Back to Journals » OncoTargets and Therapy » Volume 14
Efficacy and Toxicity Profile of Carfilzomib-Based Regimens for Treatment of Newly Diagnosed Multiple Myeloma: A Systematic Review
Authors Imtiaz H, Khan M, Ehsan H , Wahab A, Rafae A , Khan AY, Jamil A, Sana MK , Jamal A, Ali TJ, Ansar I, Khan MM, Khouri J, Anwer F
Received 26 April 2021
Accepted for publication 19 August 2021
Published 1 October 2021 Volume 2021:14 Pages 4941—4960
DOI https://doi.org/10.2147/OTT.S317570
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianmin Xu
Hassaan Imtiaz,1 Maimoona Khan,2 Hamid Ehsan,3 Ahsan Wahab,4 Abdul Rafae,5 Ali Y Khan,6 Abdur Jamil,7 Muhammad Khawar Sana,8 Abdullah Jamal,1 Taimoor Jaffar Ali,1 Iqraa Ansar,2 Muzammil M Khan,9 Jack Khouri,10 Faiz Anwer10
1Department of Internal Medicine, King Edward Medical University, Lahore, Punjab, Pakistan; 2Department of Medicine, Shifa College of Medicine, Islamabad, Pakistan; 3Department of Hematology/Oncology, Levine Cancer Institute, Atrium Health, Charlotte, NC, USA; 4Hospital Medicine/Internal Medicine, Baptist Medical Center South, Montgomery, AL, USA; 5Department of Internal Medicine, McLaren Regional Medical Center, Flint, MI, USA; 6Department of Internal Medicine, St. Joseph Mercy Oakland Hospital, Pontiac, MI, USA; 7Department of Internal Medicine, Central Michigan University, Saginaw, MI, USA; 8Department of Internal Medicine, John H. Stroger Jr. Hospital of Cook County, Chicago, IL, USA; 9Department of Gastroenterology, Beth Israel Deaconess Medical Center, Boston, MA, USA; 10Hematology, Oncology, Stem Cell Transplantation, Multiple Myeloma Program, Taussig Cancer Center, Cleveland Clinic, Cleveland, OH, 44195, USA
Correspondence: Muhammad Khawar Sana
Department of Internal Medicine, John H. Stroger Jr. Hospital of Cook County, Chicago, IL, USA
Email [email protected]
Abstract: Carfilzomib (CFZ) is a proteasome inhibitor currently approved for the treatment of relapsed and refractory multiple myeloma (RRMM). Multiple trials are ongoing to evaluate its efficacy and safety in newly diagnosed multiple myeloma (NDMM). The use of CFZ-based two- or three-drug combination regimens as induction for the management of NDMM is an emerging approach. CFZ-based regimens include combinations of immunomodulators, alkylating agents, and monoclonal antibodies along with dexamethasone. In this review, we assess the efficacy and toxicity of CFZ-based regimens in NDMM. We reviewed a total of 27 studies (n=4538 patients) with overall response rates (ORR) ranging between 80% and 100%. Studies evaluating the combination of CFZ with daratumumab reported an ORR of approximately 100%. Achievement of minimal residual disease (MRD) negativity, measured by multi-parameter flow cytometry (MPFC), ranged between 60% and 95% in 4 (n=251) out of 6 studies that measured MRD-negativity. The interim results of the ENDURANCE trial failed to show superior efficacy and progression-free survival (PFS) of carfilzomib-lenalidomide when compared to bortezomib–lenalidomide combination, albeit with a lower incidence of neuropathy. Hematological toxicity was the most common adverse event observed with these regimens, and the most common non-hematological adverse events were related to cardiovascular and electrolyte disturbances. We need to further evaluate the role of CFZ in NDMM by conducting more Phase III trials with different combinations.
Keywords: carfilzomib, newly diagnosed multiple myeloma, safety, efficacy, systematic review
Introduction
Multiple myeloma (MM) is characterized by the monoclonal proliferation of plasma cells in the bone marrow and is the second most common hematologic malignancy, accounting for about 1% of all the cancers.1 There has been a significant improvement in patient outcomes due to recent advances in therapeutic regimens and a better understanding of disease pathophysiology.1 However, there is a recorded increase in the incidence of MM, which may be attributed to a rise in the aging population in addition to better detection, among other factors.2
Recently reported data suggested that 33% of MM cases are in patients above the age of 75, while 10% of cases are above the age of 85.3 Side effects associated with treatment, especially in elderly patients, often lead to dose modifications and dose interruptions which may attenuate the sustained response necessary for long-term remission and improved quality of life.4 Patients with the genetically high-risk disease such as del (17p), t(4;14), t(14;16), t(14;20), gain 1q, or p53 mutations have shown inferior outcomes in newly diagnosed MM (NDMM) as well as relapsed and refractory multiple myeloma (RRMM).5
In patients with NDMM, significant improvement has been noted in outcomes since the introduction of proteasome inhibitors (PIs) and other immunomodulatory drugs.2 Combination regimens such as bortezomib-melphalan-prednisone (VMP) and melphalan-prednisone-thalidomide (MPT) are associated with significant hematological adverse effects (AEs) and peripheral neuropathy (PN).6
Bortezomib, a first-generation PI, was first approved for NDMM in 2003 but was noted to be associated with significant neuropathy.4 Carfilzomib (CFZ), a second-generation PI, generates more potent anti-myeloma activity with relatively deeper and more sustained therapeutic effects.7 CFZ with its tetrapeptide epoxyketone structure binds irreversibly with proteasomes, whereas bortezomib, a dipeptide boronate, offers a reversible proteasome inhibition.2 The blockage of proteasomes, large catalytic multi-enzyme complexes, interferes with protein homeostasis of myeloma cells and disrupts their protein turnover machinery leading to their apoptotic cell death. There are three proteolytic sites available on proteasomes that mediate the degradation of proteins and are named caspase-like (C-L), chymotrypsin-like (ChT-L), and trypsin-like (T-L) sites. Among these sites, CFZ primarily functions through an irreversible binding to the ChT-L site while the remaining two sites are also inhibited at higher concentrations of CFZ.7 CFZ suppresses osteoclastic bone events as well as having an anabolic effect on bones.8
CFZ was initially approved for use with dexamethasone in patients with RRMM, who had previously been treated with at least two prior lines of therapies including Bortezomib and an immunomodulatory agent. Nowadays it is also being used as a combination regimen with immunomodulators (IMiDs), daratumumab, dexamethasone, or alkylating agents in patients with RRMM.2 A number of clinical trials show the clinical efficacy of CFZ-based regimens that include ASPIRE, ENDEAVOR, CHAMPION-1, and A.R.R.R.O.W studies. The CHAMPTION-1 study showed an overall response rate of 77% with once-weekly CFZ dose of 70mg/m2 along with dexamethasone. It is now being used for patients with RRMM, even with one to three prior lines of therapy.
Given the efficacy and better-tolerated safety profile, CFZ-based regimens are also being used in NDMM.6 The National Comprehensive Cancer Network (NCCN) recommends CFZ in combination with lenalidomide (R) and dexamethasone as the primary therapy for both transplant eligible and ineligible NDMM patients.9 Whether the addition of CFZ to other drugs can alter the overall response (OR) among patients with high-risk cytogenetics, especially among NDMM, needs further analysis, and multiple clinical trials are ongoing to achieve this goal. Hereby, we discuss the safety and efficacy of CFZ-based drug combinations as induction regimens for the management of NDMM as an emerging approach.
Materials and Methods
Literature Search
A comprehensive literature search was performed on 04/20/2020 using the following resources: PubMed, EMBASE, Wiley Cochrane Library, Scopus, Web of Science, CINAHL, and Clinicaltrials.gov. Search filters were not limited to any geographical area or language other than English, if language translation was not available for foreign language articles. Studies that were published between January 2007 and April 2020 were included. All relevant articles from conference proceedings were also included. We also searched proceedings from the following conferences: European Hematology Association, American Society of Hematology, American Society of Clinical Oncology, and American Society of Bone Marrow Transplantation.
