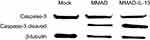Back to Journals » OncoTargets and Therapy » Volume 12
Efficacy of a new oncolytic adenovirus armed with IL-13 against oral carcinoma models
Authors Zhang KL, Li RP, Zhang BP, Gao ST , Li B, Huang CJ, Cao R, Cheng JY, Xie XD, Yu ZH, Feng XY
Received 31 January 2019
Accepted for publication 9 July 2019
Published 14 August 2019 Volume 2019:12 Pages 6515—6523
DOI https://doi.org/10.2147/OTT.S203638
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Sanjeev K. Srivastava
Kai-Liang Zhang,1,* Rui-Ping Li,1,* Bao-Ping Zhang,1,2,* Shu-Ting Gao,1 Bo Li,1 Chun-Juan Huang,1 Rui Cao,1 Jing-Yang Cheng,1 Xiao-Dong Xie,3 Zhan-Hai Yu,1 Xin-Yu Feng2
1Department of Stomatology, Lanzhou University, Lanzhou 730000, People’s Republic of China; 2Department of Civil Engineering and Mechanics, Lanzhou University, Gansu 730000, People’s Republic of China; 3Department of Basic Medical Sciences, Lanzhou University, Gansu 730000, People’s Republic of China
*These authors contributed equally to this work
Purpose: The efficacy of traditional therapies for oral carcinoma (OC) is limited. Oncolytic adenovirus, a novel strategy of cancer therapy, shows potential use in OC treatment. However, its clinical application is limited by pre-existing neutralizing antibodies. Thus, this study aimed to examine the efficacy of a new modified adenovirus against OC in vitro and in vivo.
Materials and methods: A multiple modified adenovirus (MMAD) armed with IL-13 (MMAD-IL-13) was constructed, and its effect on Cal-27 cells was examined. The potency of MMAD-IL-13 was examined in vitro and in vivo. For in vitro experiment, CCK-8 kit was used to determine the IC50 of MMAD-IL-3 in OC cell lines. For in vivo experiment, Cal-27 xenograft models were used to determine the antitumor effect of MMAD-IL-13. Apoptosis was measured in Cal-27 cells by Western blotting assay. Immunity response was detected in Cal-27 xenograft models 7 days after intratumoral injection with MMAD-IL-13. The potency of MMAD and MMAD-IL-13 was compared in Cal-27 peripheral blood mononuclear cells (PBMCs) models.
Results: MMAD-IL-13 was successfully constructed; the harvested virus could be replicated and they overexpressed human IL-13 in Cal-27 cells. Compared with MMAD, MMAD-IL-13 showed enhanced antitumor effect in vitro by inducing apoptosis and reducing percentage of M2 macrophages in tumor environment in vivo. MMAD-IL-13 also showed potent antitumor effect in Cal-27, SCC-4, and Tca8113 cells in vitro and in Cal-27 xenograft models in vivo. However, MMAD-IL13 did not harm normal human oral epithelial cells in vitro and exhibited no effect on body weight in Cal-27 xenograft models. In Cal-27 PBMC models, MMAD-IL-13 showed stronger antitumor effect than MMAD.
Conclusion: A new oncolytic adenovirus carrying the human IL-13 gene was constructed. This virus effectively led to remission of tumor development and death of OC cells in vivo and in vitro, showing its potential as a clinical cancer therapy.
Keywords: oncolytic virus, adenovirus 5, IL-13, OC
Introduction
Oral carcinoma (OC) is a lethal and deforming disease with rapidly increasing incidence. Globally, nearly 300,000 new cases of OC have occurred each year.1 Despite substantial advances in its diagnosis and management, its 5-year survival rate is still lower than 50%.2 Traditional therapy of OC majorly consists of surgery at the early stage and chemo/radiotherapy at the late stage. However, owing to high lymph node metastasis and nonspecific early symptoms, the efficacy of traditional therapy on OC is always limited.3
Oncolytic virus (OV) is a new cancer treatment strategy that has been widely used for various solid cancers.4–7 OV selectively replicates in tumors, but not in normal tissues. It also releases specific tumor antigens that can be identified by the immune system through virus-induced cytotoxicity, leading to tumor cell lysis by the host immunity. Multiple viruses have been modified to obtain these capacities, eg, herpes simplex virus-1,8 vaccine virus,9 and adenovirus. Adenovirus (AD) 5 has been well studied to limit virus replication in cancer cells and improve virus entry into cancer cells.10,11 Through deletion of its E1B 55K region, adenovirus is restricted to replicate only in p53-deficient cells.12 Based on this strategy, oncolytic adenovirus is constructed.
