Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Efficacy and Safety of Transarterial Chemoembolization with a Three-Stage Mixed Chemoembolic Regimen for Large Unresectable Hepatocellular Carcinoma
Authors Yang Y, Du N, Ma J, Peng Z, Zhou B, Yu J, Zhou X, Zhang W, Yan Z
Received 28 August 2023
Accepted for publication 30 September 2023
Published 25 October 2023 Volume 2023:10 Pages 1897—1910
DOI https://doi.org/10.2147/JHC.S433409
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Imam Waked
Supplementary video of "Transarterial Chemoembolization for HCC" [ID 433409].
Views: 145
Yanjie Yang,1,2,* Nan Du,1,2,* Jingqin Ma,1,2,* Zhijie Peng,1,2 Bo Zhou,1,2 Jiaze Yu,1,2 Xin Zhou,1,2 Wen Zhang,1,2 Zhiping Yan1,2
1Department of Interventional Radiology, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 2Shanghai Institute of Medical Imaging, Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhiping Yan; Wen Zhang, Email [email protected]; [email protected]
Objective: This study aimed to assess the treatment response, survival outcomes, and safety of a novel transarterial chemoembolization (TACE) technique with a three-stage mixed chemoembolic regimen (M-TACE) in patients with large unresectable hepatocellular carcinoma (HCC) measuring more than 5 cm in maximum diameter.
Methods: Between January 2017 and March 2023, a total of 82 patients were enrolled in this retrospective cohort study. Treatment response was assessed in the first month after M-TACE; progression-free survival and overall survival (OS) were evaluated. The prognostic factors associated with patient survival were statistically analyzed by the Cox regression model. Adverse events were recorded.
Results: The maximum diameter of the tumors ranged from 5.3 cm to 20.0 cm (mean 10.71 cm). The objective response (OR) and disease control rates were 74.4 and 92.7%, respectively, at 1-month follow-up. The median survival time was 22 months (95% CI, 13.10– 30.90 months). The OS rates were 82.0% at six months, 62.5% at one year, and 43.0% at two years. Targeted therapy and/or immunotherapy (P=0.001) and tumor response at one month (P=0.020) were protective factors for OS. In terms of safety, no major complications occurred and the only observed decrease within the normal range occurred in albumin and platelet levels one month after the embolization procedure. This decrease in levels did not show a significant relationship with the OR rates.
Conclusion: M-TACE demonstrated a promising objective tumor response, making it a viable and effective treatment option for patients with large unresectable HCC.
Plain Language Summary: 1. A groundbreaking TACE therapy for large unresectable hepatocellular carcinoma demonstrates enhanced safety and efficacy compared to conventional TACE methods.
2. The M-TACE technique effectively minimizes the required lipiodol dosage for the treatment of large unresectable hepatocellular carcinoma, optimizing patient care and reducing potential complications.
3. The M-TACE technique employs a three-stage approach, strategically implementing segmental embolization based on the diameter of the vessels, thereby enhancing the precision and efficacy of the treatment.