Eligibility Criteria
- Phase I, II, or III clinical trials.
- Clinical trials from January 2007 till April 2020.
- Studies that evaluated the safety and efficacy of CFZ.
- Studies focusing on CFZ as a primary drug therapy.
Study Selection
Studies were reviewed by three independent reviewers (AYK, HI, and MK) based on titles and abstracts. After excluding irrelevant articles, potential studies were screened by reading full texts. Conflicts among reviewers were resolved with discussion.
Data Extraction and Analysis
Data were extracted on pre-specified Microsoft Excel tables, which included the following information: author, year, study design, number of patients, median age, MM staging and cytogenetics, follow-up duration, CFZ regimen, dose, the median number of cycles, and efficacy outcomes including complete response (CR), near-complete response (nCR), stringent complete response (sCR), very good partial response (VGPR), partial response (PR), overall response rate (ORR), overall survival (OS), and progression-free survival (PFS). If the desired information was not reported in a particular study, we documented it as “not specified (NS).” Data were recorded as a median or percentage.
Search Results
The literature search identified a total of 2564 articles. After excluding 84 duplicate articles, 2480 articles were screened for relevance based on titles and abstracts. After excluding 2301 studies, 179 studies were found to be potentially useful in answering our study question. After reading the full-texts of these articles, additional 152 articles were excluded for the following reasons: review article, unrelated to CFZ, unavailable full text, duplicate study, absent desired efficacy and safety outcomes, or observational study. A total of 27 articles met the inclusion criteria. The summary of the selection process of studies is given in the PRISMA flow chart (Figure 1).
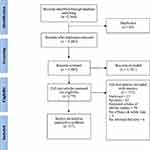 |
Figure 1 PRISMA flowchart summary of the selection process. Note: Copyright © 2009, Public Library of Science. Adapted with permission from Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.46 |
Results
Study Demographics
There are 27 studies (n= 4538 patients) included in this review. Among the included studies, there are three-phase III trials in which two trials compared the efficacy of carfilzomib-melphalan-prednisone (CMP) with bortezomib-melphalan-prednisone (VMP) and one trial compared carfilzomib-lenalidomide-dexamethasone (CRd) with bortezomib-lenalidomide-dexamethasone (VRd) (ENDURANCE trial).
Carfilzomib-Based Triplet Regimens
Carfilzomib-Based Regimens with Immunomodulators
Table 1 summarizes data, baseline characteristics, and efficacy of carfilzomib with IMiDs. Nine studies (n=527) evaluated the role of CFZ-based regimen when combined with IMiDs. Among these, eight studies focused on the combination of carfilzomib (CFZ) with lenalidomide where CFZ-based regimen were studied as consolidation therapy in one study while the other seven studies were about its role as induction. Only one study evaluated the combination of CFZ and thalidomide. CRd when used as an induction regimen showed an ORR of >90% in 5 out of 7 studies while it was not reported in the other two studies. As a consolidation regimen, it showed an ORR of 79%.10–18 Carfilzomib-thalidomide-dexamethasone (CTd) regimen showed an ORR of 94%.19 A minimum of 4 cycles was given as induction regimens with a maximum of up to 12 cycles with both thalidomide and lenalidomide.
 |
Table 1 Baseline Characteristics and Efficacy of Carfilzomib with Immunomodulators |
Carfilzomib, Lenalidomide, and Dexamethasone (CRd)
Jakubowiak et al, 2012,12 conducted a phase I/II study (n=53) for NDMM. Thirty-five patients were enrolled in a Phase I study of CFZ in the context of CRd. The CFZ dose was escalated gradually from 20 mg/m2 (n=4), 27 mg/m2 (n=13), 36 mg/m2 (n=18). The study was extended to Phase II for further assessment of maximum tolerated dose (MTD) of CFZ but later continued with a dose of 36 mg/m2 citing high response at the current dose, a lack of experience with higher doses, and due to limitations in post hoc addition of cohorts. In Phase II, patients received eight cycles of CRd as induction followed by 16 cycles of CRd as maintenance. Transplant-eligible candidates received stem cell collection. Five patients continued to receive single-agent lenalidomide maintenance therapy after induction. After a median of 12 cycles (range, 1–25) and a median follow-up of 13 months (range, 4–25), the near CR (nCR) and sCR were 62% and 42%, respectively. VGPR was 81%, and 24-month PFS was 92%. There was a progressive decline in M-protein levels after each subsequent CRd treatment. Among 29 patients who received 12+ cycles of CRd response rates were much improved. All 29 patients achieved at least a PR, while at least VGPR, nCR, and sCR were 86%, 72%, and 62%, respectively. There were a total of 76 grade (G) ≥3 AEs, including hypophosphatemia (25%), hyperglycemia (23%), anemia (21%), thrombocytopenia (17%), neutropenia (17%), elevated liver function/rash (8% each), pulmonary embolism (6%), infections/edema/thrombosis/dyspnea (4% each), fatigue/renal problems and mood problems (2% each). A later report of the subset of 23 elderly patients (≥65 years) involved in this trial showed that after receiving a median of 24 cycles of CRd therapy, all these patients achieved a PR, and VGPR, nCR, and sCR were 91%, 87%, and 65%, respectively. The 3-year PFS rate was 79.6% with an OS of 100%.
In a phase II trial conducted by Korde et al, 2015,16 NDMM patients (n=45) irrespective of transplant-eligibility were administered eight cycles of CRd induction and later proceeded to lenalidomide maintenance for 2 years if they had stable disease. Ten patients had unfavorable cytogenetics including del-17p, del-13q, and immunoglobulin heavy chain rearrangements. Forty-two patients completed eight cycles of CRd. Among 45 study participants, CR or sCR was achieved in 56% of patients [CI: 40–70%], nCR was achieved in 62% [CI: 46–76%] of patients, ≥ VGPR was achieved in 89% [CI: 76–96%] of patients and PR was achieved in 98% of patients [CI: 88–100%]. PFS at 18 months was 92%. At 24 months, CR/sCR was maintained in 88% of patients, and PR was maintained in 84% of patients. Overall, the most common AEs were lymphopenia and electrolyte derangements. G-3 hematologic AEs were lymphopenia, anemia, and neutropenia in 67%, 27%, and 24% of the study participants, respectively, whereas common G-4 AEs were lymphopenia and neutropenia in 9% of the study participants for each category. Among non-hematologic AEs, common G-3/4 AEs were electrolyte abnormalities (36%) and infections (13%). G-3 or higher peripheral neuropathy (PN) was not reported. Kzandjian et al 2018,14 reported five-year follow-up results of this trial in 2018 which showed an ORR of 98% [CI: 88–100%]. After a median follow-up of 5.2 years, the MRD-negative CR rate was 62% [CI: 47–76%]. MRD-negative CR and longer time to progression benefits were seen in both standard risk and high-risk cytogenetics.
Zimmerman et al, 2016,18 conducted a phase II clinical trial studying the CRd regimen in 76 transplant-eligible NDMM patients. Seventy-two patients received 4 cycles of CRd induction, following which 71 patients underwent autologous stem cell tranplantation (ASCT), 66 patients had 4 cycles of CRd consolidation and 44 patients underwent additional 10 cycles of CRd maintenance. Response data were available for 73 patients. VGPR, CR, and sCR at the end of the 8th cycle were 96%, 73%, and 69%, respectively. Two-year PFS with a median follow-up of 17.5 months was 97%, and 2-yr OS was 99% for all 76 patients. The most common grade 1/2 AEs were thrombocytopenia (57%) and PN (39%). The most commonly reported G-3/4 AEs were lymphopenia (28%), neutropenia (18%), and infections (8%).
