Back to Journals » OncoTargets and Therapy » Volume 17
Efficacy and Safety of the MDM2–p53 Antagonist Brigimadlin (BI 907828) in Patients with Advanced Biliary Tract Cancer: A Case Series
Authors Yamamoto N , Tolcher A , Hafez N, Lugowska I, Ramlau R, Macarulla T, Geng J, Li J, Teufel M, Märten A, LoRusso P
Received 13 October 2023
Accepted for publication 15 March 2024
Published 29 March 2024 Volume 2024:17 Pages 267—280
DOI https://doi.org/10.2147/OTT.S440979
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Daniel Neureiter
Noboru Yamamoto,1 Anthony Tolcher,2 Navid Hafez,3,4 Iwona Lugowska,5 Rodryg Ramlau,6 Teresa Macarulla,7,8 Junxian Geng,9 Jian Li,9 Michael Teufel,9 Angela Märten,10 Patricia LoRusso3
1Department of Experimental Therapeutics, National Cancer Center Hospital, Tokyo, Japan; 2NEXT Oncology, San Antonio, TX, USA; 3Yale Comprehensive Cancer Center, Yale School of Medicine, New Haven, CT, USA; 4The Angeles Clinic and Research Institute, A Cedars-Sinai Affiliate, Los Angeles, CA, USA; 5Early Phase Clinical Trials Unit, Maria Skłodowska Curie National Research Institute of Oncology, Warsaw, Poland; 6Institute of Oncology, Poznan University of Medical Sciences, Poznan, Poland; 7Vall d’Hebrón University Hospital, Barcelona, Spain; 8Vall d’Hebrón Institute of Oncology (VHIO), Barcelona, Spain; 9Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA; 10Boehringer Ingelheim International GmbH, Ingelheim am Rhein, Germany
Correspondence: Noboru Yamamoto, Department of Experimental Therapeutics, National Cancer Center Hospital, Tokyo, Japan, Tel + 81-3-3542-2511 ext 7319, Email [email protected]
Background: In patients with advanced biliary tract cancer (BTC), first-line chemotherapy plus immunotherapy has improved outcomes; however, second-line options that reflect the disease’s molecular heterogeneity are still needed. One emerging target is MDM2, amplified in ~5– 8% of BTC cases.
Methods: This is a subset analysis of two ongoing Phase Ia/Ib trials assessing patients treated with brigimadlin (BI 907828; a highly potent, oral MDM2–p53 antagonist) ± ezabenlimab (PD-1 inhibitor) ± BI 754111 (anti-LAG-3; n = 1).
Results: Results from 12 patients with BTC are shown (monotherapy: n = 6/combination: n = 6). Six patients achieved partial response (monotherapy: n = 2/combination: n = 4), four had stable disease; responses were durable. Brigimadlin had a manageable safety profile. Seven patients had dose reductions due to adverse events, but no treatment-related adverse events led to treatment discontinuation.
Conclusion: Brigimadlin demonstrated anti-tumor activity in patients with advanced MDM2-amplified BTC, and warrants further investigation.
Plain Language Summary: Biliary tract carcinoma (BTC) is a cancer that affects the bile ducts which are part of the digestive system. Usually, the first treatment for advanced BTC (ie cannot be removed surgically and/or has spread) is chemotherapy in combination with immunotherapy. However, if chemotherapy does not work, or stops working, there are few treatment options available in second-line. Accordingly, intensive research is ongoing to try and find effective drugs. One potential medicine, called brigimadlin (or BI 907828), is a tablet that activates a molecule in tumor cells called p53. The normal function of p53 is to kill cells when they first start to become cancerous. However, if p53 is turned off by genetic mutations, or other mechanisms, then cancer can develop. Although p53 is rarely mutated in BTC tumors, it is inactivated by another molecule called MDM2 which is usually present at abnormally high levels in BTC. Brigimadlin prevents interaction between MDM2 and p53. This activates p53 and causes the cancer to die. Two clinical trials are currently assessing brigimadlin in a range of cancers, including BTC, with the aim of identifying a safe dose that can be examined in more detail in larger trials. So far, 12 patients with BTC have been treated. The patients’ tumors significantly shrank in six of these patients and remained stable in a further four patients. Side effects were as expected and could be tolerated by pausing treatment or lowering the dose. These results show that brigimadlin should be tested further in patients with advanced BTC.