Keywords: hepatocellular carcinoma, transarterial chemoembolization, treatment response, prognosis, adverse events
Introduction
Hepatocellular carcinoma (HCC) is the foremost cause of death among individuals with cirrhosis, and it ranks as the fourth most common cause of cancer-associated mortality worldwide.1 The Asia-Pacific region accounts for nearly 75% of all cases due to the high incidence of chronic hepatitis B and C virus infections in this region.2,3 The prognosis of patients with HCC displays significant heterogeneity and relies on several factors including tumor burden, baseline liver function, cancer-related symptoms, and allocation of treatment. Surgery is recognized as the primary curative approach for HCC. However, it is unfortunate that only 20% of patients in China can benefit from surgery due to late-stage diagnosis and excessive tumor burden.4
The Barcelona Clinic Liver Cancer (BCLC) staging system advocates for transarterial chemoembolization (TACE) as the optimal therapeutic approach for treating intermediate-stage HCC and also recommends it as the first option for palliative treatment in late-stage HCC.5 Conventional TACE (cTACE) delivers lipiodol by transcatheter to temporarily block sinusoids, portal veins, and arterial micro-communications.6–8 The tumor’s complete lipiodol retention and peripheral portal branch deposition serve as an imaging marker of a safety margin and favorable local control.9 Nevertheless, the dependence of cTACE on lipiodol emulsion makes it susceptible to “washout” by blood flow over time, which may reduce the efficacy of TACE.10,11 Furthermore, achieving effective control over these large tumors with high lipiodol doses while conserving liver function remains a challenge, especially for patients with pre-existing conditions like hepatitis and cirrhosis.12,13
To address the current limitations, we present a hypothesis for a three-stage mixed transarterial chemoembolization (M-TACE) regimen. Our approach aims to achieve comprehensive embolization of tumor-supplying arteries, arterial collaterals, and tumor stroma using minimal doses of lipiodol. The first stage is ultraterminal embolization: a little or appropriate dose of cytotoxic drug lipiodol emulsions to embolize tumor sinusoids, communicating microarterial branches, and even portal vein branches. The second stage is terminal arterial embolization: small microspheres (≤300um) are used to embolize tumor terminal arteries. The third stage is arterial branch embolization: middle or large embolic materials such as microspheres (≥300um) or gelatin sponges are used to embolize the major arterial branches feeding the tumor (Supplementary Video).
M-TACE utilizes different embolic materials based on the diameter of the vessels in each stage. In theory, reducing the dosage of lipiodol not only minimizes the impact of “washout” but also helps preserve liver function. Hence, the objective of this study was to evaluate the efficacy and adverse events of M-TACE in patients with large HCC.
Patients and Methods
Study Population
Patients with unresectable HCC, who were treated with M-TACE in Zhongshan Hospital Affiliated to Fudan University between January 2017 and March 2023, were retrospectively enrolled in this study. The diagnosis of HCC was established according to the quality control index for standardized diagnosis and treatment of primary liver cancer in China (2022).14 The selection criteria were the largest tumor diameter >5 cm. Other inclusion criteria were as follows: (i) Eastern Cooperative Oncology Group performance status score of 0–1; (ii) no previous treatment for HCC; (iii) Barcelona Clinic Liver Cancer staging (BCLC) stage B or C; (iv) well preserved hematologic; and (iv) liver function of Child-Pugh A to B. Patients with fluoroscopic arteriovenous fistula or received other treatments during M-TACE procedure (eg radiofrequency ablation therapy and brachytherapy) were excluded. The retrospective analysis involved 370 patients, and ultimately, 82 patients were included in the final analysis, as depicted in Figure 1. The study was approved by the Ethics Committee of Zhongshan Hospital Affiliated to Fudan University and all participants provided their written informed consent before treatment.
 |
Figure 1 Flow chart showing the enrolled patients. Abbreviation: HCC, hepatocellular carcinoma. |
Pretreatment Investigations
Laboratory testing included complete blood count; biochemistry; coagulation; alpha-fetoprotein (AFP) assessment; hepatitis virus B and C serology; protein induced by vitamin K absence or antagonist-II (PIVKA-II) assessment; and serum levels of total bilirubin (TBil), alanine transaminase (ALT), aspartate transaminase (AST), and albumin (ALB).15,16 Additionally, the size of HCC lesions, the presence of extrahepatic metastases, and portal tumor thrombosis were documented.