In the IFM phase II clinical trial, Roussel et al, 2016,17 studied the role of CRd with ASCT in 46 NDMM patients. Forty-three patients (21% with high-risk cytogenetics such as 17p deletion and/or t(4;14)) completed four cycles of CRd induction, 42 patients underwent ASCT following which 41 patients received four cycles of CRd consolidation. Twenty-seven patients received 1-yr lenalidomide maintenance. Data of 42 patients were accessible for evaluation showing ORR was 97.5%, VGPR 23.5%, CR 69%, and sCR of 64%. Median PFS was not reached in this trial and four patients discontinued treatment due to AEs. Cardiovascular AEs were 43.6%, whereas 26% were infections. The most common G ≥ 3 AEs were hematologic toxicities and infections, accounting for more than 10%. G-3 or higher PN was not recorded.
Jakubowiak et al, 201713 evaluated minimal residual disease (MRD) negativity and PFS in 76 transplant-eligible NDMM patients (36% patients with high-risk cytogenetics) treated with CRd followed by ASCT. Seventy-four patients completed four cycles of CRd induction, 72 underwent ASCT, and 70 completed four cycles of consolidation. Sixty-four patients further underwent 10 cycles of CRd maintenance. Efficacy of CRd was available for 76 patients with ≥VGPR of 91%, ≥CR of 78%, and sCR of 75%. MRD negativity measured by next-generation sequencing (NGS) when combined with at least one CR was 67% (n=36) and 78% (n=32), respectively, by the end of the 8th cycle and 18th cycle of CRd whereas when measured by multiparameter flow cytometry (MFC) was 95% (n=37) and 96% (n=27), respectively. MRD negativity when achieved remained sustained in 91% and 96% patients as measured by NGS and MFC, respectively. Three-year PFS and OS were higher for those who achieved sustained MRD negativity (n=18) at 18th cycle versus whole group (n=76), ie, 94% PFS for those with sustained MRD negative state vs 86% for all patients and 100% OS for sustained MRD-negative patients versus 93% for all patients. Among 27 patients with high-risk cytogenetics, the 3-Yr PFS and OS were 81% and 87%, respectively.
Korde et al, 201715 conducted a phase I/II trial in which 29 patients with NDMM were enrolled including 18 patients in phase I and 11 patients in Phase II. Patients were administered a maximum of 12 cycles of CRd induction. CFZ was administered in two dosing escalation cohorts as 45 mg/m2 and 56 mg/m2. After completing six cycles, transplant-eligible patients underwent ASCT. The median age was 61. For the 15 evaluable patients, a median of 11 cycles was administered with 60% of participants achieving CR with MRD negativity and 40% achieving VGPR. The reported G-3 hematologic AEs included lymphopenia, anemia, and neutropenia in 41%, 3%, and 3% of the study population (n=29), respectively. The common G ≥ 3 non-hematologic events were rash (21%) and electrolyte abnormalities (17%).
Alsina et al, 201910 reported the interim results of a phase Ib clinical trial. Fifty-one NDMM patients were enrolled irrespective of transplant eligibility. The patients (n=33) in the dose-expansion arm of this trial received 56 mg/m2 of CFZ as part of the weekly CRd. By the 4th cycle of CRd, the ORR in this population was 97%, ≥VGPR was 69.7%, and CR was 3%. Nineteen patients received ASCT in this study and their ORR was 92.9%. The safety profile showed a 60.6% incidence of G ≥ 3 treatment-emergent AEs (TEAEs). The most common AEs were anemia (12.1%), hyponatremia (12.1%), and elevated ALT (9.1%).
In a prospective trial, Gavriatopoulou et al, 2020,11 evaluated the role of CRd as consolidation among 40 NDMM patients (median age, 56 years) with at least PR and less than MRD negative state after ASCT. These patients were given four cycles of CRd as consolidation followed by lenalidomide maintenance until progression. After CRd consolidation, the response quality improved in 81% of patients (total evaluable patients=37). The study showed an improvement in sCR from 2.6% (following ASCT) to 75.7% following CRd consolidation. Almost 67% achieved (n=25) an MRD-negative state after CRd treatment. Eighteen percent of patients had G ≥ 3 toxicity, but individual percentages were not reported.
Carfilzomib, Thalidomide, and Dexamethasone (CTd)
Carthadex trial,19 a multicenter phase II trial, evaluated the role of CTd as induction and consolidation in 111 transplant-eligible NDMM (high-risk cytogenetics 39%, n=43) patients (median age: 58, range 29–66) This was a dose-escalation trial of CTd with doses of CFZ ranging from 20 mg/m2 to 56 mg/m2. After completing four cycles of CTd induction, the ORR was 93% (n=103) and CR 18% (n=20), whereas ≥ VGPR was 65% [CI: 55–74%]. VGPR rate increased to 86% after CTd consolidation, whereas ORR increased to 94% (n=104). CR increased from 18% (following CTd induction) to 63% (n=70) (following CTd consolidation). G ≥ 3 hematologic AEs occurred in 10% of patients only while the most common hematologic AEs were respiratory disorders (8%) and skin disorders/vascular disorders (9% each).
Carfilzomib-Based Regimen with Alkylating Agents
There are a total of eight studies (n=580) with data on CFZ in combination with alkylating agents, ie, cyclophosphamide (Cy) or melphalan (M). Table 2 summarizes the characteristics and efficacy of carfilzomib with alkylating agents, daratumumab, quadruplet regimens, and other proteasome inhibitors. There are two studies in which CFZ combination with melphalan was evaluated while six studies used a combination of CFZ with cyclophosphamide. ORR among these studies ranged from 85% to 100% except for one study in which CFZ was used in a dose-escalation manner, showing an ORR of 66% with the dose of 36 mg/m2. The total number of cycles ranged from 4 to 9 cycles.
 |  |  |
Table 2 Baseline Characteristics and Efficacy of Carfilzomib with Alkylating Agents, Daratumumab, Quadruplet Regimens, and Other Proteasome Inhibitors |
Carfilzomib, Cyclophosphamide, and Dexamethasone (CCyd)
Bringhen et al, 2014,20 conducted a phase II trial in 58 patients (35% with unfavorable cytogenetics) with symptomatic NDDM who were 65 years old or above and were ineligible for ASCT. Authors used the carfilzomib-cyclophosphamide-dexamethasone (CCyd) regimen as induction for nine 28-day cycles followed by maintenance with 36 mg/m2 CFZ on day 1, 2, 15, and 16 of the 4-week cycle until progression or intolerance. Overall, 25 patients were evaluable at the end of nine induction cycles. Following induction, VGPR and PR were 76% and 96%, respectively, whereas nCR and sCR were 64% and 24%, respectively. One-yr PFS and OS rates were 76% and 87%. Twenty-five patients were evaluable for CFZ maintenance and after 6 months (median) of maintenance, the PR was 100%, whereas CR/nCR was 68%. Response rates were generally similar across patient subgroups according to age, ISS stage, and chromosomal profile. Anemia, thrombocytopenia, and neutropenia were common hematological toxicities. Infectious events were 18%, and PN was experienced by 9% of patients and was mostly of G-1-2. Larocca et al, 2018,21 updated the results after a 5-yr follow-up, which showed ≥ PR, ≥ VGPR, and ≥ CR of 95%, 69%, and 51% at the end of induction. Among 51% of the patients who achieved CR, 16% achieved sCR. After CFZ maintenance, ≥ PR, ≥ VGPR, and nCR/CR rates were 100%, 84%, and 60%, respectively.
Bensinger et al, 2014,22 evaluated the optimal dose, safety, and efficacy of CCyd induction (4–6 cycles) before ASCT in transplant-eligible NDMM patients in a phase Ib, open-label trial. Twenty-eight patients (high-risk cytogenetics, n=16) received a 3+3 dose-escalation schedule with cohorts of CFZ 36 mg/m2, 45 mg/m2, and 56 mg/m2 in combination with oral cyclophosphamide and dexamethasone. Among 23 evaluable patients, ≥ PR rate was 91%. Among 12 evaluable patients with high-risk cytogenetics, ≥ PR rate was 92%, ORR was 87%, and ≥ VGPR 48% after 4–6 cycles. Among dose cohorts, 56 mg/m2 dose was associated with G-3 dyspnea in 1st cycle. Fatigue (23%) and thrombocytopenia (31%) occurred in more than 20% of patients.
