Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Efficacy and Safety of Tyrosine Kinase Inhibitors Alone or Combination with Programmed Death-1 Inhibitors in Treating of Hepatitis C-Related Hepatocellular Carcinoma
Authors Lei J, Yang S, Chen B, Zhang L, Yan T, Yang G, Chen Y, Li Y, Lu Y, Zuo S
Received 12 October 2022
Accepted for publication 16 February 2023
Published 2 March 2023 Volume 2023:10 Pages 357—367
DOI https://doi.org/10.2147/JHC.S392347
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr David Gerber
Jin Lei,1,* Sibo Yang,1,* Bowen Chen,2,* Linzhi Zhang,3 Tao Yan,3 Gangqi Yang,1 Yue Chen,1 Yinyin Li,3 Yinying Lu,1,3,4 Shi Zuo1,5
1School of Clinical Medicine, Guizhou Medical University, Guiyang, People’s Republic of China; 2 302 Clinical Medical School, Peking University, Beijing, People’s Republic of China; 3Comprehensive Liver Cancer Center, the 5th Medical Center of the PLA General Hospital, Beijing, People’s Republic of China; 4Center for Synthetic and Systems Biology, School of Life Sciences, Tsinghua, Beijing, People’s Republic of China; 5Department of Hepatobiliary Surgery, The Affiliated Hospital of Guizhou Medical University, Guiyang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yinying Lu; Shi Zuo, Tel +86 13301256799 ; +86 13908516978, Email [email protected]; [email protected]
Background: Tyrosine kinase inhibitors (TKI) combined with programmed cell death-1 (PD-1) inhibitor is a potential treatment modality for patients with HCV-related unresectable hepatocellular carcinoma (uHCC).
Methods: The participants of the present work included the patients having HCV-related uHCC who were treated with TKI monotherapy (TKI group) or TKI combined with PD-1 inhibitors therapy (combination group) in our center between June 2018 and June 2021. In addition, the patients were classified into RNA-positive and RNA-negative groups based on whether or not the baseline HCV RNA was detectable. The overall survival (OS) was used as the primary efficacy endpoint, while progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR) were used as secondary endpoints. The adverse events were recorded and evaluated.
Results: Among the 67 patients contained this work, 43 patients were classified into the TKI group, while 24 patients formed the combination group. In relative to the TKI group, the combination group presented notably better median OS (21 months vs 13 months, p = 0.043) and median PFS (8 months vs 5 months, p = 0.005). No evident differences were observed between the two groups in terms of the DCR (58.1% vs 79.2%, p = 0.080), ORR (13.9% vs 25.0%, p = 0.425) and the incidence of grade 3– 4 adverse events (34.8% vs 33.3%, p = 1.000). In addition, there existed no obvious difference between the RNA-positive group and RNA-negative group in terms of median OS (14 months vs 19 months, p = 0.578) and median PFS (4 months vs 6 months, p = 0.238).
Conclusion: The patients having HCV-related uHCC after being treated with the TKI and PD-1 inhibitor combination therapy exhibited a better prognosis and manageable toxicity compared to the patients who underwent TKI monotherapy.