M-TACE Procedure
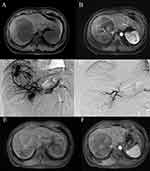
The radial artery or femoral artery puncture was performed under local anesthesia. After puncture success, a 4-Fr sheath introducer was exchanged, utilizing the Seldinger technique. Then, a 4-F MPA or RH catheter was inserted through the sheath to the aorta over a hydrophilic guide wire. The catheter was used to access the celiac trunk, hepatic artery, superior mesenteric arteries, or inferior phrenic artery as necessary. This allowed for the evaluation of vascular anatomy, tumor location, and size, as well as the assessment of portal flow patency. After finding the vessels responsible for supplying the tumor, a 2.2-F microcatheter was introduced over a hydrophilic guide wire. Before embolization, an enhanced cone beam CT (CBCT) scan was conducted using a microcatheter to accurately identify all the arteries supplying the tumor (Figure 2). In the first stage, cytotoxic drug lipiodol emulsions were used as the embolic material, and the selection of specific cytotoxic drugs, such as lobaplatin, cisplatin, and epirubicin, was based on individual patient characteristics. The dosage of the cytotoxic drug, ranging from 20 to 50 mg, was determined based on the patient’s pre-procedural liver function and the degree of cirrhosis. The lipiodol emulsion was slowly injected into the tumor under fluoroscopic monitoring, ensuring no retrograde flow occurred. The ratio of lipiodol dose to the maximum tumor diameter is typically 1:2 to 1:1 (eg, 5–10 mL for a tumor with a maximum diameter of 10 cm), and this depends on the density of the tumor vessels. The injection is stopped once the tumor outline is visualized under fluoroscopic monitoring. Moreover, it is crucial to utilize CBCT to definitively establish the sufficiency of the lipiodol dose. The details are as follows: Five to ten minutes after the injection of lipiodol emulsion, CBCT was performed to evaluate the extent of lipiodol emulsion diffusion. The CBCT images are compared with preoperative enhanced CT or enhanced MRI (Figure 3). If the distribution of lipiodol emulsion does not sufficiently cover the tumor margins, additional assessment is necessary to determine whether it is inadequate or if there are additional tumor feeders present. In the second stage, small microspheres (≤300um) are used to embolize tumor terminal arteries until slowing of the blood flow is observed under fluoroscopy. In most cases, once slowing of the blood flow is observed, only a small dose of embolic (1–2 mL of microspheres or gelatin sponge suspension) is needed to reach the embolization endpoint.17 After five to ten minutes, the embolism progresses to the third stage. Middle or large embolic materials such as microspheres (≥300um) or gelfoam particles (350–560um) were used until complete stasis was achieved in the tumor-feeding vessels. The choice of microsphere diameter was determined according to the size of the tumor-feeding artery. Each vial of microspheres was diluted with 10 mL of non-ionic contrast media and mixed with the remaining 1 mL of lipiodol emulsion from the first stage to ensure visibility under fluoroscopy. After whole embolization, angiography was performed to determine the extent of vascular occlusion.
After M-TACE, patients were transferred to the hospital ward for overnight observation and management of possible post-embolization syndrome or other complications.
Follow-Up After M-TACE
Enhanced CT and/or MRI were carried out 1 month after M-TACE. Two radiologists compared follow-up images with baseline images to assess the patient’s responses to M-TACE. The effectiveness of the treatment was assessed using the modified Response Evaluation Criteria in Solid Tumors (mRECIST), which was classified into complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD).18,19 Additionally, AFP and PIVKA-II one month after M-TACE were utilized to aid in the assessment of tumor control. The M-TACE procedure was repeated at least 45 days after the first session in patients with evidence of viable tumor persistence. M-TACE was continued until symptomatic progression was evident or liver failure developed despite tumor remission of the target lesions. The final date of follow-up was April 30, 2023, based on which progression-free survival (PFS) and overall survival (OS) were calculated for prognostic analysis.
Safety Assessment
Toxicity was assessed following the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) 3.0 criteria, focusing on conventional adverse reactions associated with TACE treatment, including liver injury and post-embolization syndrome.20 Liver injury was evaluated by assessing liver function parameters one month after M-TACE, including ALB, ALT, AST, and TBil. Furthermore, blood counts such as red blood cells (RBC), white blood cells (WBC), blood platelets (PLT), and hemoglobin (HB) were also examined one-month following M-TACE. Post-embolization syndrome, characterized by symptoms such as fever, abdominal pain, and nausea/vomiting, was documented, specifically capturing cases with a severity grade of ≥2.
Systemic Therapy
After the M-TACE procedure, we recommend considering the combination of systemic therapy including targeted therapy and/or immunotherapy for patients who have no contraindications and financial difficulties. The primarily targeted therapy option is Lenvatinib, which can be substituted with donafenib or sorafenib in case of drug resistance. Immunotherapy alternatives include atezolizumab, and pembrolizumab, among others.