In the CHAMPION-2 study,23 a Phase 1b trial evaluated three dose levels of CFZ (36, 45, and 56 mg/m2) in a dose-escalation manner followed by dose expansion along with fixed dosed cyclophosphamide and dexamethasone in 22 NDMM patients regardless of transplant eligibility. Eight cycles of 28 days were planned. Dose-limiting toxicities were not seen with any of the three doses of CFZ. Among patients (n=16, 6.3% high-risk cytogenetics) who received the maximum dose of 56 mg/m2, ORR was 87.5% (CI: 61.7–98.4%) whereas VGPR, PR, and CR were 43.8%, 37.5%, and 6.3%, respectively. ORR was 66% (CI: 9.4–99.2) and 100% (CI: 29.2–100.0) for drug cohorts of 36 mg/m2 (n=3) and 45 mg/m2 (n=3), respectively. PFS was not assessed in this trial. Common AEs irrespective of grade and drug dose cohorts were nausea (72.7%), vomiting (40.9%), diarrhea (40.9%), and anemia (40.9%). Anemia (22.7%) and neutropenia (13.6%) were more frequent G-3 or higher AEs.
Bringhen et al, 2018,24 conducted a phase I/II study in 63 patients with NDMM, who were aged ≥65 years or ineligible for ASCT. Weekly, CCyd induction was given for nine cycles (28-day) followed by CFZ maintenance (70g/m2 on day 1 and day 15 of the 4-week cycle) until progression. Twelve patients were enrolled in phase I (3:3 dose-escalation design) and 51 patients in phase II. However, a total of 54 patients were treated at the recommended phase II dose of 70 mg/m2 and were studied for efficacy and response. In the 54 response evaluable patients, ≥ PR and ≥ nCR were 93% and 44%, respectively, following nine cycles of induction. After maintenance, ≥ PR rates, and ≥ nCR and were 98% and 54%, respectively. Overall response rates for ≥ PR, ≥ VGPR, and ≥ nCR were 85%, 66%, and 30%. After a median follow-up of 19.7 months, the 2-yr PFS and OS rates were 53.2% and 81%, respectively. The common toxicities irrespective of grade during the induction phase were anemia (39%) being the most common followed by thrombocytopenia (33%), neutropenia (31%), and infections (13%). No PN was recorded.
In a phase II study of 30 high-risk MM, transplant-eligible patients, Chen et al, 2018,25 used CCyd induction. Patients then received HDM/ASCT and two consolidation cycles of CCyd. MRD analysis was done if patients had at least VGPR and subjects who achieved MRD-negativity were managed expectantly, whereas patients who were MRD-positive received maintenance with CFZ for 2 years or until progression. The interim post-induction (n=30) results showed ORR, ≥ CR rate, and ≥ VGPR rate of 86.7%, 33.3%, and 63.3%, respectively. Post-ASCT (n=25), ORR, ≥ CR, and ≥ VGPR was 84%, 44%, and 72%, while post-consolidation (n=21) ORR, ≥ CR, and ≥ VGPR was 81%, 57.1%, and 71.4%, respectively. With a median follow-up of 19.8 months, the median PFS was 28.4 months. G ≥ 3 hematologic TEAEs were about 30% (n=9) including anemia and neutropenia (16.7% each) and thrombocytopenia (13.3%). Other common TEAEs were respiratory infections (23.3%), acute kidney injury (13.3%), and diarrhea (10%).
The phase II Cardamon study26 used CCyd as induction in transplant-eligible patients and then randomized them to either ASCT or CCyd consolidation followed by CFZ maintenance. Among 281 enrolled patients, 252 patients were available for the primary outcome, ie, ≥ VGPR after CCyd induction. At the end of induction or after harvesting stem cells, ORR was 87.6% and ≥ VGPR 59.2%. ORR (87.9% versus 88.1% for high-risk and standard-risk, respectively) and ≥ VGPR rate (53.4% vs 61.9% for high-risk vs standard-risk, respectively) were similar in high-risk or standard-risk individuals. Serious AEs due to induction occurred in 28.6% (72/252) of patients, notably G-3 cardiac ischemia, hypertension, renal dysfunction, thromboembolism, and infections.
Carfilzomib, Melphalan, and Prednisone (CMP)
IFM 2012–03, a phase I trial by Leleu et al, 201927 studied the role of weekly CMP in 30 transplant-ineligible elderly NDMM patients (median age=73 years; high-risk cytogenetics, n=3) and determined the MTD of CFZ. Patients underwent nine 35-day cycles of CMP induction with CFZ given in four intravenous dosing cohorts [36 mg/m2 (n=6), 45 mg/m2 (n=6), 56 mg/m2 (n=6), and 70 mg/m2 (n=12)] followed by CFZ maintenance for 1 year (36 mg/m2 every 2 weeks). The median time to best response was 3 months. ORR rate was 93% (n=28) with ≥CR 46.6% (n=14) and VGPR 70% (n=21). MTD of CFZ was 70 mg/m2 whereas for patients older than 75 this threshold was 56 mg/m2. During induction, seven patients stopped therapy (six due to toxicity and one personal decision), whereas five patients stopped treatment during maintenance. Common causes of CFZ interruption were related to cardiovascular toxicity including heart failure and myocardial infarctions. Lymphopenia (36.7%), neutropenia (30%), thrombocytopenia (23.3%), and anemia (16.7%) were among the most common G ≥3 AEs.
In another phase I/II dose-escalation trial, 68 NDMM patients aged above 65 (median age, 72 years) were enrolled.28
In phase I (n=24), CMP with CFZ was administered at doses of 20 mg/m2, 27 mg/m2, 36 mg/m2, and 45 mg/m2 to determine MTD (36 mg/m2). In phase II (n=44), CFZ 36 mg/m2 was administered for nine induction cycles. The median time to response was 1.5 months. In 50 evaluable patients, ORR was 90% (n=45), CR 12% (n=6), VGPR 46% (n=23), and PR of 32% (n=16). The most common G ≥ 3 AEs were neutropenia (38%, CI: 26.7–50.8%), anemia (35%, CI: 24.1–47.8%), thrombocytopenia (28%, CI: 17.7–40.1%), and infections (7%, CI: 2.4–16.3%).
Carfilzomib-Based Quadruplet Regimens
There are two studies in this group where a quadruplet regimen in combination with CFZ to treat NDMM was studied. A total of 1119 patients were enrolled in these two studies. One study combined cyclophosphamide with CTd regimen. The second study compared the efficacy of carfilzomib-cyclophosphamide-lenalidomide-dexamethasone (CCyRd) versus CTd/CRd regimen. Four trials evaluated the combination of CFZ with other drugs, one of them being a monoclonal antibody such as daratumumab and isatuximab.
Carfilzomib, Cyclophosphamide, Thalidomide, and Dexamethasone (CYKLONE)
Mikhael et al, 2015,29 conducted a phase Ib/II trial evaluating CYKLONE (carfilzomib, cyclophosphamide, thalidomide, and dexamethasone) induction regimen in 64 NDMM transplant-eligible patients (median age= 62.5 years, high-risk, n=6). All patients received four induction cycles followed by SCT in 34 patients. Investigators continued on treatment for up to eight more cycles for patients who had stable disease or better. In phase I, there were four drug cohorts including 15/20 mg/m2 (n=3), 20/27 mg/m2 (n=25), 20/36 mg/m2 (n=29), and 20/45 mg/m2 (n=7). ORR was 91% (n=44) with ≥VGPR 51% (n=39). PFS at 1-year and 2-year were 85% and 76%, respectively, whereas OS both at 1-yr and 2-yr was 96%. Hematological AEs of any grade were neutropenia (55%), thrombocytopenia (47%), anemia (44%), and lymphopenia (42%). Common non-hematologic AEs were fatigue (80%), constipation (53%) and hyperglycemia (39%). Peripheral neuropathy (31%) was predominantly related to thalidomide and all events were grade 1.