Keywords: biliary tract cancer, MDM2-p53 antagonist, brigimadlin, ezabenlimab, MDM2, MDM2-amplified
Introduction
Biliary tract cancer (BTC) refers to several cancers of the biliary system, and covers a range of invasive adenocarcinomas, including intrahepatic cholangiocarcinoma (iCCA), extrahepatic cholangiocarcinoma, gallbladder carcinoma, and ampullary carcinoma. Cholangiocarcinoma incidence is low in high-income countries, with only around 0.35–2 cases per 100,000 people, but is up to 40 times higher in endemic regions of Thailand and China.1 Symptoms of BTC are dependent on the location of the primary tumor and any metastases, and are usually observed at an advanced stage of the disease due to their non-specificity.1 Until recently, the first-line standard of care for most patients with advanced BTC was gemcitabine/cisplatin. Addition of the programmed cell death-ligand 1 (PD-L1) inhibitor durvalumab to gemcitabine/cisplatin was recently approved as a first-line treatment option based on the results of the Phase III TOPAZ-1 trial. This study demonstrated that the treatment combination significantly improved overall survival (OS) versus gemcitabine/cisplatin alone.2 In the Phase III KEYNOTE-966 trial, the immunochemotherapy regimen of pembrolizumab plus gemcitabine/cisplatin significantly improved OS versus chemotherapy alone, with no new safety signals; however, the difference in response rate between the two groups was not significant.3 Envafolimab plus gemcitabine/oxaliplatin (NCT03478488; another immunochemotherapy regimen) and triplet chemotherapy regimens (NCT03768414; NCT02182778) are also currently being assessed in Phase III trials. Despite these advances, there is a lack of effective options for second and subsequent lines of treatment for patients with advanced/metastatic disease, with no clear global standard, especially for patients without targetable molecular aberrations.4–7
BTC is highly heterogenous, and selection of second-line treatment depends on the molecular characteristics of the tumor. Some patients have targetable aberrations such as fibroblast growth factor receptor (FGFR; 0–12.5%) or isocitrate dehydrogenase mutations (IDH; 0–23%).8 In these cases, pemigatinib, futibatinib (FGFR2 inhibitors) or ivosidenib (IDH inhibitor) are approved therapies.9,10 Other genetic aberrations that have been identified include TP53 mutations (44–47%), KRAS mutations (11–47%), BRAF substitutions (1–5%), and HER2 amplification (3–16%).8 The molecular complexity of BTC highlights the need for comprehensive genomic characterization of tumors to help drive appropriate treatment decisions.
The E3 ubiquitin ligase mouse double minute 2 (MDM2) is a potential drug target in some patients with BTC. MDM2 is an endogenous negative regulator of p53, hence aberrations of the MDM2 gene can result in inappropriate silencing of wild-type p53, potentially leading to tumorigenesis.11 MDM2 amplification has been observed in around 5–8% of BTCs,12,13 with TP53 mutations being mostly mutually exclusive.12 Prevalence of MDM2 amplification varies depending on the anatomical location of the tumor, ranging from 2% to 6% in iCCA,14,15 to 16% in ampullary carcinoma.16 Other studies have reported MDM2 amplification in 13–14% of gallbladder cancer cases.17,18 MDM2 amplifications are generally mutually exclusive to other clinically targetable alterations, including FGFR2-fusions, HER2 amplifications, and IDH1 mutations.12 In addition, aberrant activity of MDM2 can be caused by the loss of its negative regulator, p14ARF, due to mutations in the cyclin dependent kinase inhibitor 2A gene, which occur in around 10% of BTCs.19
Brigimadlin (BI 907828) is a highly potent, oral MDM2–p53 antagonist that binds to MDM2, leading to stabilization of p53, followed by target gene induction, cell-cycle arrest, and apoptosis in tumor cells. Brigimadlin also promotes a pro-immunogenic tumor microenvironment.20 Preliminary dose-escalation results from two Phase Ia/Ib trials investigating brigimadlin as monotherapy, and in combination with ezabenlimab (a programmed cell death protein-1 [PD-1] inhibitor), demonstrated a manageable safety profile and preliminary signs of efficacy in patients with advanced solid tumors.21,22 In the dose-escalation part of the monotherapy trial, the maximum tolerated dose was 60 mg orally, once every 3 weeks (Q3W);23 maximum tolerated dose was not reached in the combination trial.22 Key inclusion criteria included adult patients with TP53 wild-type status and either MDM2-amplified or non-amplified tumors.
Here, we present detailed case descriptions of the first 12 patients with advanced BTC treated with brigimadlin alone, or in combination with checkpoint inhibitors, and safety data from the overall population, in these two ongoing trials.
Methods: Trial Designs of 1403–0001 and 1403–0002
The Phase Ia/Ib, open-label, multicenter, dose-escalation/expansion studies 1403–0001 (NCT03449381) and 1403–0002 (NCT03964233) assessed brigimadlin in patients with advanced solid tumors as monotherapy or combined with the PD-1 inhibitor ezabenlimab, respectively (Figure 1). Briefly, both trials had Phase Ia (dose escalation) and Phase Ib (dose expansion) parts.