Statistical Analysis
Statistical analysis was performed using SPSS 26.0 (IBM, Armonk NY, USA). Demographic and clinical characteristics were reported as means ± SDs for continuous variables or as counts with percentages for categorical variables. Linear regression analysis was used to assess the relationship between the dosage of lipiodol/microspheres and the maximum tumor diameter. The chi-square test was used to analyze the tumor response between subgroups. Due to the limited sample size, Fisher’s exact test was used. Survival rates were estimated using the Kaplan-Meier method. The Cox proportional hazards regression model was employed to identify factors associated with survival. The paired t-test was utilized to compare pre-and post-treatment blood count, liver function, and tumor markers. Two-sided P-values <0.05 were considered statistically significant.
Results
Baseline Clinical Characteristics
There were 82 patients (66 men and 16 women), with a mean age of 57.70±12.35 years and an age range of 24–82 years. A total of 63 patients (76.9%) were hepatitis B and/or C carriers. The number of patients in Child-Pugh class A was 70 and in class B was 12. The number of patents in BCLC B was 20 and in C was 62. About half of the patients (40/82, 48.8%) had HCC with the largest diameter >10 cm. Extrahepatic metastases are present in more than a third of the patients(30/82, 36.6%). Portal vein tumor thrombosis (PVTT) was observed in 38 patients (46.3%). The number of M-TACE sessions ranged from 1 to 9, with a mean of 3.05±1.78. M-TACE was combined with targeted therapy and/or immunotherapy in 60 patients (73.2%). The data are presented in Table 1.
 |
Table 1 Patient Demographic and Clinical Characteristics |
Embolization Material Dosage
The mean dose of lipiodol emulsion administered during M-TACE was 8.24 ± 3.35 mL, while the mean dose of microspheres used was 2.28 ± 1.67 mL. The relationship between lipiodol emulsion and microsphere and the maximum tumor diameter is shown in Figure 4.
 |
Figure 4 Linear regression relationship between the maximum tumor size and the dosage of lipiodol (A) and microspheres (B). |
Tumor Response After M-TACE
At 1 month after TACE, 19 patients (23.2%) showed CR, 42 (51.2%) showed PR, 15 (18.3%) showed SD, and 6 (7.3%) showed PD, as summarized in Table 2. Objective response (CR or PR) was achieved in 61 patients (74.4%). Additionally, the tumor markers AFP and PIVAK-II exhibited a significant reduction following the M-TACE procedure (Table 3). Figure 5 demonstrates the response of target lesions. There was a significant reduction in viable tumor size after M-TACE (10.71±3.51 vs 6.77±5.33, P<0.001). There were no significant differences between the responder (CR+PR) and non-responder (SD+PD) groups in terms of BCLC stage B or C, Child-Pugh class A or B, maximum tumor size, tumor number, extrahepatic metastases, targeted therapy and/or immunotherapy, AFP level, PIVKA-II level, and presence of PVTT (Table 4).
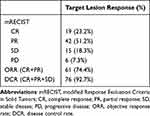 |
Table 2 Target Lesion Response of the 82 Patients 1 Month Following M-TACE |
 |
Table 3 Tumor Markers of the 82 Patients 1 Month Following M-TACE |
 |
Table 4 Subgroup Analysis of 1-Month Overall Response |
 |
Figure 5 Change in viable target lesion size after M-TACE. ***P<0.001. |
Overall Survival and Progression-Free Survival
During the follow-up period (1–53 months), the median OS was 22 months (95% CI, 13.10–30.90 months). The OS rates at 6 months, 1 year, and 2 years after chemoembolization were 82.0%, 62.5%, and 43.0%, respectively. Moreover, the PFS rates at 6 months, 1 year, and 2 years were 69.1%, 47.7%, and 37.9%, respectively (Figure 6).
 |
Figure 6 Kaplan-Meier curves of (A) progression-free survival (PFS) and (B) overall survival (OS) in patients with unresectable hepatocellular carcinoma (HCC) following M-TACE. |
Factors Associated with Patient Survival
Targeted therapy and/or immunotherapy (HR 0.217; 95% CI, 0.090–0.521), and tumor response (HR 0.268; 95% CI, 0.089–0.814) were potential protective factors for OS (Table 5). By contrast, maximum tumor size, extrahepatic metastases, and the presence of PVTT were not related to survival in these TACE patients.