However, simple adenoviruses, especially type 5 adenovirus, have been confirmed to show limited clinical effect.13,14 High seroprevalence level leads to neutralization of antibodies, inhibiting infection of adenovirus and release of virus genome into the cancer cell nucleus. Therefore, in this study, we constructed a multiple modified adenovirus (MMAD) vector to enhance the antitumor efficacy of oncolytic adenovirus. For this purpose, we inserted the RGD sequence into the HI loop of the fiber knob to improve the effectiveness of MMAD infection into cancer cells. RGD is a motif of adenovirus penton that facilitates virus entry into cells through endocytosis by the integrin receptor.15 In addition, we deleted the whole E3 region to increase MMAD payload and viral replication capacity.16 The E3 region was selected because it has complex activities, including anti-host immunity activity and preventive activity on cell apoptosis in the host. E3 is usually deleted to increase virus payload, and this deletion does not affect virus potency. Furthermore, we armed the MMAD with IL-13 (MMAD-IL-13) to improve its clinical efficacy.
In this study, we constructed a MMAD vector with deletion of the E1B 55K and E3 2.7 kb regions, addition of an RGD motif at the fiber knob, and IL-13. Moreover, we examined the antitumor effect of MMAD-IL-13 in vitro and in vivo.
Materials and methods
Animal and ethics statement
Twenty female BALB/c nude mice (6–8 weeks old) were maintained in a light- (12 hrs) and temperature-controlled room (22°C±2). All experimental procedures were approved by the Institutional Animal Care and Committee on Ethical Use of Animals of the Laboratory Animal Center at Lanzhou University.
Cell lines and culture condition
The OC cell lines Cal-27, SCC-4, and Tca8113, as well as normal human oral epithelial (NHOE) cells, were purchased from iCell Bioscience (Shanghai, People’s Republic of China). The four cell lines were maintained in the DMEM (D5796; Sigma-Aldrich, People’s Republic of China) supplemented with 10% fetal bovine serum (16000-044; Gibco, USA), 10,000 units penicillin, and 10 mg/mL streptomycin (V900929; Sigma-Aldrich). The cells were incubated in a humidified atmosphere containing 5% CO2 at 37°C.
MMAD-IL-13 vector construction
The basic adenovirus plasmid used in this study as purchased from OD260 (USA), and it contains a copy of the AD5 genome identical to that in the Adenovirus Type 5 Reference Material (GenBank AY339865). Based on the basic plasmid, we deleted E1B 55K like the ZD55, a classical OV structure. We further modified the MMAD by deleting 2.7 kb of the E3 region and inserting the peptide ACDCRGDCFCG(RGD-4C) into the fiber knob. Next, the CMV-IL13-sv40 Ploy(A) fragment was synthesized by Hongxun (Suzhou, People’s Republic of China) and used to clone E3 (Figure 1).
 |
Figure 1 The structure of MMAD-IL-13.Abbreviation: MMAD-IL-13, multiple modified adenovirus IL-13. |
Cell viability assay
Three OC cell lines and a normal cell line were seeded (2000 cells/well) in 96-well plates and cultured 6 hrs overnight. The cells were infected with MMAD-IL-13 at MOI of 1, 2, 4, 8, 16, 32, 64, 128, and 256. NHOE cells were pre-infected with MMAD-IL-13 and ZD55 at the same MOI. Next, Cal-27 cells were infected with ZD55, MMAD, and MMAD-IL-13 at MOI of 1.28, 3.2, 8, 20, 50, 128, 320, 800, and 2000. All virus samples were diluted into 100 μL medium, and then 100 μL medium was added to a mock well as a negative control. Each MOI had three replicates. Next, virus and cells were co-cultured for 120 hrs, and 10 μL CCK-8 (CK04; Dojindo, Japan) was added to each well for measurement of cell viability. The plates were then incubated in an incubator for 4 hrs, and absorbance was measured at 450 nm using a microplate reader to determine the 50% inhibitive concentration (IC50) of ZD55, MMAD, and MMAD-IL-13 at each MOI.