Keywords: hepatitis C virus, hepatitis C virus reactivation, hepatocellular carcinoma, tyrosine kinase inhibitor, programmed death-1 inhibitor
Introduction
Primary liver cancer can be regarded as the sixth most normal cancer globally according to the latest statistics, with 900,000 new cases reported annually.1 In most countries, hepatocellular carcinoma (HCC) is the leading histological type of liver cancer, accounting for nearly 75% of the total cases reported in the world.2 Most cases of HCC have reached the middle or late stage at the time of diagnosis, and systematic treatment has become the cornerstone for such patients. Until 2007, sorafenib was the sole first-line agent permitted by the Food and Drug Administration (FDA) for treating patients having unresectable HCC.3 And then, lenvatinib with non-inferior to sorafenib has been approved as a first-line treatment in 2018.4 In 2020, atezolizumab and bevacizumab were approved by FDA as the standard first-line treatment for patients with unresectable HCC, which brought fresh hope to patients while also opening a new era of combination treatment modalities.5 Although programmed cell death-1 (PD-1) inhibitors have shown promising efficacy in the treatment of patients with different tumors, there is little benefit of PD-1 inhibitors alone in HCC patients.6 However, the combination therapy of PD-1 inhibitors and tyrosine kinase inhibitors (TKI) has achieved promising OS and is, therefore, expected to be recognized of the first-line treatment options for HCC patients. The results of this Ib study showed that patients with HCC treated with lenvatinib in combination with pembrolizumab had a median OS of up to 22 months.7 Moreover, in the real world, the combination of TKI and PD-1 inhibitors could greatly improve the survival of patients compared to TKI used alone.8
Chronic hepatitis C virus(HCV) infection accounts for 25–30% of global HCC cases, and is the most important risk factor for HCC in developed countries.9 Currently, the use of direct-acting antivirals (DAAs) ensures a cure rate of over 95% in HCV patients.10 However, HCV reactivation is reported in nearly 23% of the patients with HCV-related tumors who have received anticancer therapy with immunosuppressive agents. As a consequence, 26% of the patients with HCV reactivation begin using a reduced dosage of antitumor drugs or entirely discontinue the treatment, which ultimately affects drug antitumor efficacy.11 Previous studies have demonstrated that patients treated with PD-1 inhibitors or TKI are at risk of HBV reactivation.12,13 However, whether these agents act as a risk factor for HCV reactivation remains unclear so far. HCC patients with detectable baseline HCV RNA may exhibit poorer prognosis as HCV eradication reduces the risk of HCC development and progression.14 Although studies have confirmed the efficacy and safety of using TKI alone or combined with PD-1 inhibitors among HCC patients, certain aspects remain to be investigated, such as whether the efficacy of these regimens is equivalent in HCV-related HCC patients, if a risk of HCV reactivation exists, and could HCV RNA levels affect the anti-tumor efficacy of these regimens.
Materials and Methods
Study Design and Participants
The work was designed as a retrospective one, which was conducted at the fifth medical center of the General Hospital of the Chinese people’s Liberation Army, China. The HCC patients receiving treatment with TKI alone or TKI combined with PD-1 inhibitors as the first-line treatment between June 2018 and June 2021 were contained in the retrospective analysis. The other eligibility criteria included: (1) patients diagnosed with unresectable HCC either pathologically or in two imaging techniques in line with the instruction of the American Association for the Study of Liver Diseases (AASLD)15; (2) patients suffering from Child-Pugh class A or B liver function; (3) patients suffering from a performance score of 0 or 1 on the Eastern Cooperative Oncology Group (ECOG) scale; (4) patients undergoing detectable HCV RNA or those who were HCV antibody-positive; (5) a minimum of one measurable tumor lesion according to the definition of the Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1). In addition, the exclusion criteria included: (1) presence of other primary malignant tumors; (2) the presence of other positive viral markers, including the hepatitis B surface antigen; (3) patients with incomplete follow-up records. The approval of this study was obtained by a clinical trial ethics committee registered in China. The implementation scheme was following the Declaration of Helsinki, 1975.
Treatment and Follow-Up
The patients were recommended sorafenib or lenvatinib in their respective standard doses (oral: sorafenib, 400 mg, twice daily; lenvatinib, 12 mg for patients ≥60 kg and 8 mg for patients <60 kg, once daily). Apart from that, sintilimab or camrelizumab were administered intravenously at the dose of 200 mg every 3 weeks. Toripalimab was administered intravenously at the dose of 3 mg/kg every 2 weeks. According to the treatment method used, all included patients were categorized into the following two groups: the TKI monotherapy group (TKI group) and the TKI plus PD-1 inhibitors treatment group (combination group). The demographic characteristics (age, gender, etc.), the blood indicators (liver function, coagulation function, routine blood, tumor markers, etc.), and other details were collected and evaluated at baseline. At the follow-ups, the following quantitative parameters were collected: HCV RNA, imaging changes, adverse events, and survival of the patient.