In 1403–0001, patients with TP53 wild-type solid tumors were assigned to receive 10–80 mg brigimadlin either Q3W (Arm A) or on Days 1 and 8 every 4 weeks (Arm B). In 1403–0002, 10–45 mg brigimadlin was administered with 240 mg ezabenlimab (and 600 mg BI 754111 in some patients) Q3W. In the dose-expansion phase of 1403–0001, patients with wild-type TP53, MDM2-amplified tumors are being recruited into two cohorts: sarcoma (Cohort 1) or other tumor types (Cohort 2). In 1403–0002, patients with TP53 wild-type tumors are also being recruited to two dose expansion cohorts: those with soft tissue sarcomas (Cohort 1), and those with other tumor types (Cohort 2). The Phase Ia primary endpoint for both trials is the maximum tolerated dose based on dose-limiting toxicities in Cycle 1. Secondary endpoints include pharmacokinetics, safety, and efficacy. Inclusion and exclusion criteria for both studies are largely similar. Key inclusion criteria include adult patients with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0/1, and with adequate organ function. Key exclusion criteria include previous administration of brigimadlin or any other MDM2–p53 or MDMX (MDM4)–p53 antagonist, brain metastases, and history of bleeding diathesis.
Patients provided formalin-fixed paraffin-embedded (FFPE) samples to determine eligibility. Fluorescence in situ hybridization (FISH) or FoundationOne CDx (Foundation Medicine Inc.,) next-generation sequencing (NGS; 1403–0002 Phase Ia only) was used to determine MDM2 amplification status. TP53 mutation status was evaluated with NGS (1403–0001: local-assessment; 1403–0002: Phase Ia, FoundationOne CDx; Phase Ib, NGS Panel, Almac Sciences).
The studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines, were approved by local ethics committees, (National Cancer Center Hospital Institutional Review Board (IRB), Tokyo, Chuo-ku, Japan; Central IRB: Advarra IRB Maryland, USA; Local IRB: Salus IRB Texas, USA, CEIC Área 7 Hospital Clínico San Carlos de Madrid, Madrid, Spain) and all patients provided written informed consent to publish.
Results
Case Presentations
A total of 12 cases are presented, 6 from the 1403–0001 brigimadlin monotherapy trial and 6 from the 1403–0002 combination trial. The data cut-off for Patients 1–10 was December 2022; patients 11 and 12 were added at a later stage (data cut-off February 2023). An overview of cases is shown in Table 1.
 |
Table 1 Patient and Disease Characteristics |
1403–0001 Trial (Brigimadlin Monotherapy; n = 6: All Received Brigimadlin Q3W)
Patient 1 (Japan)
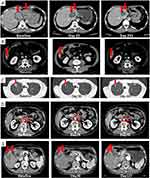
A 51-year-old Asian male presented with anemia and was diagnosed with iCCA with liver metastases. Molecular analysis of the tumor demonstrated amplified MDM2 (copy number 9) and wild-type TP53. The patient received three prior lines of systemic therapy: 7 cycles of cisplatin/gemcitabine, with a best response of stable disease (SD); 3 cycles of tegafur/gimeracil/oteracil (TS-1) with a best response of SD; and 2 cycles of an unknown investigative product with a best response of progressive disease (PD). The patient then received brigimadlin 80 mg Q3W on 14 December 2020, which was reduced to 45 mg Q3W during Cycle 2 due to decreased platelet and neutrophil counts (both grade 4). The patient achieved a best response of confirmed partial response (PR) by Day 22 (Figure 2A), which was still apparent on Day 360. Maximum tumor shrinkage was −73%. The patient remained on treatment for 12.0 months and discontinued due to PD. Progression-free survival (PFS) was 13.3 months. Analysis of CA19.9 blood levels demonstrated a drop from 17,000 U/mL at baseline to undetectable until Day 260 (114 U/mL). Grade ≥ 3 treatment-related adverse events (TRAEs) were grade 4 decreased platelet, neutrophil, and white blood cell counts, and grade 3 anemia and febrile neutropenia.
Patient 2 (USA)
A 51-year-old Hispanic male presented with suspected choledocholithiasis or bile duct stricture with obstructive jaundice and cholangitis. Bilirubin and aspartate transaminase (AST)/alkaline phosphatase were elevated. The patient had hypertension, hyperlipidemia, and nephrolithiasis. He was diagnosed with poorly differentiated ampullary adenocarcinoma with liver and lymph node metastases. MDM2 was amplified (copy number 15). The patient underwent Whipple surgery with lymph nodes and hepatic artery excision, and subsequently received endoscopic retrograde cholangiopancreatography with stent exchange in 2019, followed by 12 cycles of adjuvant mFOLFIRINOX. The patient started brigimadlin 45 mg Q3W on 25 May 2021, and treatment was ongoing at the data cut-off date. The patient achieved a confirmed PR on Day 38 that was still apparent at Day 581 (Figure 2B). Maximum tumor shrinkage is currently −61%, and treatment has been administered for 19.1 months so far. No grade ≥ 3 TRAEs have been reported.