ELISA assay
Cal-27 cells were treated with 50 MOI of MMAD-IL-13 and MMAD. At 24, 48, and 72 hrs after treatment, supernatants were collected, flash-frozen, and analyzed for IL-13 concentration using a Human IL-13 ELISA kit (Shanghai Enzyme-linked Biotechnology Co., Ltd., People’s Republic of China), per the manufacturer’s instructions.
qPCR assay
Supernatants were collected from the Cal-27 cells infected for 24 hrs with MMAD and MMAD-IL13 at 5×106 pfu/well. Next, total DNA was extracted from the cells using TIAN amp Blood DNA Kit (Tiangen, People’s Republic of China). qPCR was employed to detect E1A replication. In addition, 200 μL MMAD DNA (1×1011 vp/mL) and MMAD-IL-13 DNA (1×1011 vp/mL) were extracted as negative controls. The primer sequences used in the study were as follows. E1A primers: 5′-CGCAGATTTTTCCCGACTCT-3′ and 5′-CCG GCG GAA AAG TGA GTA AG-3′; GAPDH primers: 5′-GGAGCGAGATCCCTCCAAAAT-3′ and 5′-GGCTGTTGTCATACTTCTCATGG-3′. The qPCR process was carried out according to the instructions on the package insert of TB Green (RR091Q; Takara, People’s Republic of China).
Western blotting assay
RIPA lysis buffer (Beyotime Biotechnology, People’s Republic of China) was used to extract proteins, according to the manufacturer’s instructions. Protein concentration was estimated using the Pierce Rapid Gold BCA Protein Assay Kit (A53225; Thermo Fisher Scientific, USA). Western blotting was conducted using standard protocols, with 35 µg protein loaded in each well. The primary antibodies of β-tubulin (2146, 1:1000), caspase-3 (9662,1:1000), and cleaved caspase-3 (9661, 1:1000) were purchased from Cell Signaling Technology (USA). All anti-rabbit secondary antibodies were purchased from CW Biotechnology (People’s Republic of China).
Xenograft models
Xenograft models were established via subcutaneous injection of Cal-27 cells (5×106 cells/100 μL) into the right legs of mice. Cell viability was determined before injection by Trypan blue stain (15250061; Invitrogen, USA). When the tumor size reached approximately 100 mm3, the mice were divided into two groups (n=3) using the Study Director software (Studylog System, Inc., USA) and the spare mice were executed. Group 1 was intratumorally injected with 50 μL MMAD-IL13 (1×1010 pfu/mL) on day 1, 3, and 5. Group 2, the control group, was intratumorally injected with 50 μL PBS on day 1, 3, and 5. Tumor volume was calculated every 3 days using the following formula: tumor volume=(tumor length×tumor width)2/2. All mice were executed after 28 days or when the tumor volume reached 2000 mm3.
Peripheral blood mononuclear cells (PBMCs) models
PBMCs were extracted from the blood of our laboratory staff and then maintained in RPMI 1640 medium (11875093; Gibco, People’s Republic of China) supplemented with 10% fetal bovine serum (heat inhibited) and 10 ng/μL IL-2 (2000210; PeproTech, USA). Next, to establish tumor-bearing models, NCG mice were subcutaneously inoculated at the right leg with Cal-27 cells (1×107 cells/100 μL) in 0.1 mL DMEM containing 50% matrigel, and simultaneously injected with PBMCs (1×106 cells/100 μL) via the tail vein. When the tumor size reached approximately 100 mm3, the mice were divided into three groups (n=6) using the Study Director software (Studylog System, Inc.) and the spare mice were executed. Group 1 and Group 2 were intratumorally injected with 50 μL MMAD-IL13 (1×1010 pfu/mL) and MMAD (1×1010 pfu/mL), respectively, on day 1, 3, and 5. Group 3, the control group, was intratumorally injected with 50 μL PBS on day 1, 3, and 5. Tumor volume was calculated every 3 days using the following formula: tumor volume=(tumor length×tumor width)2/2. All mice were executed after the first case of graft-versus-host disease (GVHD) occurred or when the tumor size reached 2000 mm3.