Related Definitions and Study Endpoints
OS was used as the primary endpoint in this study in reference to the time interval from the commencement of the treatment until death due to any reason or the study endpoint, whichever happened first. The secondary endpoints used were progression-free survival (PFS) [time interval: from the initial dose to the first radiologically confirmed progressive disease (PD) or death due to any reason], disease control rate (DCR), objective response rate (ORR) as well as HCV reactivation. The radiological responses were measured using dynamic computed tomography (CT) or magnetic resonance imaging (MRI) at baseline and every 8–12 weeks after the commencement of the treatment. In addition, tumor response was assessed based on the Response Evaluation Criteria in Solid Tumors (RECIST). Adverse events were determined based on the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. HCV reactivation was determined as the an addition of HCV RNA ≥1 log 10 IU/mL above the baseline.11
Statistical Analysis
Categorical data were denoted as the frequency with proportion and investigated using the chi-squared test or Fisher’s exact test. In addition, the Kaplan-Meier method was adopted for measuring PFS and OS and plotting the curve. The Log rank test was performed for group comparisons. ORR and DCR were compared with the use of Pearson’s chi-squared analysis or Fisher’s exact test. A 2-tailed p-value of ≤0.05 was regarded to be the threshold of statistical significance. All statistical analyses were conducted by adopting SPSS version 26.0 (IBM Corp.).
Efficiency and Safety
Patient Characteristics
In the period between June 2018 and June 2021, 67 patients that fulfilled the eligibility criteria were contained in this work. Among these patients, 43 patients were categorized into the TKI group, while the remaining 24 patients formed the combination group. According to the data obtained until the study duration threshold (July 2022), the median duration of follow-up was 24 months (95% CI: 19.2–28.8). In addition, the majority of the patients were male (n = 56, 83.6%). In 39 (58.2%) patients, the BCLC stage at the time of enrollment was stage C. Lung (n = 11, 57.9%) was the most common metastatic organ in 19 patients with extrahepatic metastasis. HCV RNA was detectable in 13 (19.4%) patients, including 9 patients from the TKI group and 4 patients from the combination group. In addition, the agents used in the TKI group were sorafenib (n = 24, 55.8%) and lenvatinib (n = 19, 44.2%). In the combination group, lenvatinib plus sintilimab was used in 16 cases (66.7%), lenvatinib plus toripalimab was used in 3 cases, lenvatinib plus camrelizumab was used in 3 other cases, and sorafenib plus toripalimab or camrelizumab was used in 1 case each. There existed no differences between the sorafenib TKI group and the combination group in any of the baseline parameters (Table 1).
 |
Table 1 Baseline Patient Characteristics |
Efficacy
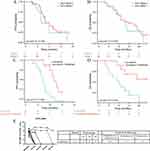
All patients had a minimum of one follow-up image for radiological tumor response assessment (Table 2). The combination group exhibited an evidently higher efficacy compared to the TKI group (Figure 1A and B) in terms of OS [median (95% CI): 21 (18.3–23.7) months vs 13 (9.6–16.4) months, p = 0.043] and PFS [median (95% CI): 8 (6.1–9.9) months vs 5 (4.3–5.7) months, p = 0.005]. The ORR rates in the combination group and TKI group were 13.9% and 25.0% (p = 0.425), separately. The DCR of the combination group was better than that of the TKI group, with only a marginal difference (79.2% vs 58.1%, p = 0.080). In the subgroup analysis of lenvatinib (n = 19) vs lenvatinib combined with sintilimab (n = 16), the median OS in the lenvatinib group was 13 months, while the 13-month survival rate in the combination group was 75% (p = 0.049). Moreover, the median PFS was 5 (95% CI: 3.9–6.2) months in the lenvatinib group and 8 (95% CI: 6.5–9.6) months in the combination group (Figure 1C and D), and the difference was of significance (p = 0.029).
