Back to Journals » Infection and Drug Resistance » Volume 16
Efficacy of a Novel Antibacterial Agent Exeporfinium Chloride, (XF-73), Against Antibiotic-Resistant Bacteria in Mouse Superficial Skin Infection Models
Authors Zhang C, Li J, Lu R, Wang S, Fu Z, Yao Z
Received 12 May 2023
Accepted for publication 12 July 2023
Published 25 July 2023 Volume 2023:16 Pages 4867—4879
DOI https://doi.org/10.2147/IDR.S417231
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Chenrui Zhang,1 Jinping Li,2 Rong Lu,2 Song Wang,2 Zheng Fu,2 Zhi Yao1
1Department of Immunology, Key Laboratory of Immune Microenvironment and Disease of the Educational Ministry of China, School of Basic Medical Sciences, Tianjin Medical University, Tianjin, People’s Republic of China; 2Kangzhe Pharmaceutical Technology Development Company, Ltd., Tianjin, People’s Republic of China
Correspondence: Zhi Yao, Tianjin Medical University, Tianjin, 300070, People’s Republic of China, Tel/Fax +8622-83336817, Email [email protected] Zheng Fu, Kangzhe Pharmaceutical Technology Development Company, Tianjin, 300110, People’s Republic of China, Tel/Fax +86 13821232027, Email [email protected]
Background: The number of incidences of antimicrobial resistance is rising continually, necessitating new and effective antibacterial drugs. The present study aimed to assess the in vitro and in vivo activity of XF-73 against antibiotic-resistant Staphylococcus aureus (S. aureus) isolates and to investigate the potential mechanism of action of XF-73.
Methods: The in vitro antibacterial activity of XF-73 and comparator antibacterial drugs, (mupirocin, fusidine, retapamulin, vancomycin, erythromycin, linezolid and daptomycin), against S. aureus (both antibiotic sensitive and resistant strains) was assessed using a broth microdilution method. Two different superficial Staphylococcal skin infection murine models were established to study the in vivo efficacy of XF-73 against antibiotic-resistant strains. The effect of XF-73 on the ultrastructure and cellular morphology of S. aureus was studied using transmission electron microscopy (TEM) and scanning electron microscopy (SEM).
Results: The MICs (minimum inhibitory concentration) determined by the broth microdilution method for XF-73 demonstrated that the compound had a high potency against S. aureus isolates with varying susceptibility to the study drugs. Also, the antibacterial activity of XF-73 was superior or similar to most of the tested antibacterial drugs. We also found that the XF-73 dermal formulation significantly inhibited S. aureus survival in both the murine skin tape-stripping and suture superficial skin infection models, maintained a consistently high inhibitory capacity against the antibiotic-resistant strains tested and was significantly more effective than mupirocin ointment, a commonly used antibiotic for the treatment of skin infections. The morphological studies using TEM suggest that XF-73 had a rapid (2 minute) bacterial cell wall disruption activity, with longer incubation (10 minute) subsequently causing membrane damage. SEM analysis demonstrated that this cell wall and cell membrane disruption did not lead to disintegration of the plasma membrane, and did not cause bacterial cell lysis.
Conclusion: Therefore, XF-73 may be an effective drug alternative to combat multi-drug-resistant skin infections in the clinical setting.
Keywords: antibiotic resistance, mupirocin-resistant strains, skin infections, bacterial cell membrane, in vivo, S. aureus
Introduction
Skin and soft tissue infections (SSTIs) are some of the most common bacterial infections. Many bacteria and fungi species reside on the surface of human skin which are usually prevented from entering the body by the intact skin barrier and do not pose a threat of infection. However, in the case of skin damage, bacteria may invade the skin and subcutaneous tissue to cause infection. SSTIs are mainly caused by Gram-positive bacteria, especially S. aureus. In addition, SSTIs are clinical conditions that vary in severity, etiology, and presentation. They include microbial invasion of the skin and underlying soft tissues and range in severity from relatively minor to extremely serious, ie, fatal infections.1 Infections limited to the skin and underlying superficial soft tissue are termed simple infections; for example, cellulitis, impetigo, and trauma-associated infections. A simple infection is characterized by the absence of systemic symptoms that indicate the spread of infection and the absence of complications that could make treatment challenging. For simple infections, topical antimicrobials, such as the topical antibiotics mupirocin and fusidic acid, are optimal.2
In vitro studies have demonstrated that mupirocin exhibits good antimicrobial activity against Gram-positive cocci such as S. aureus, Staphylococcus epidermidis, and other β-hemolytic streptococci,3 which are the most common pathogens associated with primary and secondary skin infections. Mupirocin (2% w/w) is a marketed ointment (Bactroban®). Clinical trials have demonstrated the safety and efficacy of mupirocin in the treatment of primary and secondary skin infections.4,5 However, widespread, long-term use of this antibiotic has lead to the emergence of resistance, which is a significant public health concern.6 Therefore, the treatment of SSTIs requires new antibacterial agents with broad-spectrum activity, low resistance development potential, and activity against drug-resistant and multidrug-resistant strains.