Flow cytometry assay
Macrophage was extracted from tumor at 7 days after. Three mice without treatment were used as negative controls. M2 were defined by CD45+ (480028), CD11b+ (301350), F4/80+ (123116), CD206+ (141708). All antibody used were purchased from Biolegend (USA).
Statistical analysis
Statistical significance of the data was analyzed using ANOVA and single sample Student’s t-test. All data are presented as the mean±SD. IC50 was calculated by four-parameter logistic regression. Statistical analyses were conducted by using the GraphPad Prism 7.0 software (GraphPad, USA).
Results
MMAD-IL-13 expresses IL13 and retains replication capacity
We first confirmed whether the harvested virus functioned as designed. The results showed that IL-13 concentration in the supernatant of cells treated with MMAD-IL-13 increased rapidly after infection and reached the peak at 48 hrs after infection (Figure 2A). In contrast, IL-13 concentration in the supernatant of MMAD-treated cells was very low. The qPCR also showed both MMAD and MMAD-IL-13 could replicate in Cal-27 cell (Figure 2B).
MMAD-IL-13 selectively kills tumor cells in vitro
The potency of the purified virus was compared with that of MMAD and ZD55 in Cal-27 cells. The IC50 of ZD55, MMAD, and MMAD-IL-13 was 143.3, 43.9, and 29.6, respectively (Figure 3A). Obviously, the multiple modification increased the antitumor effect of MMAD by 3.3-fold higher than that of ZD55. Moreover, the MMAD armed with IL-13 showed improved antitumor effect by 1.48-fold, compared to MMAD. Furthermore, we used MMAD-IL-13 to infect OC cell lines and a primary oral NHOE cell line. Consequently, MMAD-IL13 killed various OC cell lines, but Cal-27 cells showed the highest sensitivity to MMAD-IL13 (Figure 3B). On the contrary, all OVs at the same MOI did not harm normal cells in vitro (Figure 3C).
MMAD-IL-13 exhibits antitumor effect by inducing apoptosis
To investigate whether MMAD-IL-13 shows stronger antitumor effect than that of MMAD, we treated Cal-27 cells with MMAD-IL-13 and performed a Western blotting assay to examine the protein expression of caspase-3 and cleaved caspase-3 (Figure 4). Compared to that in the mock and MMAD groups, caspase-3 expression in the MMAD-IL-13 group was the highest. After 24 hrs, the Cal-27 cells infected with MMAD-IL-13 showed higher level of caspase-3 cleaved than the MMAD- and mock-treated cells.
MMAD-IL-13 inhibits OC cell proliferation in vivo
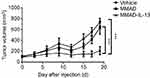
To further compare the antitumor effect of MMAD-IL-13 with that of other OVs, Cal-27 xenograft models were intratumorally injected three times with MMAD-IL-13 and tumor volume was measured every 3 days. The tumor volume of the vehicle group increased steadily and reached 875 mm3 at 36 days after treatment initiation. In contrast, the group treated with MMAD-IL-13 showed obvious inhibition of tumor growth (P<0.01), but showed no effect on body weight (Figure 5A–C).
MMAD-IL-13 shows strong antitumor effect in Cal-27 PBMC models
The function of IL-13 was examined in Cal-27 PBMC models. The first case of GVHD occurred in Group 2 at 19 days after injection. Tumor volume in the vehicle group increased steadily and reached 735 mm3 at 19 days after injection. MMAD-IL-13 showed obvious inhibitory effect on tumor growth (P<0.01) and stronger antitumor effect than that of MMAD (P<0.01) (Figure 6).
MMAD-IL-13 reduces M2 percentage in tumor
Changes in immunity after MMAD-IL-13 injection were analyzed in three mice. The result indicated that the proportion of macrophage subgroups in the tumor changed dramatically at 7 days after MMAD-IL-13 injection (Figure 7), showing significant reduction in M2 percentage (P<0.01).