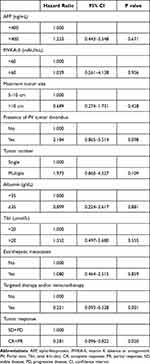 |
Table 5 Multivariate Cox Regression Analysis of Prognostic Factors for Overall Survival |
Complications
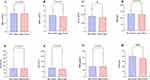
Post-embolization syndrome grade of ≥2, including fever, abdominal pain, and nausea/vomiting, developed in 46 of the 82 study patients (56.1%), as shown in Table 6. No major complications related to chemoembolization were observed, and there were no cases of in-hospital mortality. At the 1-month follow-up visit, there were no significant changes observed in most of the complete blood count and liver functions. There were significant changes only noted in the levels of ALB and PLT (Figure 7). However, upon further analysis, it was determined that the decrease in ALT and PLT levels did not significantly affect the OR rate after M-TACE, as illustrated in Figure 8.
 |
Table 6 Adverse Events and Complications |
Discussion
The injection of lipiodol through the hepatic artery enables its accumulation within the tumor, as well as its flow into the portal vein and microcollaterals. However, patients with a large HCC measuring ≥5 cm and post-hepatitis B cirrhosis pose a challenge when administering high doses of lipiodol, as it can have a significant impact on liver function.21–23 Therefore, it may be beneficial to combine different embolic materials to reduce the dosage of lipiodol while maintaining the treatment efficacy of TACE. To address this issue, we have divided the embolization process in TACE into three stages and proposed the concept of M-TACE. The M-TACE showed disease control rates of 92.7% after 1 month, and the 1-year survival rate was 62.5%. Given its demonstrated safety and feasibility, it can be considered a preferred option for the management of large HCC.
Combining microspheres with a reduced dosage of lipiodol can enhance the efficacy of tumor embolization. In a retrospective study conducted by Hao et al, involving 110 patients, the effectiveness of the combination of 300–500μm microspheres with lipiodol (Embo-TACE) was compared to lipiodol alone.4 The results showed that Embo-TACE was associated with a longer median survival period and higher survival rates at 1 year, 2 years, and 3 years.4 In our proposed M-TACE approach, we administered the optimal dosage of lipiodol emulsion along with microspheres, aiming to establish an ischemic environment while ensuring sustained and controlled release of the cytotoxic agents within the tumors. The combination can tailor the choice of embolic material based on the varying diameter of tumor vessels to complete occlusion of the blood-supplying arteries of the tumor for improving treatment outcomes.
In recent years, the discourse regarding the dosage of lipiodol employed in TACE procedures has gradually diminished. With advancements in catheter quality and the refinement of superselective techniques, it has become easier to navigate the catheter directly into tumor-feeding arteries. As a result, the lipiodol dose can be increased up to 20 mL to fill tumors measuring less than 10 cm in diameter, and for larger tumors exceeding 10 cm, a dosage of 30 mL or more can be administered.24 In a study conducted by Chen et al, a high dose of lipiodol (exceeding 20 mL) was utilized for TACE treatment of large HCC (>5 cm). The findings revealed that 53.7% of the patients experienced abdominal pain, 71.3% experienced fever, and 20.8% experienced vomiting.25 While prioritizing the effectiveness of TACE, we maintained a mean lipiodol dosage of 8.24±3.35 mL in our study, ensuring that the maximum lipiodol dosage did not exceed 20 mL. This approach significantly decreased the incidence of post-embolization syndrome and minimized the impact on blood cells and liver function. In our study, abdominal pain was reported by 32.9% of patients, fever by 28.0%, and nausea and/or vomiting by 7.3%.
The expert consensus on non-operative therapies for the combined modality treatment of HCC indicates that systemic therapy is associated with improved OS.26 Therefore, following M-TACE treatment, we routinely advise the combination of targeted therapy and/or immunotherapy for HCC patients. Our findings also highlight the significance of combining systemic therapy with TACE treatment. Assessing the local tumor response to treatment is crucial for localized therapies like TACE, especially one month after the treatment. Various criteria are available with mRECIST being one of the most commonly utilized.27,28 Our study also highlighted the significance of achieving OR one month after M-TACE as a valuable predictor of better OS.