Destiny Pharma plc has developed a novel, proprietary, synthetic dicationic porphyrin drug, XF-73 (exeporfinium chloride). Preliminary in vitro studies demonstrated that XF-73 has high potency against clinically isolated Gram-positive bacteria, including those resistant to many antibiotics.7 The potential for four methicillin-resistant S. aureus (MRSA) isolates to generate mutational resistance to XF-73 has been examined, and the results demonstrated that, compared to control antibiotics (mupirocin, daptomycin and vancomycin), the intrinsic activity of XF-73 encountered no resistance over 55 passages at sub-inhibitory concentrations.8 XF-70, (a regioisomer of XF-73), has been shown to have a significant antibacterial effect in an in vivo mouse model of MRSA infected burn wounds when administered in an aqueous solution.9 Therefore, we have further examined the in vitro antibacterial activity of XF-73 against sensitive and resistant S. aureus strains and compared it to selected antibiotics. In vivo antibacterial activities of a XF-73 dermal formulation (0.2% w/w) was assessed alongside mupirocin (2% w/w) ointment in murine Staphylococcal skin infection models in order to assess the pharmacological potential of XF-73 as a novel topical treatment for SSTIs. Finally, the potential mechanism of action of XF-73 was explored by investigating the morphological changes post XF-73 exposure via transmission and scanning electron microscopy.
Materials and Methods
Experimental Animals
This study purchased 6–8-week-old female Balb/C mice from the Academy of Military Medical Science (Beijing, China) and housed them in polycarbonate cages with one animal each. The animals were acclimatized for one week in an approved climate-controlled facility with 12 h light/dark cycle with free access to water and sterile rodent chow. All animal experiments were undertaken following approval by the Animal Ethics Committee at Tianjin Medical University, Tianjin, China.(No. TMUaMEC 20210517) and were in accordance with the Guide for the Care and Use of Laboratory Animals.
Bacterial Strains and Growth Media
The bacterial strains used in the study are listed in Table 1. SA006, SA007, SA009, SA0011, and SA0013 were derived from clinical isolates. NRS384-MT-1, NRS384-MT-2, and NRS384-MT-3 are strains with low-levels of mupirocin-resistance induced by exposure of the NRS384 strain to increasing concentrations of mupirocin. S. aureus ATCC BAA-1708, a high-level mupirocin-resistant strain, was purchased from Microbiologics (CA, USA). The organism was revived from storage at −80° C and grown on Muller-Hinton agar (MHA, Oxoid, UK) plates for solid culture or in Muller-Hinton broth (MHB, Oxoid) for liquid culture.
 |
Table 1 S. aureus Strains Used in This Study |
Drugs and Test Compounds
XF-73 was provided by Destiny Pharma plc (UK). Mupirocin, fusidine, retapamulin, vancomycin, erythromycin, linezolid, and daptomycin were purchased from Selleck. Test compounds were prepared in dimethyl sulfoxide (DMSO) (Sigma) stock solutions at a concentration of 12.8 mg/mL. To determine the minimum inhibitory concentration (MIC) of mupirocin against the high-level mupirocin-resistant strain, a second stock concentration of 1.6384 g/mL was prepared. For the in vivo animal experiments, XF-73 was obtained from Destiny Pharma Plc (UK) supplied as a dermal formulation designed for skin application and stored in a dark vial. Mupirocin ointment was obtained as a commercial preparation (Sino-US Tianjin SmithKline Pharmaceutical Co., Ltd.). The control group was topically administered with an equivalent volume of water, (control group).
Antibiotic Susceptibility Testing Methods
The MIC determination of XF-73 and other test antibacterial drugs against S, aureus (both antibiotic sensitive and resistant strains) was performed using the microdilution broth method, as suggested by Clinical and Laboratory Standards Institute (CLSI, 2021 edition).10 Briefly, the target strains for the antimicrobial activity assay were grown at 37°C to the log-phase in MHB and were diluted to 1×106 colony-forming units (CFUs)/mL. A volume of 99 μL of cell suspension and 1 μL of two-fold serially diluted antibacterial agent stock solution was added to each well. After incubation at 37°C for 18–24 h, the MICs were determined by identifying the lowest antimicrobial concentration of antibacterial agents at which visible growth was absent. All tests were conducted in triplicate.