 |
Table 2 Tumor Response |
HCV RNA and Prognosis
The enrolled patients were categorized into the RNA-negative group (n = 54) and RNA-positive group (n = 13) according to whether their baseline HCV RNA levels could be detected. The genotype of the 13 HCV RNA-positive patients was mainly 1b (n = 7, 53.9%). All 13 patients received anti-HCV agents, and after approximately 3 months of treatment, the HCV RNA was observed to be undetectable in 69.2% of these patients. No evident differences were observed in the median OS (14 months vs 19 months, p = 0.578), median PFS (4 months vs 6 months, p = 0.238), ORR (23.1% vs 14.8%, p = 0.760), and DCR (53.8% vs 68.5%, p = 0.500) between the RNA-positive group and the RNA-negative group (Figure 2A and B, Table 2). Additionally, we screened HCC patients with undetectable baseline HCV RNA who were treated with either lenvatinib (lenvatinib group, n=16) or lenvatinib plus sintilimab (combination group, n=12) to compare PFS and OS between the two groups. The results showed that the combination group had better median OS (not reached vs 11 months, P=0.030) and median PFS (8 months vs 5 months, P=0.040) than the lenvatinib group (Figure 2C and D).
Safety
In the TKI group, 95.3% of the patients experienced grade ≥ 1 adverse event. In the combination group, all patients experienced adverse events (Table 3). The most common adverse event in both TKI and combination groups was hand and foot syndrome (32.6% vs 33.3%). The most common grade 3–4 adverse events found in the TKI group included decreased platelet count (9.3% of the TKI group patients), hand and foot syndrome (7.0%), and decreased white blood cell count (7.0%). In the combination group, the most common grade 3–4 adverse events included decreased lymphocyte count (12.5% of the combination group patients), hand and foot syndrome (8.3%), lowered white blood cell count (8.3%), and decreased neutrophil count (8.3%). Besides, no notable differences were found in the incidence of grade 3–4 adverse events between the TKI group and combination group (34.8% vs 33.3%, p = 0.900). HCV reactivation occurred in 3 cases (4.5%), including 2 cases (4.7%) from the TKI group and 1 case (4.2%) from the combination group (Table 4). Among the 3 patients with HCV reactivation, 2 patients had undetectable baseline HCV RNA levels, and one patient had elevated alanine aminotransferase (ALT) (142 U/L) during HCV reactivation. In the TKI group, the agent dosage was lowered in one case, while the treatment regimen was changed in one other case due to grade 3 hand and foot syndrome. In the combination group, immunotherapy of one patient had to be interrupted due to immune-related pneumonia. No patients died due to adverse events.
 |
Table 3 Adverse Event |
 |
Table 4 Clinical Characteristics of Patients with HCV Reactivation |
Discussion
HCV infection is the main reason for liver cirrhosis and HCC. Approximately 71 million cases of HCV infection are reported worldwide.16 Currently, almost all HCV patients are cured through treatment with DAAs.10 Therefore, in clinical studies, patients with HCV-related HCC and those with non-virus-related HCC are regarded as the same group, and the treatment efficacy of the two groups is not evaluated separately. However, even patients with HCV eradication are at risk of developing HCC.17 HCV reactivation is frequently reported during the anti-tumor process in patients with HCV-related HCC.18 HCV with active replication may lead to hepatitis and consequently impact the anti-tumor plan. In this context, this work first evaluated the efficacy and safety of using TKI monotherapy or TKI plus PD-1 inhibitors combination therapy in patients with HCV-related HCC. In accordance with the results, the combination therapy comprising TKI plus PD-1 inhibitors resulted in a notably better median PFS and OS compared to TKI monotherapy, and the grade 3–4 adverse events did not differ significantly between the two groups.