Patient 3 (Japan)
A 69-year-old Asian female presented with hypertension, hyperlipidemia, elevated AST/alkaline phosphatase, and dysesthesia. She was diagnosed with biliary tree adenocarcinoma with liver metastases. MDM2 was reported as amplified (copy number unknown). The patient received one prior line of systemic therapy: 22 cycles of cisplatin/gemcitabine. Treatment was discontinued because of AEs. Treatment with brigimadlin was initiated on 12 February 2019 at 45 mg Q3W. Cycle 2 was delayed with a subsequent dose reduction to 30 mg Q3W due to grade 3 thrombocytopenia and grade 2 neutropenia. There was a second dose reduction to 20 mg Q3W during Cycle 4 due to grade 3 thrombocytopenia. Best response was SD (maximum tumor shrinkage: −13%) up to Day 162. Treatment was administered for 6.0 months before discontinuation due to patient withdrawal. Grade ≥ 3 TRAEs were grade 3 and 4 decreased neutrophil count, grade 3 decreased platelet count, and grade 3 decreased white blood cell count.
Patient 4 (USA)
A 78-year-old white male with a history of type 2 diabetes, hypertension, hyperlipidemia, dyspnea on exertion, and non-cardiac chest pain was diagnosed with moderately differentiated ampullary adenocarcinoma with liver and lymph node metastases. MDM2 amplification status was unknown. The patient underwent the Whipple procedure followed by 12 cycles of adjuvant FOLFOX with a best response of SD, then fluorouracil maintenance. He later received 39 cycles of fluorouracil/leucovorin with a best response of SD. Treatment with brigimadlin was initiated on 25 May 2021 at 45 mg Q3W. Best response was PD and treatment was administered for 0.8 months. PFS was 1.3 months. The patient discontinued due to PD. No grade ≥ 3 TRAEs were reported in this patient.
Patient 5 (Poland)
A 72-year-old white female with a history of cholecystectomy and hypertension was diagnosed with undifferentiated ampullary carcinoma with liver and lymph node metastases. MDM2 was amplified (copy number 13); TP53 was wild-type. The patient received first-line FOLFIRINOX for 2 months until treatment was stopped due to thrombocytopenia. Second-line treatment was gemcitabine for 2.5 months, which was discontinued due to PD. Treatment with brigimadlin was initiated in July 2022 at 45 mg Q3W, which was reduced to 30 mg during Cycle 3 due to grade 3 increased alanine transaminase and grade 2 increased AST; treatment was ongoing at the data cut-off date. Best response so far is SD, and treatment has been administered for 5.0 months. Grade ≥ 3 AEs were grade 3 neutropenia and grade 3 thrombocytopenia.
Patient 6 (Japan)
A 74-year-old Asian male with a history of anemia and thrombocytopenia was diagnosed with bile duct adenocarcinoma with liver and lymph node metastases. MDM2 was reported as amplified (copy number unknown); no mutations were detected in TP53. First-line treatment with brigimadlin 45 mg Q3W was initiated on 6 July 2022. Treatment was administered for 1 day, but was suspended due to hematologic AEs. Treatment was later discontinued due to PD. PFS was 1.3 months. Grade 3/4 TRAEs were anemia and hypokalemia (both grade 3).
1403–0002 Trial (Brigimadlin + Ezabenlimab; n = 6)
Patient 7 (Japan)
A 57-year-old Asian female with a history of arrhythmia, anemia, thrombocytopenia, and hypoalbuminemia was diagnosed with iCCA with liver, lung, and lymph node metastases. MDM2 was amplified (copy number 36); TP53 was wild-type. The patient received 15 cycles of cisplatin/gemcitabine, which was stopped due to toxicity. She subsequently received second-line TS-1, third-line gemcitabine, and radiotherapy for left cervical lymph nodes. Treatment with brigimadlin 30 mg and ezabenlimab 240 mg Q3W was initiated on 22 November 2021. The brigimadlin dose was reduced to 20 mg Q3W during Cycle 3 due to grade 1 thrombocytopenia and grade 2 neutropenia. The patient achieved confirmed PR up to Day 247 (Figure 2C). Treatment was administered for 8.2 months before discontinuation due to AEs. PFS was 9.6 months. Maximum tumor shrinkage was −54%. Grade 3/4 TRAEs (related to brigimadlin) were grade 4 neutropenia and grade 3 reduced white blood cell count.
Patient 8 (Japan)
A 66-year-old Asian male with a history of angina pectoris, dyslipidemia, lower leg edema, and elevated AST was diagnosed with gallbladder adenocarcinoma with lymph node metastases. MDM2 was amplified (copy number 14). No mutations were detected in TP53. The patient underwent gallbladder resection, hepatectomy, bile duct resection and D2 dissection followed by 4 cycles of adjuvant TS-1. He subsequently received first-line TS-1 and 7 cycles of second-line cisplatin/gemcitabine with a best response of SD. The patient then received brigimadlin starting on 9 June 2021 at 45 mg and ezabenlimab 240 mg Q3W. Cycle 3 was delayed and the brigimadlin dose was reduced to 30 mg Q3W due to grade 3 thrombocytopenia. Best response was PR up to Day 227, with maximum tumor shrinkage of −50% (Figure 2D). Treatment was administered for 4.6 months, and then suspended due to hematologic AEs. Treatment was later discontinued due to PD. PFS was 7.9 months. Grade 3/4 TRAEs (related to brigimadlin) were grade 3 and grade 4 decreased neutrophil count, grade 3 and grade 4 thrombocytopenia, grade 3 reduced white blood cell count, grade 3 elevated AST, and grade 3 anemia.