Induction of Mupirocin Resistance
In order to induce mupirocin-resistance, the MIC of mupirocin on NRS384 was first determined by the agar dilution method. Specifically, NRS384 was inoculated on MHA and incubated at 37 °C for 18 h. A single colony was then picked from the plate and incubated overnight at 37 °C, 220 rpm, in 5 mL MHB to prepare a bacterial suspension. The turbidity of the bacterial suspension was adjusted to 0.3 McFarland standard (approximately 1.0×108 CFU/mL) followed by a ten-fold dilution with MHB. Mupirocin was solubilized in DMSO, and serial two-fold dilutions ranging from 6.4–0.00625 mg/mL were added to the media at 1:100 dilutions. Then, 5 µL of the prepared bacterial suspension was added to the medium containing the appropriate drug concentration and incubated at 35 °C for 18 h in a humid microaerobic atmosphere. The MIC was defined as the lowest concentration of the drug at which visible growth was absent after 18 hours.11
After the baseline MIC was determined, NRS384 grown on MHA was swabbed onto MHA containing 2 x MIC mupirocin and incubated for 48 h. Surface growth was isolated by transferring to antimicrobial-free medium, and transferred to fresh agar plates containing twice the previous mupirocin concentration, this process was repeated serially until no growth was present. The isolates were passaged on antimicrobial-free media at least 10 consecutive times, and the MIC was reevaluated to assess the stability of the selected resistance. When NRS384 was transferred successively to a series of MHA plates containing doubled concentrations of mupirocin, the MIC of mupirocin to the strain increased from 32- to 64-fold. The resistant strain with a 64-fold mupirocin MIC increase was selected for further experimentation.12,13
Mupirocin MIC Interpretative Criteria
Mupirocin-resistant phenotypes of staphylococci can be divided into two types: those with high-level resistance (MIC ≥512 µg/mL) and those with low-level resistance (MIC = 8–256 µg/mL).
Skin Tape-Stripping Infection Model
Six- to eight-week-old female Balb/c mice were used in all experiments. Zoletil 50 (a mixture of the sedative tiletamine and the muscle relaxant zolazepam in sterile water) was used to anesthetize the mice for 1h by intraperitoneal injection of 75 mg/kg during surgery and infection. The anesthetics sedated the mice for around one hour, which provided enough time to perform the procedures.
The dorsal area of the mice was shaved with a clipper, any remaining fur was removed with hair-removal lotion, and the hairless areas were cleaned with wet gauzes. These areas (approximately 2 cm2) were then tape-stripped 80 times with pieces of elastic surgical adhesive bandage until the skin became visibly damaged, ie, reddened and glistening but without regular bleeding. For each mouse, 16 pieces of tape were used, and each piece was used five times.14–16
An inoculum of 107 cells from overnight cultures of S. aureus NRS384, S. aureus NRS384-MT-3, or S. aureus ATCC BAA-1708 (in the stationary phase) as a 15 µL droplet was placed on the surface of the stripped skin and spread across the abraded area with a pipette tip to initiate the infection. Approximately 12 h post-infection, the mice were treated with 50 mg 2% mupirocin ointment or 50 mg 0.2% (w/w) XF-73 formulation. Tubes containing the different dermal drug formulations were weighed before and after administration to determine the dose received per mouse. Mice administered with an equivalent volume of water were served as infection controls.
On day 2 after modeling, 18 h after the last topical application, infected mice were sacrificed by cervical dislocation and a 1×2 cm2 area of the wound of about 0.05 g was excised aseptically, and any remaining formulation attached to the wounds was scraped off. The skin samples were then placed in 5 mL round-bottomed tubes containing 3 mL sterile saline, minced with sharp scissors, and homogenized using a T 10 basic ULTRA-TURRAX Disperser (IKA, Germany) set at 30,000 rpm for 120 s. Subsequently, 100 µL of a range of dilutions of the homogenates were plated onto MHA plates to determine the number of CFU. The plates were incubated at 37 °C for 20 h, and the colonies were counted. Three independent experiments were conducted to investigate the reproducibility of infection with bacteria. In each assay, the number of CFU/mL was determined, and log10 of the counts was calculated to analyze the data.