The PD-1/PDL-1 axis is the main pathway for maintaining immune homeostasis.19 However, this balance may be disrupted due to the overexpression of PDL-1 in tumor cells, thereby promoting the exhaustion of T cells, which ultimately leads to tumor cell immune escape.20 PD-1 inhibitors achieve the objective of anti-tumor action through the inhibition of an excessive combination of PD 1 and PDL-1. Sorafenib and lenvatinib are multi-kinase inhibitors that exhibit an anti-vascular endothelial growth factor receptor effect, which leads to anti-tumor action based on the inhibition of tumor angiogenesis.4 However, the efficacy of PD-1 inhibitors monotherapy and TKI monotherapy is not satisfactory. The results of CheckMate 459 demonstrated the insignificance of the median OS benefit of nivolumab vs sorafenib (16.4 months vs 14.7 months, p=0.075).6 In recent times, combination therapy is becoming the preferred choice over monotherapy. For instance, lenvatinib monotherapy resulted in the median OS of 13.6 months in REFLECT, while the combination therapy of lenvatinib plus pembrolizumab led to the median OS of 22 months in KEYNOTE-524.4,7 Zhao et al analyzed and compared the real-world research results for lenvatinib monotherapy and the combination therapy of lenvatinib plus sintilimab.8 The authors reported that the combination group presented significantly better results compared to the lenvatinib group concerning the median OS (21.7 months vs 12.8 months, p = 0.005) and median PFS (11.3 months vs 6.6 months, p = 0.01). This finding was in consistence with the conclusion reached in the present study. In another study comparing the sintilimab monotherapy with the combination therapy of sorafenib plus sintilimab, the median OS and median PFS in the combination group were notably higher than those in the sintilimab group.21 Another retrospective study confirmed that the efficacy of the combination therapy of PD-1 inhibitors plus TKI was better than that achieved using PD-1 inhibitors alone.22 While the findings of the above three retrospective studies demonstrated that combination therapy could achieve good efficacy, there were only 9 cases of HCV-related HCC among the 359 patients included in these studies, which could be viewed as a limitation. In this context, the findings of this work contribute data for the combination treatment of patients undergoing HCV-related HCC in support of the previous conclusions reported in the literature for these patients. Therefore, it is suggested that patients with HCV-related HCC receiving combination therapy of TKI plus PD-1 inhibitors would achieve a better prognosis compared to using TKI alone.
The HCV patients with a sustained virological response (SVR) may exhibit promotion in the occurrence and development of HCC,17,23 which may be related to HCV causing persistent liver inflammation, oxidative stress as well as deregulation of cell signaling pathways.24 The patients with active HCV RNA replication may exhibit inflammatory response induction and promotion of the release of reactive oxygen species under oxidative stress, which increases the probability of further deterioration of the original condition compared to the patients with SVR.25 On the contrary, no evident difference is reported to exist in the median PFS and OS between the baseline HCV RNA-positive and HCV RNA-negative patients. The reasons underlying this issue were analyzed in the present study, and the following aspects were revealed: (1) 92.3% of the baseline HCV RNA-positive patients achieved undetectable HCV RNA levels within half a year after receiving the anti-HCV treatment; (2) the sample size was too small; (3) among the six genotypes of HCV, genotype 3b is the most carcinogenic,26 and the patients with this genotype accounted for only 15.4% in the present study. Moreover, the results of CheckMate 040 revealed that the HCC patients with and without HCV infection who received monotherapy (nivolumab) exhibited almost the same median PFS (5.4 months vs 4 months) and 9-month survival rate (82% vs 81%).27 In addition, the efficacy of TKI monotherapy (lenvatinib or sorafenib) was not affected in patients with HCV infection.4 Therefore, it was speculated that the efficacy would not be affected when using a combination of two agents. Although HCV is associated with a risk of carcinogenesis, this effect may not be sufficient to affect the efficacy of a systematic treatment, which could explain the finding of no statistical differences in the prognostic indicators between the HCV RNA-positive and HCV RNA-negative patients. However, since the sample size of the present study was small, this conclusion has to be validated in prospective studies with larger clinical sample sizes in future investigations.