Patient 9 (Japan)
A 67-year-old Asian male with a history of anemia and hypoalbuminemia was diagnosed with iCCA with liver and lymph node metastases. MDM2 was amplified (copy number 7); no mutations were detected in TP53. The patient received 10 cycles of first-line cisplatin/gemcitabine with a best response of PR. He then received 7 cycles of second-line gemcitabine/TS-1. Treatment with brigimadlin and ezabenlimab was initiated on 28 April 2021 at 45 mg and 240 mg Q3W, respectively. Cycle 3 was delayed and the dose of brigimadlin was reduced to 30 mg Q3W due to grade 3 thrombocytopenia. The patient achieved SD on Day 36, and PR up to Day 162 (Figure 2E). The maximum tumor shrinkage was −49%. The patient was on treatment for 4.2 months; however, due to hematologic AEs the treatment was suspended, and was later discontinued due to PD. PFS was 7.6 months. Grade 3/4 TRAEs (related to brigimadlin) were decreased platelet count, anemia, and hypoxia (all grade 3). Additionally, in November 2021 the patient underwent nasobiliary drainage for cholangitis and had a central venous catheterization for pancreatitis.
Patient 10 (USA)
A 56-year-old Caucasian female presented with biliary obstruction for which a stent was fitted in 2019. She was diagnosed with iCCA with liver, pancreatic, and lymph node metastases. MDM2 was amplified (copy number 9) and there were no TP53 mutations. First-line treatment was 7 cycles of cisplatin/gemcitabine; second-line treatment was 15 cycles of FOLFOX. A left neck lymph node resection was undertaken in 2020. Treatment with brigimadlin 45 mg, ezabenlimab 240 mg, and BI 754111 600 mg Q3W was initiated on 29 July 2020. The best response was SD on Days 36, 110, and 148. Treatment was administered for 4.1 months before discontinuation due to physician decision, and the patient was then transferred to a hospice. PFS was 5.9 months. Grade 3/4 TRAEs (related to brigimadlin) were anemia, and decreased neutrophil and white blood cell counts (all grade 3).
Patient 11 (Spain)
A 59-year-old Caucasian male was diagnosed with moderately differentiated iCCA in February 2020. MDM2 was amplified and there were no TP53 mutations. The patient underwent right hepatectomy and hepatic hilum lymphadenectomy followed by adjuvant capecitabine. First-line treatment was 18 cycles of cisplatin/gemcitabine with PR as best response, second-line treatment was 4 cycles of Sym021 (targeting PD-1) and Sym023 (targeting TIM3), and third-line therapy was 5 cycles of FOLFOX; best response for second- and third-line therapy was SD. Treatment with brigimadlin 45 mg and ezabenlimab 240 mg Q3W was initiated on 21 October 2022, and was ongoing at the data cut-off date. The dose of brigimadlin was reduced to 30 mg due to grade 2 neutropenia. Liver and lymph node metastases were reported at time of trial entry. The patient achieved a confirmed PR starting on Day 48 that was still apparent at Day 127, with maximum tumor shrinkage of −39%. Treatment has been administered for 4.2 months so far. Grade 3/4 TRAEs (related to brigimadlin) were grade 3 and 4 thrombocytopenia, and grade 3 neutropenia.
Patient 12 (Spain)
A 74-year-old Asian male was diagnosed with poorly differentiated gall bladder carcinoma in February 2021. MDM2 and HER2 were amplified and there were no TP53 mutations. The patient underwent cholecystectomy, lymphadenectomy, and resection of liver segments IVB and V. First-line treatment was cisplatin/gemcitabine from December 2021 to May 2022, second-line treatment was cycles of FOLFOX from May to September 2022, and third-line therapy was 1 cycle of irinotecan. Treatment with brigimadlin 45 mg and ezabenlimab 240 mg Q3W was initiated on 24 October 2022, and was ongoing at the data cut-off date. Pancreatic and abdominal metastases were reported at time of trial entry. The patient had confirmed SD starting on Day 36 that was still apparent at Day 128, with best percentage change from baseline of 5%. Treatment has been administered for 4.1 months so far. No Grade 3/4 TRAEs (related to brigimadlin) have been reported.