Carfilzomib, Cyclophosphamide, Lenalidomide, and Dexamethasone (CCyRd)
The NCRI myeloma XI phase III trial30 compared carfilzomib-based quadruplet induction (carfilzomib-cyclophosphamide-lenalidomide-dexamethasone, CCyRd) with response-adapted triplet inductions such as cyclophosphamide-thalidomide-dexamethasone/cyclophosphamide-lenalidomide-dexamethasone prior to ASCT in 1055 patients. CCyRd was associated with a longer PFS than triplet regimens, 3-yr PFS 64.5% versus 50.3% [HR: 0.63, CI: 0.51–0.76], respectively. Patients were randomized 3 months post-ASCT to receive lenalidomide maintenance or observation. A higher proportion of patients who received quadruplet induction underwent ASCT versus those who received triplet regimens. There was no heterogeneity in terms of PFS in high-risk versus standard-risk patients. Despite the quadruplet regimen, patients showed no significant toxicity.
Carfilzomib-Based Regimen with Monoclonal Antibodies (Daratumumab, Isatuximab)
Four trials tested the efficacy of CFZ in combination with monoclonal antibodies and showed promising results. These included trials a total study population of 1314 patients. The number of induction cycles in trials by Costa et al, Chari et al, and Weisel et al were 4, 13, and 6, respectively. CFZ was given in a dose-escalated fashion in the last two trials. Landgren et al. assessed the MRD negativity while Weisel et al. reported results for safety profile.
MMY1001 phase Ib trial by Chari et al, 201731 evaluated the safety and efficacy of Dara-CRd in 22 NDMM patients (median age= 59.5 years) irrespective of transplant candidacy. Dara-CRd regimen was administered up to a maximum of 13 cycles. The dose of CFZ was increased from 20 mg/m2 to 70 mg/m2 in progressing cycles. Among 21 evaluable patients, the ORR was 100%. Other outcome measures were sCR=43%, CR (excluding sCR)=14%, VGPR=33%, and PR=10%. The most common G-3/4 AEs included lymphopenia (64%), neutropenia (18%), diarrhea (18%), and pulmonary embolism (14%).
Landgren et al, 2019,32 conducted a phase II trial to test two different Dara-CRd regimens/cohorts in NDMM and their roles in the achievement of MRD-negativity. The plan was to enroll 82 patients in this trial with 41 in each cohort. Cohort-1 received a weekly dose of 56 mg/m2 CFZ augmented with lenalidomide, dexamethasone, and daratumumab. Cohort-2 patients received a biweekly 30 mg/m2 of CFZ with the same regimen of all other drugs as in cohort-1. In the interim analysis, only cohort-1 patients were included. The median number of cycles delivered in cohort-1 was six. Out of 18 evaluable patients, 15 patients were MRD-negative (MRD negative rate= 83%). The toxicity profile was not a part of the interim analysis.
Weisel et al, 2019,33 reported the results of a safety run-in cohort (n=10) for the four-drug regimen (isatuximab-carfilzomib-lenalidomide-dexamethasone, I-CRd) in patients with high-risk NDMM in the GMMG-CONCEPT trial. This trial plans to enroll 153 patients who would receive I-CRd (6 cycles) as induction followed by HDM and I-CRd consolidation (4 cycles) and I-CR maintenance. This safety run-in trial aimed to report dose-limiting toxicity after two cycles of I-CRd induction. All patients had at least one TEAE (total events, 49) during the run-in phase. Main G ≥ 3 toxicities were hematologic in nature with neutropenia being the most common (n=6). Non-hematologic G-3 AE was cerebrovascular disorders (n=2). Nine out of 10 patients received six induction cycles and all 10 patients achieved ≥VGPR.
Costa et al, 2020,34 performed a phase II trial among 81 transplant-eligible NDMM patients (median age= 61 years). Patients received four cycles of daratumumab combined with carfilzomib-lenalidomide-dexamethasone (Dara-CRd) as induction. After ASCT, patients were given Dara-CRd as consolidation. The number of cycles was tailored by MRD status. The maximum number of consolidation cycles was eight and patients without a confirmed MRD-negative status after that were started on lenalidomide maintenance. Results were reported at different points in this trial. After two cycles of induction (n=81), 67% and 33% of patients achieved VGPR and PR, respectively. After four cycles of induction (n=72), nCR, CR, VGPR, and PR were 39%, 3%, 8%, and 10%, respectively. MRD-negativity assessed in this trial was 40% at post-induction, 73% post-ASCT, and 82% at MRD-guided consolidation, respectively. The common G ≥3 AEs were neutropenia (25%), lymphopenia (23%), and infections (12%).
Carfilzomib vs Other Proteasome Inhibitors
Bortezomib-based regimens are used as a treatment for NDMM and have shown good efficacy. CFZ is being studied for the treatment of NDMM. Herein, we describe two clinical trials including the long-awaited ENSURANCE trial that compared the efficacy of bortezomib against carfilzomib-based regimens. A total of 2042 patients were enrolled in both studies. One study compared CMP against VMP showing an ORR of 84% vs 79%, respectively.
Carfilzomib-Melphalan-Prednisone (CMP) vs Bortezomib-Melphalan-Prednisone (VMP)
Facon et al, 201935 conducted a phase III CLARION trial in 955 transplant-ineligible patients (median age=72 years), comparing CMP (n=478, high-risk 11.3%) with VMP (n=477, high-risk 14%) (Bortezomib-melphalan-prednisone). CMP cohort received CFZ 20 mg/m2 on C1D1, C1D2, and 36 mg/m2 thereafter, whereas the VMP cohort received bortezomib 1.3 mg/m2 with melphalan and prednisone being the same in both of the cohorts. CMP cohort had a median PFS of 22.3 months versus 22.1 months in VMP (HR: 0.906, CI: 0.746–1.101). OS was not reached in either of the cohorts, HR: 1.08, CI: 0.82–1.43. ORR for CMP cohort was 84.3% (CI: 80.7–87.5%) vs 78.8% (CI: 74.9–82.4%) for VMP cohort. CR rate was also higher for CMP versus VMP, ie, 23.2% versus 21%, respectively. Odds of ≥ PR achievement were increased up to 41% in CMP versus VMP, OR: 1.41, CI: 1.01–1.97. MRD-negative rates were not different in either of the cohorts. The most common G ≥ 3 AEs were neutropenia (22.6% for CMP vs 29.4% for VMP), thrombocytopenia (22.6% for CMP vs 29.4% for VMP) and anemia (16.9% for CMP vs 13.6% for VMP).
Carfilzomib-Lenalidomide-Dexamethasone (CRd) vs Bortezomib-Lenalidomide-Dexamethasone (VRd)
In the ENDURANCE phase III trial, Kumar et al, 2020,36 compared the efficacy of CRd (n=545) versus VRd (n=542) among 1087 patients. The patients were administered with bortezomib of 1.3 mg/m2 dose in VRd arm with the 3-weekly cycle for 12 cycles while CFZ of 36 mg/m2 dose in CRd arm with 4-weekly cycles for 9 cycles. Both arms received maintenance of lenalidomide. Median PFS (34.6 months for CRd versus 34.4 months for VRd) was the same in both arms, HR: 1.04, CI: 0.8 to 1.3. High-risk patients were excluded in the ENDURANCE trial. The 3-yr OS was also similar with CRd OS of 86% versus 84% in VRd.