In the present study, the incidence of adverse events of all grades in the combination group was 100%, while the value was 95.3% in the TKI group, and the difference between the two groups was not of statistical significance. Hand and foot syndrome was the most common adverse event, while grade 3–4 adverse events occurred in 34.3% of patients. In addition, HCV reactivation occurred in 3 cases (4.5%). Improvement in all adverse events was achieved after symptomatic treatment, and no patient died due to adverse events. Furthermore, an acceptable toxicity event was observed in the patients with HCV-related HCC who received the combination therapy of PD-1 inhibitors plus TKI, which was consistent with the findings of other investigations.8
HCV reactivation may lead to delays in the treatment or the discontinuation of systemic therapy, ultimately causing liver failure. The use of immunosuppressive drugs, particularly rituximab or high-dose steroids, during the antitumor process may cause the incidence of HCV reactivation to reach a value as high as 23%.11 Based on the recommendation of the European Association for the Study of the Liver (ESAL), patients with malignancies should be screened for HCV infection prior to cancer treatment to prevent the possible HCV reactivation upon treatment.28 In recent years, various novel anticancer therapies have been effectively applied to patients with HCV-related tumors. However, whether HCV reactivation is related to these therapies is currently a research hotspot. No HCV reactivation events have been reported during immunotherapy in patients with HCC (21 cases) and other solid tumors (14 cases).29,30 Although the above findings indicate that HCV reactivation may not be related to immunotherapy, the number of cases evaluated to reach this conclusion in the respective studies was quite small and, therefore, the level of evidence is inadequate.31 Therefore, determining whether the use of immunotherapy is a factor causing this HCV reactivation in HCC patients requires further investigation using larger sample sets. In the present study, 3 cases of HCV reactivation occurred, one of which had received lenvatinib plus sintilimab while the remaining two had received sorafenib. One patient developed HCV-related hepatitis flare [the levels of ALT reaching a value 3 times greater than the upper limit of the normal range].11 In addition, one patient’s Child-Pugh score increased from A6 to B8. Fortunately, the 3 patients with HCV reactivation did not change their antitumor regimen, and 2 of these patients achieved undetectable HCV RNA levels within 4 months after the administration of the antiviral therapy. The patients who received immunotherapy exhibited a lower rate of HCV reactivation compared to those who received immunosuppressive therapy. However, the HCC patients may experience HCV reactivation during systemic therapy as well, which renders it necessary to closely monitor the HCV RNA levels in these patients.
As with all research, this work also had certain limitations. The first one was that the present study was designed as a retrospective one with a small sample size. This could have caused a certain degree of information bias and selection bias. Another limitation was that the treatment modalities that the included patients had received were either TKI alone or TKI in combination with PD-1 inhibitors, and there were no two specific treatment regimens available for comparison. However, to increase the confidence level, a subgroup analysis was performed additionally to compare the efficacies of the lenvatinib plus sintilimab treatment versus the use of lenvatinib alone.
Conclusion
The present study utilized real-world data to assess the efficacy and safety of TKI monotherapy or TKI plus PD-1 inhibitors therapy for treating patients suffering from HCV-related HCC. In agreement with the findings reported by other studies on etiology-related HCC, the TKI plus PD-1 inhibitors therapy was observed to have greater effectiveness compared to the TKI monotherapy in patients with HCV-related HCC. A risk of HCV reactivation existed in the HCV-related HCC patients who received systemic treatment. Therefore, close monitoring of HCV RNA levels is recommended as necessary.
Ethics Statement
This study conformed to the Declaration of Helsinki and was approved by Chinese Ethics Committee of Registering Chinese Trials (approval number: ChiECRCT20210406). Since the study was a retrospective study, the Ethics Committee withdrew the requirement for informed consent of patients. All data in the article were obtained from the eHealth system, and we are committed to using these data only to support the conclusions of this article.
Acknowledgments
We thank the patients and their families.
Funding
This work was supported by the Issued by the Science, Technology and Innovation Commission of Shenzhen Municipality (KCXFZ202002011006448).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Altekruse SF, Devesa SS, Dickie LA, McGlynn KA, Kleiner DE. Histological classification of liver and intrahepatic bile duct cancers in SEER registries. J Registry Manag. 2011;38(4):201–205.
3. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
4. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
5. Cheng A-L, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi:10.1016/j.jhep.2021.11.030
6. Yau T, Park J-W, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77–90. doi:10.1016/S1470-2045(21)00604-5
7. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi:10.1200/JCO.20.00808
8. Zhao L, Chang N, Shi L, et al. Lenvatinib plus sintilimab versus lenvatinib monotherapy as first-line treatment for advanced HBV-related hepatocellular carcinoma: a retrospective, real-world study. Heliyon. 2022;8(6):e09538. doi:10.1016/j.heliyon.2022.e09538
9. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273.e1. doi:10.1053/j.gastro.2011.12.061
10. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. 2017;166(9):637–648. doi:10.7326/M16-2575
11. Torres HA, Hosry J, Mahale P, Economides MP, Jiang Y, Lok AS. Hepatitis C virus reactivation in patients receiving cancer treatment: a prospective observational study. Hepatology. 2018;67(1):36–47. doi:10.1002/hep.29344
12. He M-K, Peng C, Zhao Y, et al. Comparison of HBV reactivation between patients with high HBV-DNA and low HBV-DNA loads undergoing PD-1 inhibitor and concurrent antiviral prophylaxis. Cancer Immunol Immunother. 2021;70(11):3207–3216. doi:10.1007/s00262-021-02911-w
13. Yao Z-H, Liao W-Y, Ho -C-C, et al. Incidence of hepatitis B reactivation during epidermal growth factor receptor tyrosine kinase inhibitor treatment in non-small-cell lung cancer patients. Eur J Cancer. 2019;117:107–115. doi:10.1016/j.ejca.2019.05.032
14. van der Meer AJ, Feld JJ, Hofer H, et al. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J Hepatol. 2017;66(3):485–493. doi:10.1016/j.jhep.2016.10.017
15. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
16. Blach S, Zeuzem S, Manns M. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–176. doi:10.1016/S2468-1253(16)30181-9
17. Lu M-Y, Yeh M-L, Huang C-I, et al. Dynamics of cytokines predicts risk of hepatocellular carcinoma among chronic hepatitis C patients after viral eradication. World J Gastroenterol. 2022;28(1):140–153. doi:10.3748/wjg.v28.i1.140
18. Mustafayev K, Torres H. Hepatitis B virus and hepatitis C virus reactivation in cancer patients receiving novel anticancer therapies. Clin Microbiol Infect. 2022;28:1321–1327. doi:10.1016/j.cmi.2022.02.042
19. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi:10.1146/annurev.immunol.26.021607.090331
20. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi:10.1038/nrc3239
21. Dai L, Cai X, Mugaanyi J, et al. Therapeutic effectiveness and safety of sintilimab-dominated triple therapy in unresectable hepatocellular carcinoma. Sci Rep. 2021;11(1):19711. doi:10.1038/s41598-021-98937-2
22. Chen S-C, Huang Y-H, Chen M-H, et al. Anti-PD-1 combined sorafenib versus anti-PD-1 alone in the treatment of advanced hepatocellular cell carcinoma: a propensity score-matching study. BMC Cancer. 2022;22(1):55. doi:10.1186/s12885-022-09173-4
23. Waziry R, Hajarizadeh B, Grebely J, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67(6):1204–1212. doi:10.1016/j.jhep.2017.07.025
24. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
25. Hino K, Hara Y, Nishina S. Mitochondrial reactive oxygen species as a mystery voice in hepatitis C. Hepatol Res. 2014;44(2):123–132. doi:10.1111/hepr.12247
26. Kanwal F, Kramer JR, Ilyas J, Duan Z, El-Serag HB. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology. 2014;60(1):98–105. doi:10.1002/hep.27095
27. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi:10.1016/S0140-6736(17)31046-2
28. Lampertico P, Agarwal K. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69(2):461–511. doi:10.1016/j.jhep.2018.03.026
29. Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81–88. doi:10.1016/j.jhep.2013.02.022
30. Tio M, Rai R, Ezeoke OM, et al. Anti-PD-1/PD-L1 immunotherapy in patients with solid organ transplant, HIV or hepatitis B/C infection. Eur J Cancer. 2018;104:137–144. doi:10.1016/j.ejca.2018.09.017
31. Wuyts L, Janssens A, Vonghia L, et al. Nivolumab and anti-HCV activity, a case report. Acta Clin Belg. 2021;76(5):392–396. doi:10.1080/17843286.2020.1741897
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.