Summary
Patients with BTC received brigimadlin as monotherapy in 1403–0001, or in combination with ezabenlimab (plus BI 754111 in one patient) in 1403–0002. A confirmed objective response was seen in two out of six patients in the monotherapy trial (both had MDM2-amplified tumors), and four out of six patients in the combination trial (all four had MDM2-amplified tumors; Figure 3). A further four patients achieved SD: two in the monotherapy trial, and two in the combination trial. Treatment was administered for over 4 months in four of the six patients in the monotherapy trial, and in all six patients in the combination trial; four patients were still on treatment at data cut-off (Figure 4).
 |
Figure 4 Tumor responses in patients with biliary tract cancer treated with brigimadlin monotherapy or in combination with ezabenlimab. Patient 10 also received BI 754111. |
In these 12 patients with BTC, the most common grade ≥ 3 TRAEs were neutropenia, thrombocytopenia, anemia, and reduced white blood cell count. TRAEs were managed by appropriate treatment delays and/or dose reductions; there were no treatment discontinuations due to TRAEs. These findings are consistent with the safety profile of brigimadlin in the overall patient populations in the 1403–0001 and 1403–0002 trials (summarized in Table 2; data cut-off December 2022). Briefly, in the 1403–0001 trial, 110 patients had been treated with brigimadlin Q3W, of whom 102 (92.7%) experienced at least one TRAE. Grade ≥ 3 TRAEs were experienced by 49 (44.5%) patients, most commonly thrombocytopenia (n = 26, 23.6%), neutropenia (n = 24, 21.8%), and leukopenia (n = 12, 10.9%). AEs leading to dose reduction or discontinuation occurred in 32 and 6 (29.1% and 5.5%) patients, respectively. In the 1403–0002 trial, 42 patients had been treated with brigimadlin in combination with ezabenlimab, of whom 39 (92.9%) experienced at least one TRAE. Grade ≥ 3 TRAEs were experienced by 21 (50.0%) patients, most commonly thrombocytopenia and neutropenia (both n = 8, 19.0%), and leukopenia (n = 7, 16.7%). AEs leading to dose reduction or discontinuation occurred in 11 and 2 (26.2% and 4.8%) patients, respectively. An infographic summary of the patient cases presented here can be found in Supplementary Figure 1.
 |
Table 2 Adverse Events in the Overall Patient Populations of Trials 1403–0001 (Brigimadlin Monotherapy) and 1403–0002 (Combination with Ezabenlimab) |
Discussion
In this sub-analysis of two Phase Ia/Ib trials, brigimadlin alone or in combination with ezabenlimab demonstrated encouraging clinical efficacy in patients with MDM2-amplified BTC. Responses were seen in patients treated in both trials, which were mostly durable. Overall, brigimadlin had a manageable safety profile in patients with BTC. The most common grade 3/4 TRAEs included neutropenia, thrombocytopenia, anemia, and reduced white blood cell count. Seven of 12 patients had dose reductions due to AEs, and were able to continue on treatment afterwards. No TRAEs led to treatment discontinuation. These results are similar to those observed in the overall populations of patients treated in the 1403–0001 and 1403–0002 trials. To the best of our knowledge, this is the first description of patients with BTC who have been treated with an MDM2–p53 antagonist. These results suggest that MDM2–p53 inhibition warrants further investigation in patients with BTC with MDM2 amplification and wild-type TP53.
The prevalence of MDM2 amplification in patients with BTC is currently uncertain owing to a paucity of data. In the TOPAZ-1 trial, 8.2% of the biomarker-evaluable population had MDM2-amplified tumors.13 Importantly, MDM2 amplification in BTC generally appears to be mutually exclusive to other targetable aberrations, such as FGF2 and IDH mutations.15 Therefore, patients with MDM2-amplified BTC represent a subgroup of patients for whom effective treatment options are lacking.12 Furthermore, MDM2 amplification has been reported as a negative prognostic factor in patients with BTC.17,19,24–26 As MDM2 is transcriptionally transactivated and strictly regulated by p53, MDM2 is only expected to be overexpressed in TP53 wild-type BTC. The function of wild-type p53 may therefore be suppressed by MDM2 overexpression, which potentially could lead to aggressive tumor behavior. MDM2 overexpression has been significantly correlated with an increased presence of metastases, more advanced tumor stage, and lower OS.24,25 Given the role of MDM2 in the pathogenesis of BTC, and the emergence of potential MDM2-targeted treatment options, it is important that further studies are undertaken to establish its prevalence in BTC. In the clinic, diagnostic tests including FISH and NGS tests such as FoundationOne Cdx can be used to detect MDM2 amplification.17,27,28 Analysis of MDM2 overexpression or amplification should be included in molecular analysis of BTC tumors.