Discussion
OC is the most common squamous cell carcinoma. Considering limitations in the clinical treatment of OC, it is particularly important to develop new methods to improve prognosis in patients with OC. OV has shown powerful antitumor effect against melanoma, lung cancer, and multiple other cancers.8 The common delivery method of oncolytic adenovirus is intratumoral injection, restricting its use in some cancers such as diffuse gastric cancer.17 However, intratumor injection is relatively convenient for OC, and OV injection can help control tumor metastasis through selective replication. Hence, we tested the antitumor effect of the OV MMAD-IL-13 on several OC cell lines.
The concept of OV was first developed in the 1950s. After half a century of development, various strategies were implemented to optimize the efficacy of OV. All these strategies were mainly concentrated on two areas: improvement of tumor specificity and improvement of cancer-killing effect.18,19 AD5, the most common human host virus, is used as a vehicle to carry a heterologous gene to a target cell or tissue. Following previous reports, we constructed a complex strategy using MMAD to improve the antitumor effect of adenovirus.11,12,16,20 The E3 region is nonessential for the replication of oncolytic adenovirus; thus, the whole E3 region can be deleted to improve the payload of MMAD.16 In addition, E3 also plays a key role in inhibiting the antivirus immunity system.21,22 However, the antitumor immunity system overlaps with the antivirus immunity system, especially in the innate immunity. Therefore, we thought that deletion of E3 would be effective to increase the antitumor effect of AD5 in vivo. Moreover, we modified the fiber of AD5 to increase the rate of virus entry into cells.11,23,24 The result of our in vitro experiment proved these modification strategies were successful in improving the antitumor efficacy of AD5.
Numerous OV therapies have been developed; however, the clinical effect of a simple OV is still debated. Therefore, the “Gene-Viro Therapy” suggested that OV combined with gene therapy may be more effective in treating cancer than an injection of a simple OV alone. IL-13, which is commonly used as a treatment of autoimmunity diseases, can invoke the Th2 immunity and inhibit the Th1 immunity.25–27 This may help silence the antitumor immunity of CD4+ T cells to prevent OV death before its entry into cells.28 This effect may be eliminated by compensatory mechanism. In fact, mouse immunity is different from human immunity; for example, mouse IL-13 shows low similarity to human IL-13. Unfortunately, this study did not examine the antitumor effect in syngeneic models. Nevertheless, our in vivo experiment showed that MMAD-IL-13 effectively inhibited OSSC tumor growth.
To investigate the effect of MMAD-IL-13, we used the Western blotting assay to examine the expression of caspase-3, an apoptosis marker.29 The endogenous expression of IL-13 seemed to enhance tumor cell apoptosis after infection with oncolytic adenovirus. Furthermore, MMAD-IL-13 injection led to a decline in the percentage of M2 macrophages, which can secrete IL-10 and TGF-β to suppress the immune system.30,31 MMAD-IL-13 reduced M2 percentage to improve the effectiveness of macrophages in presenting tumor-specific antigens, as reported with other OVs.32 Thus, this finding suggested that MMAD-IL-13 showed increased tumor cell lysis effect and activated the host innate immunity system of tumor microenvironment. However, the human antiviral system is activated by CD8+ T cells and CD4+ T cells, and nude mice lack the adaptive immune system. Therefore, the effect of treatment with MMAD-IL-13 in the model may be different from that in the whole immune system.
To further investigate the pre-exist immunity effect, Cal-27 PBMC model were built. Compare to the nude mice model and syngenetic model, this model is better to show the oncolytic adenovirus effect in the human body. Observably, the unarmed OV MMAD show very limited antitumor effect (P>0.05) while MMAD-IL-13 was not affected. To balance the antitumor effect is hard, especially when these two fields overlap. Inhere, the adenovirus lysis effect was enhanced and the IL-13 was used to inhibit the antitumor effect. This strategy can improve the antitumor effect and extend the action time of OV to improve its clinical effect.