According to the Chinese Expert Consensus on Multidisciplinary Diagnosis and Treatment of HCC with PVTT (2018 Edition), PVTT is observed in approximately 44% to 62% of HCC patients in China and significantly impacts prognosis and clinical staging.29 This Chinese guideline also suggests that the administration of TACE is considered safe and effective for patients with unresectable HCC, provided they have adequate liver function and appropriate collateral blood circulation around the occluded PVTT.29 In our study, the presence of PVTT in large HCC did not significantly affect OS. This could be attributed to the precise super-selection assisted by CBCT, which helped avoid embolization of normal liver tissue, leading to improved outcomes.
The heterogeneity in the efficacy of TACE for large HCC is evident, primarily influenced by the TACE superselection process and the dosage of lipiodol. In a cohort of 302 patients, Kim et al concluded that the efficacy of TACE in treating a single large HCC (>10 cm) remains uncertain.30 Due to compromised liver function, these patients typically exhibited a relatively high rate of major complications (21%).30 Similarly, according to the EASL guidelines, large HCC (>10 cm) is considered a relative contraindication for TACE.31 Nevertheless, we found that the large HCC (>10 cm) was not a significant prognostic factor for survival after TACE. These findings underscore the potential for improved outcomes by employing embolization strategies such as M-TACE.
The potential efficacy mechanism of the “stepwise” embolization strategy of tumors can be analyzed as follows. In the first stage, enhanced CBCT performed before embolization significantly aids in accurately identifying all tumor supply arteries, surpassing the benefits of DSA alone.32 Then, lipiodol emulsion is used to embolize the tumor’s microcirculation. The dosage of lipiodol emulsion depends on the tumor size and vessel density as determined by angiography. Waiting for 5 to 10 minutes is crucial as it allows the lipiodol emulsion to effectively penetrate the tumor stroma, portal vein, and arterial collaterals.33 After 5 to 10 minutes of lipiodol “redistribution”, conducting CBCT enables accurate measurement and assessment of lipiodol deposition in the targeted tumor site. This serves as a vital prognostic indicator, aiding in the evaluation of the risk of local recurrence.34,35 In the second stage, smaller microspheres are used to occlude the terminal arterial branches of the tumor and slow down lipiodol washout. A crucial point to emphasize is that the microspheres need adequate time, typically five to ten minutes, for proper “redistribution” within the blood flow.36 In general, smaller microspheres require a longer duration to travel distally within the vascular network.35 According to Dion et al, the redistribution of 40–100μm microspheres can be observed for up to 10 minutes following embolization.37 Consequently, failing to wait for a sufficient duration of time can result in incomplete and less efficacious embolization. Finally, as with cTACE, larger microspheres or gelatin sponges are used to occlude the major artery supplying the tumor.
The limitations of this study are a small sample size, nonrandomized controls, and a single-center design. Furthermore, the follow-up period in this study was relatively short, with the longest duration being less than 5 years.
Conclusion
In conclusion, for large HCC, TACE therapy should not be considered adjuvant therapy. Instead, it is crucial to enhance the precision and refinement of TACE treatment. Our findings suggest that M-TACE, which utilizes different embolic materials based on the diameter of the vessels in each stage, is an effective and safe therapy. M-TACE demonstrates excellent local control and provides a survival benefit for patients with large unresectable HCC.
Abbreviations
HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; BCLC, Barcelona Clinic Liver Cancer; AFP, alpha-fetoprotein; PIVKA-II, vitamin K absence or antagonist-II; TBil, total bilirubin; ALT, alanine transaminase; AST, aspartate transaminase; ALB, albumin; CBCT, cone beam CT; mRECIST, modified Response Evaluation Criteria in Solid Tumors; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; RBC, red blood cells; WBC, white blood cells; PLT, blood platelets; HB, hemoglobin; PVTT, portal vein tumor thrombosis.