Other MDM2–p53 antagonists are currently in development for the treatment of patients with solid tumors, including alrizomadlin, ASTX295, navtemadlin, and siremadlin.29–32 Until recently, the most advanced MDM2–p53 antagonist in terms of clinical development was milademetan, which was being investigated versus trabectedin in patients with dedifferentiated liposarcoma in a randomized, multicenter, open-label, Phase III trial (NCT04979442; MANTRA). However, enrollment to MANTRA-2, and plans for Phase I/II combination trials, have recently been suspended (May 2023) after the primary endpoint was not met in the MANTRA trial (median PFS: 3.6 months vs 2.2 months; HR: 0.89).33,34 The remaining compounds are either at Phase I or Phase II stage and are predominantly being investigated in combination with other therapies. Of note, many of the TRAEs observed in this study, including thrombocytopenia, neutropenia, anemia, and nausea, have been reported in patients treated with other MDM2–p53 antagonists, suggesting that they are class-related. These AEs are manageable, and rarely lead to treatment discontinuation.29
The clinical development of brigimadlin in patients with MDM2-amplified BTC is ongoing. Brightline-2 (NCT05512377) is a Phase IIa/IIb, open-label, single-arm, multicenter trial of brigimadlin in an estimated 100 patients for whom previous treatment was not successful, or for whom no approved treatment exists. The aims are to demonstrate the efficacy, safety, and tolerability of brigimadlin in patients with MDM2-amplified BTC, and other solid tumors. Patients must have locally advanced/metastatic, MDM2-amplified, TP53 wild-type BTC, or pancreatic ductal adenocarcinoma. The primary endpoint is objective response, and secondary endpoints include duration of objective response, PFS, OS, and safety. Key inclusion criteria are as follows: adult patients (≥ 18 years old); MDM2 amplification (copy number ≥ 8) and TP53 wild-type status; ≥ 1 measurable target lesion (Response Evaluation Criteria In Solid Tumors v1.1); ECOG PS of 0/1; and adequate organ function. Key exclusion criteria are as follows: previous treatment with an MDM2/4–p53 antagonist; active bleeding; significant risk of hemorrhage or current bleeding disorder; major surgery performed ≤ 4 weeks prior to start of trial treatment or planned ≤ 6 months after screening; clinically significant previous or concomitant malignancies in the opinion of the investigator affecting the efficacy and/or outcome of the trial; and currently enrolled in another investigational device or drug trial. Recruitment is ongoing.
Conclusion
In conclusion, brigimadlin alone or in combination with ezabenlimab showed promising efficacy and a manageable safety profile in patients with BTC, including in those with MDM2-amplified tumors. Further assessment in this patient population is warranted, and is ongoing in trials such as Brightline-2. Given the emergence of MDM2 amplification as a potential drug target, patients with BTC should be routinely tested for MDM2 amplification, along with other genetic aberrations.
Data Sharing Statement
To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data, typically, one year after the approval has been granted by major Regulatory Authorities or after termination of the development program. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
Acknowledgments
The authors thank the patients and their caregivers for making these studies possible. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive payment related to the development of the manuscript. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Steven Kirkham, PhD, of Ashfield MedComms, an Inizio Company, and funded by Boehringer Ingelheim. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations. The study was supported and funded by Boehringer Ingelheim.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Author Disclosures
Noboru Yamamoto reports advisory council or committee for Eisai, Takeda, Otsuka, Boehringer Ingelheim, Cmic, Chugai, MERCK, Healios; received honoraria from ONO, Chugai, Daiichi-Sankyo, Eisai; received grants or funds from Astellas, Chugai, Eisai, Taiho, BMS, Pfizer, Novartis, Eli Lilly, AbbVie, Daiichi-Sankyo, Bayer, Boehringer Ingelheim, Kyowa Kirin, Takeda, ONO, Janssen Pharma, MSD, MERCK, GSK, Sumitomo Pharma, Chiome Bioscience, Otsuka, Carna Biosciences, Genmab, Shionogi, TORAY, KAKEN, AstraZeneca, Cmic. Anthony Tolcher reports employment with NEXT Oncology; reports consulting or advisory role (recipient: institution) for Nanobiotix, Pierre Fabre, Ascentage Pharma, AbbVie, EMD Serono, BioInvent, Adagene, Agenus, Aximmune, Bayer, HBM Partners, Mekanistic Therapeutics, NBE Therapeutics, Pelican Therapeutics, Pfizer, Immunome, Gilde