Suture-Superficial Skin Infection Model
Mice were anesthetized, and fur removed as described earlier. Sterile silk suture thread (3/0-gauge silk suture material) was cut into 8.5 cm lengths and soaked in broth cultures of S. aureus NRS384, S. aureus NRS384-MT-3 or S. aureus ATCC BAA-1708 (109 CFU/mL) for 30 min. Then, the sutures were removed aseptically, dried on sterile filter paper, and stored at 4 °C until use. A 2 cm length of inoculated suture was then inserted under the skin of the mid-back and knotted at both ends to secure it. An incision was then made along the length of the suture without reaching the panniculus carnosus. Adhesive temporary skin closure strips (Steri Strip, 3M) were used to close the wounds, allowing the animals to recover more quickly. To quantify viable bacteria loaded on the sutures, 1 cm lengths (n=3) of the sutures were incubated in 1 mL of MHB at 37 °C, 220 rpm, for 45 min. Subsequently, 100 µL of each dilution (three times), including the original suspension, was transferred to MHA plates and incubated at 37 °C for 20 h. Colonies on plates containing 30–300 CFU were counted, then the number of CFU applied per wound was determined, and the Log10 of the counts calculated to analyze the data.17–20
Mice received single topically applied doses of mupirocin or XF-73 dermal formulation, as previously described, about 24 h after suture insertion. On the third day of the study, 18 h post-dosing, the animals were sacrificed by cervical dislocation. Each lesion and the surrounding skin (approximately 2 cm2, 0.05 g) was excised and homogenized, and the colonies counted as described above to determine the bacterial burden.
Statistical Analysis
For each model, the efficacies of XF-73 and mupirocin treatments were compared with the infection control. Each comparison was made by using one-way ANOVA and the least significant difference (LSD) to determine the P-values for both groups with P-values of ≤0.05 considered as significant.
Transmission Electron Microscopy and Scanning Electron Microscopy
To study the morphological changes induced in bacterial cells following exposure to XF-73, TEM was used to observe changes in the bacterial cell walls, membranes, and cytoplasm after treatment with different XF-73 concentrations and different incubation times.
Briefly, S. aureus ATCC29213 was incubated in LB broth at 37 °C, 220 rpm, until the OD 600 nm value reached 0.8 and was then treated with 1 x or 4 x MIC concentrations of XF-73 for 2 or 10 min. The S. aureus cells treated with XF-73 were then collected by centrifugation (5000 x g, 15 min) and washed twice with phosphate buffered saline (PBS). The washed precipitates were fixed overnight at 4 °C with 2.5% glutaraldehyde and postfixed in 1% OsO4.
After washing, the bacterial pellets were dehydrated in graded ethanol and embedded in EPON 812 resin. Subsequently, ultrathin sections (70 nm) were sliced and collected on copper grids, stained with uranyl acetate and lead citrate, and observed under an electron microscope (HT7700; Hitachi, Japan).21 Bacterial cells not treated with XF-73 were used as controls.
SEM was used to observe any changes in bacterial cell morphology after XF-73 exposure. The post-exposure washing and fixing process for the bacteria was the same as that used for TEM. After the routine washing and dehydration steps, the bacteria were transferred to a mixture (1:1, v/v) of absolute ethanol and tert-butanol for 20 min and then to pure tert-butanol for 30 min. After freeze-drying and gold plating, the bacterial specimens were analyzed using a scanning electron microscope (Gemini SEM 300).22
Results
In vitro Antimicrobial Potency
The in vitro antibacterial activity of XF-73 was determined using a broth microdilution method and the results demonstrated that the antibacterial activity of XF-73 against S. aureus isolates (both sensitive and resistant strains). (Table 1) was superior or comparable to that of most of the tested antibacterial drugs, with the presence of antibiotic resistance mechanisms having no impact on the MIC for XF-73 (Table 2). For example, the antibacterial activity of XF-73 against MRSA was similar to mupirocin, fusidine, and daptomycin, and it was significantly better than linezolid and erythromycin.
 |
Table 2 Broth Microdilution MICs of S. aureus Strains Against XF-73 and a Range of Commonly Used Antibiotics for Test Strains |
Antibacterial Effect in in vivo Wound Infection Models
This study evaluated the antibacterial effects of the XF-73 dermal formulation and mupirocin ointment in two different superficial Staphylococcal skin infection murine models (tape-stripping infection model and suture-wound model) infected with mupirocin-resistant S. aureus at different resistance levels. We found that administration of XF-73 significantly reduced the bacterial burden in both of the murine models of superficial skin infection, regardless of the bacterial mupirocin resistance levels, compared with the infection control group and also the mupirocin ointment treatment group (Figures 1 and 2). However, the mupirocin ointment had little effect on reducing the bacterial bioburden in both of the murine models of superficial skin infection when infected with the high-level mupirocin-resistant strain (Figures 1 and 2). Therefore, it is worth investigating the potential of XF-73 as a novel topically applied agent for the treatment of bacterial infections.
The antibacterial activity of XF-73 dermal formulation at a concentration of 0.2% (w/w) and mupirocin ointment at a concentration of 2% (w/w) were evaluated in two murine infection models. Wounds in the two animal models were infected with NRS384 or NRS384-MT-3 (a low-level mupirocin-resistant strain, MIC = 16 µg/mL) and treated 12 hours later with mupirocin ointment or XF-73 dermal formulation.