Conclusion
In summary, we constructed a new oncolytic adenovirus carrying the human IL-13 gene. This virus effectively led to remission of tumor development and cancer cell death in vivo and in vitro, showing its potential for clinical therapy. However, the antitumor effect of this virus in immunized models should be further investigated.
Acknowledgment
This study was funded by the National Natural Science Foundation of China (No. 81360588 and 81473457), the lzujbky-2017-142 and lzujbky-2017-23.
Disclosure
The authors report no conflicts of interest in this work.
References
1. D’Cruz AK, Dandekar MR. Elective versus therapeutic neck dissection in the clinically node negative neck in early oral cavity cancers: do we have the answer yet? Oral Oncol. 2011;47(9):780–782. doi:10.1016/j.oraloncology.2011.06.013
2. Iyer S, Thankappan K, Balasubramanian D, Balasubramanian D. Early detection of oral cancers: current status and future prospects. Curr Opin Otolaryngol Head Neck Surg. 2016;24(2):110–114. doi:10.1097/MOO.0000000000000237
3. Krishnamurthy A. Current status and future perspectives of sentinel lymph node biopsy in oral cancers. Indian J Dent Res. 2017;28(3):239–240. doi:10.4103/ijdr.IJDR_213_17
4. Kuhn I, Harden P, Bauzon M, et al. Directed evolution generates a novel oncolytic virus for the treatment of colon cancer. PLoS One. 2008;3(6):e2409. doi:10.1371/journal.pone.0002409
5. Marelli G, Howells A, Lemoine NR, Wang Y. Oncolytic viral therapy and the immune system: a double-edged sword against cancer. Front Immunol. 2018;9:866. doi:10.3389/fimmu.2018.00866
6. Kirn DH, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9(1):64–71. doi:10.1038/nrc2545
7. Shen Y, Nemunaitis J. Herpes simplex virus 1 (HSV-1) for cancer treatment. Cancer Gene Ther. 2006;13(11):975–992. doi:10.1038/sj.cgt.7700946
8. Rehman H, Silk AW, Kane MP, Kaufman HL. Into the clinic: talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J Immunother Cancer. 2016;4:53. doi:10.1186/s40425-016-0158-5
9. Kung CH, Kuo SC, Chen TL, Weng WS. Isolation of vaccinia JX594 from pustules following therapy for hepatocellular carcinoma. BMC Cancer. 2015;15:704. doi:10.1186/s12885-015-1584-3
10. Jiang H, Rivera-Molina Y, Gomez-Manzano C, et al. Oncolytic adenovirus and tumor-targeting immune modulatory therapy improve autologous cancer vaccination. Cancer Res. 2017;77(14):3894–3907. doi:10.1158/0008-5472.CAN-17-0468
11. Dai B, Roife D, Kang Y, et al. Preclinical evaluation of sequential combination of oncolytic adenovirus delta-24-RGD and phosphatidylserine-targeting antibody in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2017;16(4):662–670. doi:10.1158/1535-7163.MCT-16-0526
12. Zhang ZL, Zou WG, Luo CX, et al. An armed oncolytic adenovirus system, ZD55-gene, demonstrating potent antitumoral efficacy. Cell Res. 2003;13(6):481–489. doi:10.1038/sj.cr.7290191
13. Varghese R, Mikyas Y, Stewart PL, Ralston R. Postentry neutralization of adenovirus type 5 by an antihexon antibody. J Virol. 2004;78(22):12320–12332. doi:10.1128/JVI.78.22.12320-12332.2004
14. Smith JG, Cassany A, Gerace L, Ralston R, Nemerow GR. Neutralizing antibody blocks adenovirus infection by arresting microtubule-dependent cytoplasmic transport. J Virol. 2008;82(13):6492–6500. doi:10.1128/JVI.00557-08
15. Fueyo J, Alemany R, Gomez-Manzano C, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95(9):652–660. doi:10.1093/jnci/95.9.652