Carfilzomib with Dexamethasone
Carfilzomib, Clarithromycin, Lenalidomide, and Dexamethasone (Car-BiRD)
In this single-arm phase II trial reported by Forsberg et al, 2019,37 72 NDMM patients (high-risk cytogenetics, n=9 (27%)) with a median age of 59 years were given CFZ-dexamethasone (Cd) induction. CFZ delivered in two dosing cohorts, Cd1 20/45 mg/m2 (n=25) and Cd2 20/56 mg/m2 (n=47). This was followed by ASCT in eligible patients and BiRD consolidation (Clarithromycin, lenalidomide, and dexamethasone). The median time to best response was three cycles. Cd1 and Cd2 induction cohorts had ORR 84% versus 93%, CR 12% versus 14%, VGPR 48% versus 64%, and PR 24% versus 26%, respectively. The most common G ≥ 3 AEs were hypertension (7%), lymphopenia (6%), and lung infection (6%). Table 3 summarizes the toxicity of CFZ containing regiments in the trials of NDMM so far.
 |  |  |
Table 3 Toxicity Profile of Carfilzomib-Based Regimens |
Discussion
CFZ-based combination regimens have demonstrated favorable efficacy and a toxicity profile in patients with NDMM. We analyzed data from 27 clinical trials. The most commonly studied therapeutic regimen was CRd with an ORR ranging from 79% to 100%. The four-drug combination regimens CCyRd and CYKLONE yielded results comparable to those of three-drug regimens. However, the combination of the monoclonal antibody, daratumumab, with CRd generated deep and durable responses, including high MRD-negativity and an ORR of 100% without additional toxicity.2
Various dosing and scheduling of CFZ in patients with NDMM were studied, including a once-weekly dose of 70 mg/m2 of CFZ and twice-weekly doses of 36 mg/m2.38 Data pooled from two phase I/II studies (n=121), namely Bringhen et al,20,24 2014 (n=58) and Bringhen et al, 2017 (n=63), included transplant-ineligible NDMM patients who received 9 induction cycles of CCyd, followed by CFZ maintenance. The study found no significant difference in 3-yr PFS (47% versus 51% P=0.92) and OS (72% versus 73%; P=0.71) between the once-weekly 70 mg/m2 dose and twice-weekly dose of 36 mg/m2. The rates of G ≥ 3 hematologic (24% versus 30%; P=0.82) and non-hematologic (38% versus 41%; P=0.83) AEs were also similar among the two groups. Another important finding was that the rate of CVAEs (cardiovascular AEs) did not increase with the higher, once-weekly dose of 70 mg/m2. The phase III ARROW trial,39 which compared twice-weekly CFZ at a dose of 27 mg/m2 versus once-weekly CFZ at 70 mg/m2 in RRMM patients, demonstrated slightly different results. The median PFS was found to be longer with a once-weekly dose than with a twice-weekly dose (11.2 months [CI: 8.6–13.0] versus 7.6 months [5.8–9.2, CI: 0.54–0.83; p=0.0029]), without any additional toxicity. Studies have compared the efficacy and tolerability of various CFZ doses, but the CARTHADEX trial did not show an improvement in efficacy in terms of CR rates beyond the 20/36 mg/m2 dose of CFZ. It is interesting to note that the rate of CVAEs remained consistent and was generally low (all grades=12%, G >3: 5%), however, the rate of infections, particularly pneumonia, gradually increased with higher doses. A post hoc analysis was conducted, which studied data from the ENDEAVOR, ARROW, and CHAMPION-1 trials to compare the efficacy and safety profile of a once-weekly dose of 70 mg/m2 CFZ to the twice-weekly dose of 56 mg/m2 in patients with RRMM.40 This cross-trial comparison showed no significant difference in the PFS (P=0.47) and ORR (odds ratio: 1.12; CI: 0.74‐1.69; P=0.61) among the two groups. In addition, G > 3 AEs were observed more frequently in the twice-weekly 56 mg/m2 group compared to the once-weekly 70 mg/m2 group.
The combination of carfilzomib-lenalidomide-dexamethasone (CRd) was the most widely evaluated and most efficacious among the triplet regimens, with ORR ranging from 79% to 100% and CR ranging from 50% to 63%. The four-drug regimens containing the antibody daratumumab with CRd demonstrated results superior to the triplet regimens, with ORR reaching up to 100%. Data by Landgren et al study42 were particularly impressive as it resulted in MRD negativity of 80%, regardless of HDCT and ASCT use. The results from the Car-BiRD and CYCLONE trials showed similar efficacy compared with the triplet regimens. The ongoing MASTERS trial34 is studying the four-drug regimen of daratumumab with CRd as induction therapy as well as post-transplant consolidation therapy in NDMM patients. This study will use MRD not only as a primary endpoint but also determine the intensity and duration of post-transplant Dara-CRd consolidation required in each patient. This patient-centered approach can be clinically useful as it will spare MRD-negative patients from the burden of continuous therapy and the development of potential toxicities. The results from this trial are very encouraging with VGPR of 90% post-induction and sCR reaching up to 95% following MRD-based consolidation. The rates of MRD-negative remission (10−5) were 82% post-MRD directed consolidation and MRD (10−6) were 63%. The common G >3 AEs of therapy were hematological (neutropenia: 25%, lymphopenia: 23%) in nature. MRD-negative patients who have discontinued therapy will have close follow-up to assess for any resurgence of MRD-positive disease. The MRD negativity values observed in the MASTERS trial are higher compared to those achieved in the GRIFFIN trial,41 whereby a head-to-head comparison was made between VRD and Dara-VRD regimens in NDMM patients. By the end of post-AST consolidation, sCR was found to be 42.4% in the D-VRD group and 32% in the VRD group. MRD negativity (10−5) was 47.1% with D-VRD and 16.5% with VRD by the end of consolidation. Another phase 1b trial42 studying Dara-CyBorD in MM patients showed 56% patients achieving MRD negativity of 10−5.
The most common G ≥ 3 AEs encountered as a result of CFZ therapy were hematological, while the most common non-hematological side effect being CVAEs. An integrated analysis was conducted which studied the CVAEs in transplant-ineligible patients receiving CCyd for NDMM.43,45 It included three phase I/II clinical trials (total patients=148), with one study administering CFZ at a dose of 36 mg/m2 and the other two escalating the dose from 46 mg/m2 to 70 mg/m2. The study found that 45% of patients had at least one CVAE, with the most common being hypertension and dyspnea. Patients with pre-existing cardiovascular risk factors at enrollment had a fourfold increased risk (odds ratio: 3.79; P<0.001) of developing CVAEs as compared to those without a CV risk factor (baseline hypertension and peripheral vascular disease conferring the highest risk). The incidence of major cardiac events was also higher in older patients compared to younger ones (29% vs 6%; P<0.001). In light of these findings, it is suggested that elderly patients receiving CFZ should undergo a pretreatment screening cardiac evaluation to detect any abnormalities that may be aggravated during the treatment.44
Peripheral neuropathy (PN) is another non-hematological AE associated with proteosome inhibitors and often lead to dose interruptions, discontinuations of treatment, or dose reductions which may result in inadequate management of the disease and eventually early disease progression. A phase III clinical trial, CLARION,35 compared carfilzomib-melphalan-prednisone versus bortezomib-melphalan-prednisone in transplant-ineligible NDMM patients. The study showed that despite no significant PFS difference between CFZ and bortezomib groups (median, 22.3 versus 22.1 months; CI: 0.75–1.10), the adverse effect profile was markedly different in the two groups. The rate of G >2 PN was 2.5% in the CFZ group, compared to 35.1% in the bortezomib group (OR: 0.048, CI, 0.026–0.088; P: 0.0001). This finding is consistent with the ENDEAVOR trial, which also reported a higher rate of G > 2 PN in the bortezomib group (32%) versus the CFZ group (6%). ENDURANCE,36 another phase III clinical trial comparing CRd with VRd, revealed similar results. CRd did not improve the median PFS (VRd: 34.4 months versus CRd: 34.6 m) and 3-yr OS [VRd: 84% (CI: 80–88%) versus CRd: 86% (CI: 82–89)] in patients with NDMM. PN was again more commonly seen with VRd. However, these results cannot be applied to NDMM patients with high-risk cytogenetics as ENDURANCE specifically excluded high-risk cases in their patient population. With these results, CFZ can be used as PI in frontline setting, especially in patients at high risk for development of PN. CFZ, however, was associated with hematological, cardiovascular, and renal AEs in both these trials. Patients receiving CFZ-based therapy had a relatively higher risk of developing G >3 renal toxicity (Risk ratio: 2.29, P:0.001) with acute kidney injury being the most common event.45 Similarly, patients in CFZ group had a higher rate of cardiac failure (10.8% vs 4.3%) as compared to bortezomib group.