In the tape-stripping model infected with NRS384, the bacterial count was 0.91 log10 lower (8.59 ± 0.42 log10) following treatment with mupirocin ointment than in the infected control group (9.50 ± 0.40 log10 CFU/wound) (p = 0.0239). In contrast, antibacterial efficacy of the XF-73 dermal formulation was greater, with a 3.19 log10 lower bacterial count following administration compared with the infected control group (p < 0.0001) (Figure 1A; Table 3). The same effect was seen in the model infected with NRS384-MT-3, in which the bacterial count was 0.9 log10 (p = 0.0003) lower after treatment with mupirocin ointment (8.59 ± 0.41 log10 CFU/wound) than that of the infected control (9.49 ± 0.29 log10 CFU/wound), and the bacterial counts after treatment with XF-73 dermal formulation (6.27 ± 0.41 log10 CFU/wound) were 3.22 log10 lower than that of the infected control group (p < 0.0001) (Figure 1B; Table 3). Similar results were observed in the suture-wound model infected with the two strains described above (Figure 2A and B; Table 4).
 |
Table 3 Comparative Effectiveness of XF-73 Dermal Formulation and Mupirocin Ointment in Tape-Stripped Mice Infected with S. aureus NRS384 or Mupirocin-Resistant S. aureus |
 |
Table 4 Comparative Effectiveness of XF-73 Dermal Formulation and Mupirocin Ointment in Mice with Experimental Surgical Wound Infections Caused by S. aureus NRS384 or Mupirocin-Resistant S. aureus |
The antibacterial activity of the XF-73 dermal formulation and mupirocin ointment were then evaluated in the two animal models infected with BAA-1708 (a high-level mupirocin-resistant strain). In the tape-stripping infection model, the XF-73 dermal formulation demonstrated significant therapeutic antibacterial activity. The bacterial count (8.76 ± 0.40 log10 CFU/wound) was 1.02 log10 lower (p < 0.0001) than the infected control (9.778 ± 0.38 log10 CFU/wound) after one administration (Figure 1C; Table 3). The effect was even more pronounced after two doses, with the bacterial count (6.65 ± 0.35 log10 CFU/wound) being 3.3 log10 lower than that of the infected control (9.95 ± 0.47 log10 CFU/wound) (Figure 1D; Table 3). In contrast, mupirocin ointment was ineffective with no log reduction seen compared to the infected control after one administration (Figure 1C; Table 3) and no significant reduction was also observed after two administrations (Figure 1D; Table 3).
In the suture-wound model, mupirocin ointment had little or no effect, whereas a single dose of XF-73 dermal formulation had a highly significant effect, with a 2.52 log10 reduction in the wound bacterial count (6.60 ± 0.27 log10CFU/wound) compared to the infected control (9.12 ± 0.25 log10 CFU/wound) (Figure 2C; Table 4).
Effect of XF-73 on Staphylococcus aureus Cell Morphology
TEM
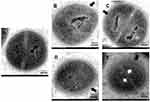
The TEM images showed the effect of different concentrations of XF-73 and different incubation times on the ultrastructure of S. aureus ATCC 29213. The untreated control bacteria (Figure 3A) were intact and clear, with smooth cell walls and dense outer cell membrane. However, the bacterial cell wall appeared to show signs of disruption just 2 min after 1 x MIC XF-73 application (Figure 3B), and this disruption became more pronounced with increasing time (Figure 3C) and drug concentration (Figure 3D and E). In addition, XF-73 further disrupted the bacterial cell membrane, causing gradual blurring of the membrane, as shown in Figure 3C–E. Intracellular cavities were also observed after 10 min of treatment with 4 x MIC, (Figure 3E), suggesting that disruption of membrane integrity might lead to the cytoplasmic leakage and consequent bacterial death previously reported.23 The above results suggest that the effect of XF-73 on bacteria may begin with the disruption of the bacterial cell wall, followed by further disruption of the bacterial cell membrane with increasing drug concentration and duration of action, leading to cytoplasmic leakage, but the action of XF-73 did not affect the gross morphology of the organism.
SEM
SEM was performed to directly observe the cell morphology and integrity of S. aureus in the presence and absence of XF-73 treatment. No efflux of intracellular material was observed around the bacterial cells using this technique, even after prolonged treatment with high concentrations of XF-73. The bacterial cells remained intact, with smooth, rounded, spherical surfaces and clear cell borders even after incubation at 4 x MIC for 16 hours (Figure 4), with no morphological differences observed compared to the untreated control demonstrating that the action of XF-73 did not lead to lysis of the bacteria. This is a positive property of XF-73, as it means it would have fewer pro-inflammatory effects when used clinically.