Data Sharing Statement
The raw data can be obtained by contacting the corresponding author, Dr. Zhiping Yan, via email, subject to compliance with applicable laws and regulations.
Ethics Approval and Informed Consent
Institutional Review Board (IRB) of Zhongshan Hospital of Fudan University approved the study (IRB No. B2022-317R). All patients provided written informed consent before treatment, in compliance with the Declaration of Helsinki. The donation process conformed to the Declaration of Istanbul. All organs were donated voluntarily with written informed consent.
Funding
This work was funded by the Shanghai Key Clinical Specialty Construction Program – Extending Two Wings: Interventional Therapy (shslczdzk06003).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kim E, Viatour P. Hepatocellular carcinoma: old friends and new tricks. Exp Mol Med. 2020;52(12):1898–1907. doi:10.1038/s12276-020-00527-1
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Vogel A, Meyer T, Sapisochin G, et al. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–1362. doi:10.1016/S0140-6736(22)01200-4
4. Hao MZ, Lin HL, Chen QZ, et al. Safety and efficacy of transcatheter arterial chemoembolization with embospheres in treatment of hepatocellular carcinoma. J Dig Dis. 2017;18(1):31–39. doi:10.1111/1751-2980.12435
5. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
6. Kan Z, Ivancev K, Lunderquist A. Peribiliary plexa--important pathways for shunting of iodized oil and silicon rubber solution from the hepatic artery to the portal vein. An experimental study in rats. Invest Radiol. 1994;29(7):671–676. doi:10.1097/00004424-199407000-00002
7. Terayama N, Matsui O, Gabata T, et al. Accumulation of iodized oil within the nonneoplastic liver adjacent to hepatocellular carcinoma via the drainage routes of the tumor after transcatheter arterial embolization. Cardiovasc Intervent Radiol. 2001;24(6):383–387. doi:10.1007/s00270-001-0070-2
8. Miyayama S, Matsui O, Yamashiro M, et al. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol. 2007;18(3):365–376. doi:10.1016/j.jvir.2006.12.004
9. Matsui O. Current status of hepatocellular carcinoma treatment in Japan: transarterial chemoembolization. Clin Drug Investig. 2012;32(Suppl 2):3–13. doi:10.1007/BF03265492
10. Lencioni R, de Baere T, Soulen MC, et al. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–116. doi:10.1002/hep.28453
11. Caine M, Chung T, Kilpatrick H, et al. Evaluation of novel formulations for transarterial chemoembolization: combining elements of lipiodol emulsions with drug-eluting beads. Theranostics. 2019;9(19):5626–5641. doi:10.7150/thno.34778
12. Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int. 2019;13(2):125–137. doi:10.1007/s12072-018-9919-1
13. Sidali S, Trepo E, Sutter O, et al. New concepts in the treatment of hepatocellular carcinoma. United European Gastroenterol J. 2022;10(7):765–774. doi:10.1002/ueg2.12286
14. National Cancer C, Liver Cancer Expert Committee Of National Cancer Quality Control C. 中国肝癌规范诊疗质量控制指标(2022版)[Quality control index for standardized diagnosis and treatment of primary liver cancer in China 2022]. Zhonghua Zhong Liu Za Zhi. 2022;44(7):600–608. Chinese. doi:10.3760/cma.j.cn112152-20220418-00265
15. Kim DY, Toan BN, Tan CK, et al. Utility of combining PIVKA-II and AFP in the surveillance and monitoring of hepatocellular carcinoma in the Asia-Pacific region. Clin Mol Hepatol. 2023;29(2):277–292. doi:10.3350/cmh.2022.0212
16. Feng H, Li B, Li Z, et al. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma. BMC Cancer. 2021;21(1):401. doi:10.1186/s12885-021-08138-3
17. Soulen MC, Baere TD. Physics and physiology of transarterial chemoembolization and drug-eluting beads for liver tumors. Image-Guided Interventions in Oncology. 2020;3:29–42.
18. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
19. Kudo M, Ikeda M, Ueshima K, et al. Response Evaluation Criteria in Cancer of the Liver version 5(RECICL 2019 revised version). Hepatol Res. 2019;49(9):981–989. doi:10.1111/hepr.13394
20. Hamashige S, Arquilla ER. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Principles and Practice of Clinical Trial Medicine. 2008;42:461–533.
21. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016;150(4):835–853. doi:10.1053/j.gastro.2015.12.041
22. Kudo M, Han KH, Ye SL, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: asia-Pacific primary liver cancer expert consensus statements. Liver Cancer. 2020;9(3):245–260. doi:10.1159/000507370
23. Zhou F, Zhou J, Wang W, et al. Unexpected rapid increase in the burden of NAFLD in China From 2008 to 2018: a systematic review and meta-analysis. Hepatology. 2019;70(4):1119–1133. doi:10.1002/hep.30702
24. Cheng HY, Shou Y, Wang X, et al. Adjustment of lipiodol dose according to tumor blood supply during transcatheter arterial chemoembolization for large hepatocellular carcinoma by multidetector helical CT. World J Gastroenterol. 2004;10(18):2753–2755. doi:10.3748/wjg.v10.i18.2753
25. Chen MS, Li JQ, Zhang YQ, et al. High-dose iodized oil transcatheter arterial chemoembolization for patients with large hepatocellular carcinoma. World J Gastroenterol. 2002;8(1):74–78. doi:10.3748/wjg.v8.i1.74
26. Llovet JM, de Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(5):293–313. doi:10.1038/s41575-020-00395-0
27. Kim CJ, Kim HJ, Park JH, et al. Radiologic response to transcatheter hepatic arterial chemoembolization and clinical outcomes in patients with hepatocellular carcinoma. Liver Int. 2014;34(2):305–312. doi:10.1111/liv.12270
28. Peng CW, Teng W, Lui KW, et al. Complete response at first transarterial chemoembolization predicts favorable outcome in hepatocellular carcinoma. Am J Cancer Res. 2021;11(10):4956–4965.
29. Cheng S, Chen M, Cai J, et al. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus (2018). Liver Cancer. 2020;9(1):28–40. doi:10.1159/000503685
30. Kim GH, Kim JH, Shim JH, et al. Chemoembolization for single large hepatocellular carcinoma with preserved liver function: analysis of factors predicting clinical outcomes in a 302 patient cohort. Life. 2021;11(8):840. doi:10.3390/life11080840
31. Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol. 2015;62(5):1187–1195. doi:10.1016/j.jhep.2015.02.010
32. Kakeda S, Korogi Y, Ohnari N, et al. Usefulness of cone-beam volume CT with flat panel detectors in conjunction with catheter angiography for transcatheter arterial embolization. J Vasc Interv Radiol. 2007;18(12):1508–1516. doi:10.1016/j.jvir.2007.08.003
33. Najmi Varzaneh F, Pandey A, Aliyari Ghasabeh M, et al. Prediction of post-TACE necrosis of hepatocellular carcinoma usingvolumetric enhancement on MRI and volumetric oil deposition on CT, with pathological correlation. Eur Radiol. 2018;28(7):3032–3040. doi:10.1007/s00330-017-5198-9
34. de Baere T, Arai Y, Lencioni R, et al. Treatment of liver tumors with lipiodol TACE: technical recommendations from experts opinion. Cardiovasc Intervent Radiol. 2016;39(3):334–343. doi:10.1007/s00270-015-1208-y
35. Laurent A, Wassef M, Saint Maurice JP, et al. Arterial distribution of calibrated tris-acryl gelatin and polyvinyl alcohol microspheres in a sheep kidney model. Invest Radiol. 2006;41(1):8–14. doi:10.1097/01.rli.0000188027.34400.f3
36. Stampfl S, Bellemann N, Stampfl U, et al. Arterial distribution characteristics of Embozene particles and comparison with other spherical embolic agents in the porcine acute embolization model. J Vasc Interv Radiol. 2009;20(12):1597–1607. doi:10.1016/j.jvir.2009.08.018
37. Dion JE, Rankin RN, Vinuela F, et al. Dextran microsphere embolization: experimental and clinical experience with radiologic-pathologic correlation. Work in progress. Radiology. 1986;160(3):717–721. doi:10.1148/radiology.160.3.2426727
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.