Regarding limitations in our systematic review, the clinical trials included in this review are mainly phase I/II and phase II studies, with only three randomized phase III clinical trials. Secondly, many of the studies used different doses of CFZ and had variable durations of inductions, consolidation, and maintenance phases that might have impacted the PFS and OS rates. Thus, the dosing regimen should be individualized in every patient until we have better data to support a particular dose. Also, due to the lack of individual patient data and the limited number of patients recruited in each study, we were not able to determine which dose of CFZ proved to be the safest and most efficacious. Third, CVAEs, an important safety concern of CFZ, were reported differently in each study. Some used a broader term as “cardiac failure” while other studies reported more specific outcomes such as congestive heart failure, arrhythmias, atrioventricular nodal block, or cardiac arrest. Thus, it was difficult to ascertain which CVAE occurred more frequently.
Conclusion
CFZ-based therapeutic regimens have proven to be highly efficacious with excellent CR and VGPR rates and impressive MRD negativity. The three and four drug combination regimens are well-tolerated in patients with NDMM, based on phase II clinical trials conducted in recent years. However, when phase III clinical trials, such as ENDURANCE and CLARION, compared it to the standard-of-care regimen containing bortezomib, it failed to improve the PFS and OS rates in NDMM patients. This was a rather unexpected finding, given the promising results that CFZ regimens demonstrated in phase II trials. Studies comparing several dosing schedules of CFZ have showed contradictory efficacy data; however, cardiovascular side effects remained consistent with higher doses. The incidence of PN reported with carfilzomib is quite low when compared to bortezomib so it can still prove to be a useful alternative in patients who develop bortezomib-induced PN. Cardiotoxicity is an important adverse effect related to CFZ as it can impact the duration of treatment and the overall survival rate of patients. Elderly patients with pre-existing cardiovascular risk factors are especially affected; therefore, cardiovascular assessment before initiating therapy is highly recommended. Dara-CRd combination regimen has shown promising resultsso far, especially in the MASTERS trial studying MRD-based response adapted therapy in NDMM. Another trial studying this combination showed MRD negativity rate of 80% in the absence of ASCT. In light of these findings, it may have the potential to bypass the need for transplant. Randomized phase III clinical trials should be conducted to directly compare Dara-CRd with the standardized VRd therapy in patients with NDMM.
Acknowledgments
The authors acknowledge the support in the literature search and data extraction during the preparation of this manuscript provided by Muhammad Ali Khaqan, Muhammad Ali Aziz, and Muhammad Yasir Anwar.
Disclosure
Dr Faiz Anwer report grants from Celgene, grants from BMS, personal fees from BMS, grants, personal fees from Janssen Pharmaceuticals, and personal fees from Janssen Pharmaceuticals, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Zhang S, Kulkarni AA, Xu B, et al. Bortezomib-based consolidation or maintenance therapy for multiple myeloma: a meta-analysis. Blood Cancer J. 2020;10(3):33. doi:10.1038/s41408-020-0298-1
2. Landgren O, Sonneveld P, Jakubowiak A, et al. Carfilzomib with immunomodulatory drugs for the treatment of newly diagnosed multiple myeloma. Leukemia. 2019;33(9):1. doi:10.1038/s41375-019-0517-6
3. Facon T, Anderson K. Treatment approach for the older, unfit patient with myeloma from diagnosis to relapse: perspectives of a European hematologist. Hematology. 2018;2018(1):83–87. doi:10.1182/asheducation-2018.1.83
4. Engelhardt M, Yong K, Bringhen S, Wäsch R. Carfilzomib combination treatment as first-line therapy in multiple myeloma: where do we go from the carthadex (KTd)-trial update? Haematologica. 2019;104(11):2128–2131. doi:10.3324/haematol.2019.228684
5. Ziogas DC, Terpos E, Kastritis E, Dimopoulos Ma. An overview of the role of carfilzomib in the treatment of multiple myeloma. Expert Opin Pharmacother. 2017;18(17):1883–1897. doi:10.1080/14656566.2017.1404575
6. Bringhen S, Petrucci MT, Larocca A, et al. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: a multicenter, phase 2 study. Blood. 2014;124(1):63–69. doi:10.1182/blood-2014-03-563759
7. Muchtar E, Gertz MA, Magen H. A practical review on carfilzomib in multiple myeloma. Eur J Haematol. 2016;96(6):564–577. doi:10.1111/ejh.12749
8. Accardi F, Toscani D, Bolzoni M, Dalla Palma B, Aversa F, Giuliani N. Mechanism of action of bortezomib and the new proteasome inhibitors on myeloma cells and the bone microenvironment: impact on myeloma-induced alterations of bone remodeling. Biomed Res Int. 2015;2015:172458. doi:10.1155/2015/172458
9. Shaji KK, Natalie SC, Kehinde A, et al. Multiple myeloma, version 3.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(12):1685–1717. doi:10.6004/jnccn.2020.0057
10. Alsina M, Landgren CO, Raje N, et al. A phase 1b study of once-weekly carfilzomib combined with lenalidomide and dexamethasone (wKRd) in patients (pts) with newly diagnosed multiple myeloma (NDMM). Clin Lymphoma Myeloma Leuk. 2019;19(10):e52. doi:10.1016/j.clml.2019.09.079
11. Gavriatopoulou M, Terpos E, Ntanasis-Stathopoulos I, et al. Consolidation with carfilzomib, lenalidomide, and dexamethasone (KRd) following ASCT results in high rates of minimal residual disease negativity and improves bone metabolism, in the absence of bisphosphonates, among newly diagnosed patients with multiple myeloma. Blood Cancer J. 2020;10(3). doi:10.1038/s41408-020-0297-2
12. Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120(9):1801–1809. doi:10.1182/blood-2012-04-422683
13. Jakubowiak AJ, Raje N, Vij R, et al. High rate of sustained minimal residual disease negativity predicts prolonged survival for the overall patient population in the phase 2 KRd plus autologous stem cell transplantation MMRC trial. Blood. 2017;130(Supplement 1):4533.
14. Kazandjian D, Korde N, Mailankody S, et al. Remission and progression-free survival in patients with newly diagnosed multiple myeloma treated with carfilzomib, lenalidomide, and dexamethasone: five-year follow-up of a phase 2 clinical trial. JAMA Oncol. 2018;4(12):1781–1783. doi:10.1001/jamaoncol.2018.5457
15. Korde N, Mailankody S, Smith EL, et al. MRD response-driven phase I/II study for newly diagnosed multiple myeloma patients using higher doses of twice-weekly carfilzomib (45 and 56 mg/m2) in combination with lenalidomide and dexamethasone. Blood. 2018;132:1983.
16. Korde N, Roschewski M, Zingone A, et al. Treatment with carfilzomib-lenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncol. 2015;1(6):746–754. doi:10.1001/jamaoncol.2015.2010
17. Roussel M, Lauwers-Cances V, Robillard N, et al. Frontline therapy with carfilzomib, lenalidomide, and dexamethasone (KRd) induction followed by autologous stem cell transplantation, krd consolidation and lenalidomide maintenance in newly diagnosed multiple myeloma (NDMM) patients: primary results of the intergroupe francophone du myélome (IFM) krd phase II study. Blood. 2016;128(22):1142.
18. Zimmerman T, Raje NS, Vij R, et al. Final results of a phase 2 trial of extended treatment (TX) with Carfilzomib (CFZ), Lenalidomide (LEN), and Dexamethasone (KRd) plus autologous stem cell transplantation (ASCT) in newly diagnosed multiple myeloma (NDMM). Blood. 2016;128(22):675. doi:10.1182/blood.V128.22.675.675
19. Wester R, Van Der Holt B, Asselbergs E, et al. Phase II study of carfilzomib, thalidomide, and low-dose dexamethasone as induction and consolidation in newly diagnosed, transplant eligible patients with multiple myeloma; the carthadex trial. Haematologica. 2019;104(11):2265–2273. doi:10.3324/haematol.2018.205476
20. Bringhen S, Cerrato C, Petrucci MT, et al. A Phase II Study with Carfilzomib, Cyclophosphamide and Dexamethasone (CCD) for Newly Diagnosed Multiple Myeloma. Washington, DC: American Society of Hematology; 2013.