Discussion
Skin and Soft Tissue Infections are one of the most common bacterial infections and are mainly caused by Gram-positive bacteria, most commonly Staphylococcus, especially MRSA.1 As one of the most common drug-resistant bacteria in clinical circulation, MRSA remains a dangerous source of infection. Therefore, we first evaluated the antibacterial activity of XF-73 in vitro against S. aureus, including antibiotic-resistant bacteria. The results demonstrated that the antibacterial activity of XF-73 against S. aureus was superior or comparable to most of the comparator antibiotics. In particular, XF-73 was more efficacious than mupirocin when targeting mupirocin-resistant strains. This is consistent with previous results regarding the in vitro activity of XF-73, indicating that XF-73 has excellent bactericidal activity against Gram-positive strains tested, irrespective of species, phenotype, or genotype.7
Next, the in vivo activity of a dermal formulation of XF-73 was explored in two murine models of superficial skin infection caused by mupirocin-resistant S. aureus, the tape-stripping infection model and the suture-wound model, which are currently the most widely used in vivo models for evaluating the efficacy of new topical antimicrobial agents for the treatment of SSTIs and have been shown to simulate local skin infections caused by S. aureus.14,17,24,25
The in vivo pharmacodynamic study demonstrated that the XF-73 dermal formulation had a significant antibacterial effect in the two animal models infected with mupirocin-resistant S. aureus strains with different mupirocin resistance levels. However, the therapeutic efficacy of mupirocin decreases as the level of mupirocin resistance in bacteria rises, with little to no therapeutic effect observed across the two models of infection using a high-level mupirocin-resistant strain. In contrast, the XF-73 dermal formulation maintained a consistently high inhibitory capacity against the mupirocin-resistant strains tested and was significantly more effective than mupirocin ointment, which is currently commonly used for the treatment of skin infections. This suggests that XF-73 may be an effective drug against antibiotic-resistant skin infections in future clinical practice. Furthermore, in both superficial skin infection models, no obvious signs of systemic toxicity or local irritation associated with the administration of XF-73 dermal formulation was found. This is consistent with the results from a clinical trial with a nasal gel formulation of XF-73 (0.2% w/w) which was reported as well tolerated by healthy subjects with no serious adverse events, demonstrating the safety of XF-73.26
The results of previous studies have demonstrated the excellent antibacterial activity of XF-73 in vitro and in vivo.7 We also explored the possible mechanism of the antimicrobial action of XF-73. According to previous studies, the mechanism of action of XF-73 is unique compared to all antibiotic classes, XF-73 has a rapid onset of action, killing >99.99% of S. aureus within 5 min.23 Mechanism of action studies have shown that the drug has rapid cell membrane-disrupting activity and that XF-73 may interact with the cytoplasmic membrane of S. aureus,27 disrupting membrane integrity and causing membrane damage, resulting in the release of intracellular K+ and ATP from the cells and the simultaneous inhibition of macromolecular synthesis.28
We used TEM and SEM to observe the morphological and ultrastructural changes in S. aureus following exposure to XF-73. TEM images show that XF-73 initially disrupts the cell wall integrity after only 2 minutes incubation and within a further 8 minutes subsequently affects the integrity of the bacterial membrane at low concentrations, causing the originally intact and dense bacterial plasma membrane to become gradually blurred and disrupted, but unlike Nisin, which also targets the cell membrane,29 this damage does not lead to bacterial lysis. SEM images also confirm that XF-73 does not lyse the S. aureus, which is a positive attribute suggesting that inflammation-related adverse effects can be avoided when using XF-73 in clinical applications. These findings are consistent with existing studies showing that XF drugs have a unique and ultra-rapid mechanism of action on bacterial membranes, resulting in the loss of important components of the bacteria and resulting in cell death, but not lysing the bacteria themselves, which may explain why mutants resistant to XF-73 have not been able to be generated.8
A limitation of the mouse skin tape-stripping model is the high degree of variation between individual data points in some groups which may be due to the naked eye judgment of the tape-stripping procedure. As a result, it cannot be guaranteed that all mice received the same level of barrier damage. Therefore, the same stripping technique and number of strippings may produce different results in different mice, with some mice losing more epidermis than others after each stripping and yielding varying areas of infection after the same stripping technique, these could not be confirmed or prevented, but it was good to see that the changes were within acceptable limits. In addition to the skin tape-stripping model a skin suture-wound model was also used, where a much narrower range of variation among the individual data points for each group in was observed, demonstrating the strong reproducibility of this model. The advantage of the suture-superficial skin infection model relative to the tape-stripping model is that the time required to process each animal is significantly reduced, which provides an obvious advantage when dealing with many animals.
