard.
Patient informed consent
Written informed consent was obtained from the family of the patient described in this report.
Availability of data and materials
All the data generated during this study are included in this published article. The datasets analyzed during the current study are available from the corresponding authors on reasonable request.
Acknowledgments
The authors thank the patient and her family. We also thank the investigators, study coordinators, operation staff and the whole project team who worked on this case and Dr. Lu Zhang of Burning Rock Biotech for helpful discussions. This work was supported by the Suzhou Commission of Health and Family Planning under grant number LCZX201713 and Nanjing Medical University under grant number 2017NJMU164.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jiang X, Tang H, Chen T. Epidemiology of gynecologic cancers in China. J Gynecol Oncol. 2018;29:e7.
2. Kimura T, Kamiura S, Yamamoto T, Seino-Noda H, Ohira H, Saji F. Abnormal uterine bleeding and prognosis of endometrial cancer. Int J Gynaecol Obstet. 2004;85:145–150.
3. Greer BE, Koh WJ, Abu-Rustum N, et al. Uterine Neoplasms. Clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2009;7:498–531.
4. Kesterson JP, Fanning J. Fertility-sparing treatment of endometrial cancer: options, outcomes and pitfalls. J Gynecol Oncol. 2012;23:120–124.
5. Lax SF, Kendall B, Tashiro H, Slebos RJ, Hedrick L. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer. 2000;88:814–824.
6. Doll A, Abal M, Rigau M, et al. Novel molecular profiles of endometrial cancer-new light through old windows. J Steroid Biochem Mol Biol. 2008;108:221–229.
7. Rolitsky CD, Theil KS, McGaughy VR, Copeland LJ, Niemann TH. HER-2/neu amplification and overexpression in endometrial carcinoma. IntJ Gynecol Pathol. 1999;18:138–143.
8. Santin AD, Bellone S, Van Stedum S, et al. Amplification of c-erbB2 oncogene: a major prognostic indicator in uterine serous papillary carcinoma. Cancer. 2005;104:1391–1397.
9. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science (New York, NY). 1987;235:177–182.
10. Berchuck A, Kamel A, Whitaker R, et al. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990;50:4087–4091.
11. Tanner M, Hollmen M, Junttila TT, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–278.
12. Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283.
13. Opdam FL, Guchelaar H-J, Beijnen JH, Schellens JHM. Lapatinib for advanced or metastatic breast cancer. Oncologist. 2012;17:536–542.
14. Fleming GF, Sill MW, Darcy KM, et al. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2010;116:15–20.
15. Leslie KK, Sill MW, Lankes HA, et al. Lapatinib and potential prognostic value of EGFR mutations in a Gynecologic Oncology Group phase II trial of persistent or recurrent endometrial cancer. Gynecol Oncol. 2012;127:345–350.
16. Growdon WB, Groeneweg J, Byron V, et al. HER2 over-expressing high grade endometrial cancer expresses high levels of p95HER2 variant. Gynecol Oncol. 2015;137:160–166.
17. Black JD, Lopez S, Cocco E, et al. PIK3CA oncogenic mutations represent a major mechanism of resistance to trastuzumab in HER2/neu overexpressing uterine serous carcinomas. Br J Cancer. 2015;113:1020–1026.
18. Diver EJ, Foster R, Rueda BR, Growdon WB. The therapeutic challenge of targeting HER2 in endometrial cancer. Oncologist. 2015;20:1058–1068.
19. Schwab CL, English DP, Roque DM, et al. Neratinib shows efficacy in the treatment of HER2/neu amplified uterine serous carcinoma in vitro and in vivo. Gynecol Oncol. 2014;135:142–148.
20. Schwab CL, Bellone S, English DP, et al. Afatinib demonstrates remarkable activity against HER2-amplified uterine serous endometrial cancer in vitro and in vivo. Br J Cancer. 2014;111:1750.
21. Yap TA, Vidal L, Adam J, et al. Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J Clin Oncol. 2010;28:3965–3972.
22. Keating GM. Afatinib: a review in advanced non-small cell lung cancer. Target Oncol. 2016;11:825–835.
23. Lin NU, Winer EP, Wheatley D, et al. A phase II study of afatinib (BIBW 2992), an irreversible ErbB family blocker, in patients with HER2-positive metastatic breast cancer progressing after trastuzumab. Breast Cancer Res Treat. 2012;133:1057–1065.
24. Hurvitz SA, Shatsky R, Harbeck N. Afatinib in the treatment of breast cancer. Expert Opin Investig Drugs. 2014;23:1039–1047.
25. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216.
26. Satoh H, Ishikawa H, Kurishima K, Yamashita YT, Ohtsuka M, Sekizawa K. Cut-off levels of NSE to differentiate SCLC from NSCLC. Oncol Rep. 2002;9:581–583.
27. Kang S, Seo SS, Chang HJ, Yoo CW, Park SY, Dong SM. Mutual exclusiveness between PIK3CA and KRAS mutations in endometrial carcinoma. Int J Gynecol Cancer. 2008;18:1339–1343.
28. Oda K, Stokoe D, Taketani Y, McCormick F. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res. 2005;65:10669–10673.
29. Shattuck DL, Miller JK, Carraway KL
30. Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–280.
31. Coco S, Truini A, Alama A, et al. Afatinib resistance in non-small cell lung cancer involves the PI3K/AKT and MAPK/ERK signalling pathways and epithelial-to-mesenchymal transition. Target Oncol. 2015;10:393–404.
32. Lancaster JM, Powell CB, Chen LM, Richardson DL. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015;136:3–7.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