21. Larocca A, Petrucci MT, Conticello C, et al. Updated results of a phase ii study with carfilzomib, cyclophosphamide and dexamethasone (CCD) for newly diagnosed transplant ineligible multiple myeloma patients. HemaSphere. 2018;2:234.
22. Bensinger WI, Vescio R, Gasparetto C, et al. A multicenter phase 1b, open-label, dose-finding pilot study to evaluate the combination of carfilzomib and cyclophosphamide with dexamethasone (CCyD) prior to autologous stem cell transplant (ASCT) in patients with transplant eligible newly diagnosed multiple myeloma. Blood. 2014;124(21):4739–4739.
23. Boccia RV, Bessudo A, Agajanian R, et al. A multicenter, open-label, Phase 1b Study of carfilzomib, cyclophosphamide, and dexamethasone in newly diagnosed multiple myeloma patients (CHAMPION-2). Clin Lymphoma Myeloma Leuk. 2017;17(7):433–437. doi:10.1016/j.clml.2017.05.009
24. Bringhen S, D’Agostino M, De Paoli L, et al. Phase 1/2 study of weekly carfilzomib, cyclophosphamide, dexamethasone in newly diagnosed transplant-ineligible myeloma. Leukemia. 2018;32(4):979–985. doi:10.1038/leu.2017.327
25. Chen Y, Gopalakrishnan S, Ooi M, et al. Carfilzomib, cyclophosphamide, and dexamethasone in transplant eligible newly diagnosed multiple myeloma: preliminary result of sghmm1 trial, high-risk cohort (NCT02217163). HemaSphere. 2018;2:589.
26. Yong K, Popat R, Wilson W, et al. Efficacy and safety of carfilzomib at 56 mg/m2 with cyclophosphamide and dexamethasone (K56Cd) in newly diagnosed multiple myeloma patients followed by ASCT or K56Cd consolidation: initial results of the Phase 2 Cardamon Study. Blood. 2019;134(Supplement_1):861. doi:10.1182/blood-2019-127992
27. Leleu X, Fouquet G, Richez V, et al. Carfilzomib weekly plus melphalan and prednisone in newly diagnosed transplant-ineligible multiple myeloma (IFM 2012-03): a phase I trial. Clin Cancer Res. 2019;25(14):4224–4230. doi:10.1158/1078-0432.CCR-18-3642
28. Moreau P, Kolb B, Attal M, et al. Phase 1/2 study of carfilzomib plus melphalan and prednisone in patients aged over 65 years with newly diagnosed multiple myeloma. Blood. 2015;125(20):3100–3104. doi:10.1182/blood-2015-02-626168
29. Mikhael JR, Reeder CB, Libby EN, et al. Phase Ib/II trial of CYKLONE (cyclophosphamide, carfilzomib, thalidomide and dexamethasone) for newly diagnosed myeloma. Br J Haematol. 2015;169(2):219–227. doi:10.1111/bjh.13296
30. Pawlyn C, Davies F, Cairns D, et al. Quadruplet KCRD (carfilzomib, cyclophosphamide, lenalidomide and dexamethasone) induction for newly diagnosed myeloma patients. Clin Lymphoma Myeloma Leuk. 2019;19(10):e2. doi:10.1016/j.clml.2019.09.003
31. Chari A, Usmani SZ, Krishnan A, et al. Daratumumab (DARA) in combination with carfilzomib, lenalidomide, and dexamethasone (KRD) in patients with newly diagnosed multiple myeloma (mmy1001): updated results from an open-label, phase 1b study. Blood. 2017;130:3110.
32. Landgren O, Hultcrantz M, Lesokhin AM, et al. Weekly carfilzomib, lenalidomide, dexamethasone and daratumumab (wKRd-D) combination therapy provides unprecedented MRD negativity rates in newly diagnosed multiple myeloma: a clinical and correlative phase 2 study. Blood. 2019;134(Supplement_1):862. doi:10.1182/blood-2019-126378
33. Weisel K, Asemissen AM, Schieferdecker A, et al. Isatuximab, carfilzomib, lenalidomide and Dexamethasone (I-KRd) in front-line treatment of high-risk multiple myeloma: results of the safety run-in cohort in the phase II, multicenter GMMG-CONCEPT trial. Clin Lymphoma Myeloma Leuk. 2019;19(10):e17. doi:10.1016/j.clml.2019.09.024
34. Costa LJ, Chhabra S, Godby KN, et al. Daratumumab, carfilzomib, lenalidomide and dexamethasone (dara-KRd) induction, autologous transplantation and post-transplant, response-adapted, measurable residual disease (MRD)-based dara-KRd consolidation in patients with newly diagnosed multiple myeloma (NDMM). Blood. 2019;134:860.
35. Facon T, Lee JH, Moreau P, et al. Carfilzomib or bortezomib with melphalan-prednisone for transplant-ineligible patients with newly diagnosed multiple myeloma. Blood. 2019;133(18):1953–1963. doi:10.1182/blood-2018-09-874396
36. Kumar S, Jacobus SJ, Cohen AD, et al. Carfilzomib, lenalidomide, and dexamethasone (KRd) versus bortezomib, lenalidomide, and dexamethasone (VRd) for initial therapy of newly diagnosed multiple myeloma (NDMM): results of ENDURANCE (E1A11) phase III trial. J Clin Oncol. 2020;38(18_suppl):LBA3–LBA. doi:10.1200/JCO.2020.38.18_suppl.LBA3
37. Forsberg PA, Rossi AC, Boyer A, et al. Phase II study of carfilzomib and dexamethasone therapy for newly diagnosed multiple myeloma. Am J Hematol. 2019;94(5):539–545. doi:10.1002/ajh.25435
38. Bringhen S, Mina R, Petrucci MT, et al. Once-weekly versus twice-weekly carfilzomib in patients with newly diagnosed multiple myeloma: a pooled analysis of two phase I/II studies. Haematologica. 2019;104(8):1640–1647. doi:10.3324/haematol.2018.208272
39. Team AT. Routine versus clinically driven laboratory monitoring and first-line antiretroviral therapy strategies in African children with HIV (ARROW): a 5-year open-label randomised factorial trial. Lancet. 2013;381(9875):1391–1403. doi:10.1016/S0140-6736(12)62198-9
40. Moreau P, Stewart KA, Dimopoulos M, et al. Once-weekly (70 mg/m2) vs twice-weekly (56 mg/m2) dosing of carfilzomib in patients with relapsed or refractory multiple myeloma: a post hoc analysis of the ENDEAVOR, A.R.R.O.W., and CHAMPION-1 trials. Cancer Med. 2020;9(9):2989–2996. doi:10.1002/cam4.2945
41. Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936–945. doi:10.1182/blood.2020005288
42. O’dwyer M, Henderson R, Naicker S, et al. CyBorD-DARA is potent initial induction for MM and enhances ADCP: initial results of the 16-BCNI-001/CTRIAL-IE 16-02 study. Blood Adv. 2019;3(12):1815–1825. doi:10.1182/bloodadvances.2019000010
43. Bringhen S, Mina R, Petrucci M, et al. Incidence and risk factors of cardiovascular adverse events in a large population of newly-diagnosed, transplant ineligible myeloma patients treated with carfilzomib. In: Haematologica. Via Giuseppe Belli 4, 27100 Pavia, Italy: Ferrata Storti Foundation; 2017;102:510–511.
44. Bringhen S, Milan A, Ferri C, et al. Cardiovascular adverse events in modern myeloma therapy–incidence and risks. A review from the European Myeloma Network (EMN) and Italian Society of Arterial Hypertension (SIIA). Haematologica. 2018;103(9):1422–1432. doi:10.3324/haematol.2018.191288
45. Ball S, Behera TR, Anwer F, Chakraborty R. Risk of kidney toxicity with carfilzomib in multiple myeloma: a meta-analysis of randomized controlled trials. Ann Hematol. 2020;99(6):1265–1271. doi:10.1007/s00277-020-04062-x
46. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