Conclusion
XF-73 is a new structurally distinct antimicrobial drug with a unique mechanism of action which has the potential to be an effective and safe candidate for the topical treatment of microbial infections. In this study, XF-73 was shown to have good in vitro activity against a number of S. aureus isolates, including multidrug-resistant strains, with efficacy superior or comparable to that of the concurrently tested antibacterial drugs; and the XF-73 dermal formulation also demonstrated effective inhibition of antibiotic-resistant bacteria in two models of superficial skin infection, including a high-level mupirocin-resistant strain that was virtually unresponsive to mupirocin. Preliminary morphological studies suggest that the unique mechanism of action of XF-73 may be related to an initial interaction with the bacterial cell wall, followed by a subsequent interaction with the the bacterial cell membrane which affects the cell membrane integrity which could increase its permeability, explaining the release of important intracellular components and ultimately bacterial death without causing bacterial lysis. However, the precise molecular mechanism of the interaction between XF-73 and bacterial membranes is not yet fully understood and will be the focus of future research.
Ethical Approval and Consent to Participate
This study has been approved by the ethics review committee of the Tianjin Medical University General Hospital and we confirm that informed consent was obtained from the participants. The study protocols were according to the guidelines in the Declaration of Helsinki (TMUaMEC20220324).
Acknowledgments
This study was supported by grants from the National Key Technologies R&D Program, intergovernmental international innovation cooperation (2018YFE0102000).
We would like to thank Destiny Pharma plc for their support and supply of XF-73 drug substance and dermal formulation, and we would like to thank Destiny Pharma researchers Dr William Love, Daniel Hynes and Dr William Rhys-Williams for their advice on this study and their help in revising this manuscript.
Disclosure
Jinping Li, Rong Lu, Song Wang, and Zheng Fu are affiliated with Kangzhe Pharmaceutical Technology Development Company, Ltd. The authors report no other conflicts of interest in this work.
References
1. Moffarah AS, Al Mohajer M, Hurwitz BL, Armstrong DG. Skin and soft tissue infections. Microbiol Spectr. 2016;4(4). doi:10.1128/microbiolspec.DMIH2-0014-2015
2. Sartelli M, Guirao X, Hardcastle TC, et al. 2018 WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. 2018;13:58. doi:10.1186/s13017-018-0219-9
3. Sutherland R, Boon RJ, Griffin KE, Masters PJ, Slocombe B, White AR. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob Agents Chemother. 1985;27(4):495–498. doi:10.1128/AAC.27.4.495
4. Goldfarb J, Crenshaw D, O’Horo J, Lemon E, Blumer JL. Randomized clinical trial of topical mupirocin versus oral erythromycin for impetigo. Antimicrob Agents Chemother. 1988;32(12):1780–1783. doi:10.1128/AAC.32.12.1780
5. McLinn S. A bacteriologically controlled, randomized study comparing the efficacy of 2% mupirocin ointment (Bactroban) with oral erythromycin in the treatment of patients with impetigo. J Am Acad Dermatol. 1990;22(5 Pt 1):883–885. doi:10.1016/0190-9622(90)70118-2
6. Dadashi M, Hajikhani B, Darban-Sarokhalil D, van Belkum A, Goudarzi M. Mupirocin resistance in Staphylococcus aureus: a systematic review and meta-analysis. J Glob Antimicrob Resist. 2020;20:238–247. doi:10.1016/j.jgar.2019.07.032
7. Farrell DJ, Robbins M, Rhys-Williams W, Love WG. In vitro activity of XF-73, a novel antibacterial agent, against antibiotic-sensitive and -resistant gram-positive and gram-negative bacterial species. Int J Antimicrob Agents. 2010;35(6):531–536. doi:10.1016/j.ijantimicag.2010.02.008
8. Farrell DJ, Robbins M, Rhys-Williams W, Love WG. Investigation of the potential for mutational resistance to XF-73, retapamulin, mupirocin, fusidic acid, daptomycin, and vancomycin in methicillin-resistant Staphylococcus aureus isolates during a 55-passage study. Antimicrob Agents Chemother. 2011;55(3):1177–1181. doi:10.1128/AAC.01285-10
9. Hurtuk MG, He LK, Szilagyi A, et al. The novel antibacterial drug XF-70 is a potent inhibitor of Staphylococcus aureus infection of the burn wound. J Burn Care Res. 2010;31(3):462–469. doi:10.1097/BCR.0b013e3181db5265