16. Lochmuller H, Jani A, Huard J, et al. Emergence of early region 1-containing replication-competent adenovirus in stocks of replication-defective adenovirus recombinants (delta E1+ delta E3) during multiple passages in 293 cells. Hum Gene Ther. 1994;5(12):1485–1491. doi:10.1089/hum.1994.5.12-1485
17. Miest TS, Cattaneo R. New viruses for cancer therapy: meeting clinical needs. Nat Rev Microbiol. 2014;12(1):23–34. doi:10.1038/nrmicro3140
18. Turnbull S, West EJ, Scott KJ, Appleton E, Melcher A, Ralph C. Evidence for oncolytic virotherapy: where have we got to and where are we going? Viruses. 2015;7(12):6291–6312. doi:10.3390/v7122938
19. Mavani HJ, Wick JY. Oncology’s trojan horse: using viruses to battle cancer. Consult Pharm. 2016;31(12):676–684. doi:10.4140/TCP.n.2016.676
20. Rojas JJ, Cascallo M, Guedan S, et al. A modified E2F-1 promoter improves the efficacy to toxicity ratio of oncolytic adenoviruses. Gene Ther. 2009;16(12):1441–1451. doi:10.1038/gt.2009.103
21. Bortolanza S, Bunuales M, Alzuguren P, et al. Deletion of the E3-6.7K/gp19K region reduces the persistence of wild-type adenovirus in a permissive tumor model in Syrian hamsters. Cancer Gene Ther. 2009;16(9):703–712. doi:10.1038/cgt.2009.12
22. Spurrell E, Gangeswaran R, Wang P, et al. STAT1 interaction with E3-14.7K in monocytes affects the efficacy of oncolytic adenovirus. J Virol. 2014;88(4):2291–2300. doi:10.1128/JVI.02829-13
23. Bayo-Puxan N, Gimenez-Alejandre M, Lavilla-Alonso S, et al. Replacement of adenovirus type 5 fiber shaft heparan sulfate proteoglycan-binding domain with RGD for improved tumor infectivity and targeting. Hum Gene Ther. 2009;20(10):1214–1221. doi:10.1089/hum.2009.038
24. Xu Y, Chu L, Yuan S, et al. RGD-modified oncolytic adenovirus-harboring shPKM2 exhibits a potent cytotoxic effect in pancreatic cancer via autophagy inhibition and apoptosis promotion. Cell Death Dis. 2017;8(6):e2835. doi:10.1038/cddis.2017.518
25. Bartolome RA, Jaen M, Casal JI. An IL13R alpha 2 peptide exhibits therapeutic activity against metastatic colorectal cancer. Br J Cancer. 2018;119(8):940–949.
26. Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin Cancer Res. 1995;1(11):1253–1258.
27. Ranasinghe C, Trivedi S, Wijesundara DK, Jackson RJ. IL-4 and IL-13 receptors: roles in immunity and powerful vaccine adjuvants. Cytokine Growth Factor Rev. 2014;25(4):437–442. doi:10.1016/j.cytogfr.2014.07.010
28. Mahr JA, Gooding LR. Immune evasion by adenoviruses. Immunol Rev. 1999;168:121–130.
29. Negara KS, Suwiyoga K, Pemayun TGA, et al. The role of caspase-3, apoptosis-inducing factor, and B-cell lymphoma-2 expressions in term premature rupture of membrane. Rev Bras Ginecol Obstet. 2018;40(12):733–739. doi:10.1055/s-0038-1675611
30. Qi L, Yu H, Zhang Y, et al. IL-10 secreted by M2 macrophage promoted tumorigenesis through interaction with JAK2 in glioma. Oncotarget. 2016;7(44):71673–71685. doi:10.18632/oncotarget.12317
31. Guo Z, Wen Z, Qin A, et al. Antisense oligonucleotide treatment enhances the recovery of acute lung injury through IL-10-secreting M2-like macrophage-induced expansion of CD4+ regulatory T cells. J Immunol. 2013;190(8):4337–4348. doi:10.4049/jimmunol.1203233
32. Van den Bossche W, Kleijn A, Teunissen CE, et al. Oncolytic virotherapy in glioblastoma patients induces a tumor macrophage phenotypic shift leading to an altered glioblastoma microenvironment. Neuro Oncol. 2018;20(4):1494–1504. doi:10.1093/neuonc/noy082
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.