10. CLSI Institute. Performance Standards for Antimicrobial Susceptibility Testing.
11. Haas CE, Nix DE, Schentag JJ. In vitro selection of resistant Helicobacter pylori. Antimicrob Agents Chemother. 1990;34(9):1637–1641. doi:10.1128/AAC.34.9.1637
12. Li L, Li R, Qi C, et al. Mechanisms of polymyxin resistance induced by Salmonella typhimurium in vitro. Vet Microbiol. 2021;257:109063. doi:10.1016/j.vetmic.2021.109063
13. Ding YX, Wu Q, Guo Y, et al. Effects of in vitro-induced drug resistance on the virulence of Streptococcus. Vet Med Sci. 2021;7(3):935–943. doi:10.1002/vms3.404
14. Kugelberg E, Norstrom T, Petersen TK, Duvold T, Andersson DI, Hughes D. Establishment of a superficial skin infection model in mice by using Staphylococcus aureus and Streptococcus pyogenes. Antimicrob Agents Chemother. 2005;49(8):3435–3441. doi:10.1128/AAC.49.8.3435-3441.2005
15. Imanishi I, Hattori S, Hisatsune J, Ide K, Sugai M, Nishifuji K. Staphylococcus aureus penetrate the interkeratinocyte spaces created by skin-infiltrating neutrophils in a mouse model of impetigo. Vet Dermatol. 2017;28(1):126–e27. doi:10.1111/vde.12398
16. Hahn BL, Onunkwo CC, Watts CJ, Sohnle PG. Systemic dissemination and cutaneous damage in a mouse model of staphylococcal skin infections. Microb Pathog. 2009;47(1):16–23. doi:10.1016/j.micpath.2009.04.007
17. Gisby J, Bryant J. Efficacy of a new cream formulation of mupirocin: comparison with oral and topical agents in experimental skin infections. Antimicrob Agents Chemother. 2000;44(2):255–260. doi:10.1128/AAC.44.2.255-260.2000
18. Tarrago C, Esquirol LP, Arano A, Lachamp L, D’Aniello F, Zsolt I. Therapeutic efficacy of ozenoxacin in animal models of dermal infection with Staphylococcus aureus. Future Microbiol. 2018;13:21–30. doi:10.2217/fmb-2017-0290
19. Hakansson J, Bjorn C, Lindgren K, Sjostrom E, Sjostrand V, Mahlapuu M. Efficacy of the novel topical antimicrobial agent PXL150 in a mouse model of surgical site infections. Antimicrob Agents Chemother. 2014;58(5):2982–2984. doi:10.1128/AAC.00143-14
20. Hakansson J, Ringstad L, Umerska A, et al. Characterization of the in vitro, ex vivo, and in vivo efficacy of the antimicrobial peptide DPK-060 used for topical treatment. Front Cell Infect Microbiol. 2019;9:174. doi:10.3389/fcimb.2019.00174
21. Zang H, Qian S, Li J, et al. The effect of selenium on the autophagy of macrophage infected by Staphylococcus aureus. Int Immunopharmacol. 2020;83:106406. doi:10.1016/j.intimp.2020.106406
22. Liang H, He K, Li T, et al. Mechanism and antibacterial activity of vine tea extract and dihydromyricetin against Staphylococcus aureus. Sci Rep. 2020;10(1):21416. doi:10.1038/s41598-020-78379-y
23. Ooi N, Miller K, Hobbs J, Rhys-Williams W, Love W, Chopra I. XF-73, a novel antistaphylococcal membrane-active agent with rapid bactericidal activity. J Antimicrob Chemother. 2009;64(4):735–740. doi:10.1093/jac/dkp299
24. Onunkwo CC, Hahn BL, Sohnle PG. Clearance of experimental cutaneous Staphylococcus aureus infections in mice. Arch Dermatol Res. 2010;302(5):375–382. doi:10.1007/s00403-010-1030-y
25. Rittenhouse S, Singley C, Hoover J, Page R, Payne D. Use of the surgical wound infection model to determine the efficacious dosing regimen of retapamulin, a novel topical antibiotic. Antimicrob Agents Chemother. 2006;50(11):3886–3888. doi:10.1128/AAC.00183-06
26. Yendewa GA, Griffiss JM, Jacobs MR, et al. A two-part Phase 1 study to establish and compare the safety and local tolerability of two nasal formulations of XF-73 for decolonisation of Staphylococcus aureus: a previously investigated 0.5mg/g viscosified gel formulation versus a modified formulation. J Glob Antimicrob Resist. 2020;21:171–180. doi:10.1016/j.jgar.2019.09.017
27. Maxwell A, Dowson CG, Spencer J. The molecular basis of antibiotic action and resistance. J Mol Biol. 2019;431(18):3367–3369. doi:10.1016/j.jmb.2019.06.018
28. Lee J-H. Perspectives towards antibiotic resistance: from molecules to population. J Microbiol. 2019;57(3):181–184. doi:10.1007/s12275-019-0718-8
29. Jensen C, Li H, Vestergaard M, Dalsgaard A, Frees D, Leisner JJ. Nisin damages the septal membrane and triggers DNA condensation in methicillin-resistant Staphylococcus aureus. Front Microbiol. 2020;11:1007. doi:10.3389/fmicb.2020.01007
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.