Healthcare, Immunomet, Mirati Therapeutics, Pieris Pharmaceuticals, Aro Biotherapeutics, Asana Biosciences, Elucida Oncology, Partner Therapeutics, Ryvu Therapeutics, Sotio, Eleven Biotherapeutics, Zymeworks, Mersana, Trillium Therapeutics, Boehringer Ingelheim, Janssen, Aclaris Therapeutics, BluPrint Oncology, Daiichi Sankyo, IDEA Pharma, Immuneering, IMPAC Medical Systems, Karma Oncology, Lengo Therapeutics, Menarini, Seattle Genetics, SK Life Sciences, Spirea, Sunshine Guojian, Transcenta, Zentalis, Transgene, Deka Biosciences, HiberCell, Ikena Oncology, Jazz Pharmaceuticals, Pyxis, Vincerx Pharma, ZielBio, Senti Biosciences, Ocellaris Pharma, Lilly; reports leadership role with Next Oncology; received travel, accommodations, expenses fees (recipient: institution) from Sotio; served as an expert testimony for Immunogen; reports stock and other ownership interests (recipient: institution) from Pyxis; received research fundings (recipient: institution) from AbbVie, Pfizer, Syndax, Asana Biosciences, ADC Therapeutics, Adagene, Aminex, Ascentage Pharma, Arrys Therapeutics, CStone Pharmaceuticals, Deciphera, GlaxoSmithKline, Inhibrx, Innate Pharma, Kiromic, Mersana, Naturewise, NextCure, Nitto BioPharma, Pieris Pharmaceuticals, Symphogen, Tizona Therapeutics, Inc., Zymeworks, Agenus, Amphivena Therapeutics, Astex Pharmaceuticals, Boehringer Ingelheim Basilea, eFFECTOR Therapeutics, EMD Serono, Gilead Sciences, Kechow Pharma, K-Group Beta, Janssen Research & Development, Merck Sharp & Dohme, ORIC Pharmaceuticals, Samumed, Spring Bank, Seattle Genetics, Sunshine Guojian, Synthorx, Bioinvent, Birdie, BJ Bioscience, Boston Biomedical, Daiichi Sankyo, ImmuneOncia, Mirati Therapeutics, NBE Therapeutics, Odonate Therapeutics, Qilu Puget Sound Biotherapeutics, Shanghai HaiHe Pharmaceutical, Takeda, ABL Bio, Apros Therapeutics, Arcellx, ARMO BioSciences, Artios. Iwona Lugowska received honoraria and travel/accommodations/expenses from BMS, Macrogenics, Celon, Janssen, RM, Novartis, MSD, Roche, Boehringer Ingelheim, Amgen. Rodryg Ramlau reports consulting or advisory role for Bristol-Myers Squibb, Boehringer Ingelheim, MSD Oncology, Merck, Roche, Novartis, Takeda, AstraZeneca, Pfizer. Teresa Macarulla reports personal fees from Ability Pharmaceuticals SL, AstraZeneca, Basilea Pharma, Baxter, BioLineRX Ltd, Celgene, Eisai, Incyte, Ipsen Bioscience Inc, Janssen, Lilly, MSD, Novocure, QED Therapeutics, Roche Farma, Sanofi-Aventis, Servier, and Zymeworks, outside the submitted work. Junxian Geng, Jian Li, Michael Teufel, and Angela Märten report employment with Boehringer Ingelheim. Junxian Geng reports a patent US20230058171A1 pending to Boehringer Ingelheim International GmbH. Patricia LoRusso reports consulting or advisory role for Genentech, CytomX Therapeutics, Roche/Genentech, Halozyme, Five Prime Therapeutics, Agenus, Agios, Cybrexa Therapeutics, Sotio, Abbvie, Genmab, Takeda, Tyme, IQvia, Trial to Reduce IDDM in the Genetically at Risk (TRIGR), Pfizer, ImmunoMet, Black Diamond Therapeutics, GlaxoSmithKline, QED Therapeutics, AstraZeneca, EMD Serono, Shattuck Labs, Astellas Pharma, Salarius Pharmaceuticals, Silverback Therapeutics, Macrogenics, Kyowa Kirin International, Kineta, Zentalis, Molecular Templates, Molecular Templates, ABL Bio, SK Life Sciences, ST Cube, Bayer, I-Mab, Seattle Genetics, ImCheck therapeutics, Relay Therapeutics, Stemline Therapeutics, Mekanistic Therapeutics, Compass Therapeutics, BAKX Therapeutics, Scenic Biotech, Qualigen Therapeutics, Roivant, Neurotrials Research; received travel/accommodations/expenses from Genentech; reports stock and other ownership interests from BAKX Therapeutics; received honoraria from Five Prime Therapeutics; received research funding (recipient: institution) from Genentech.
References
1. Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397(10272):428–444. doi:10.1016/S0140-6736(21)00153-7
2. Oh D-Y, Ruth He A, Qin S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evidence. 2022;1(8):EVIDoa2200015. doi:10.1056/EVIDoa2200015
3. Kelley RK, Ueno M, Yoo C, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet. 2023;401(10391):1853–1865. doi:10.1016/S0140-6736(23)00727-4
4. Khankhel ZS, Goring S, Bobiak S, et al. Second-line treatments in advanced biliary tract cancer: systematic literature review of efficacy, effectiveness and safety. Future Oncol. 2022;18(18):2321–2338. doi:10.2217/fon-2021-1302
5. Nagino M, Hirano S, Yoshitomi H, et al. Clinical practice guidelines for the management of biliary tract cancers 2019: the 3rd English edition. J Hepatobil Pancreat Sci. 2021;28(1):26–54.
6. Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796–807. doi:10.1016/S1470-2045(20)30157-1
7. Benson AB, D’Angelica MI, Abrams T, et al. NCCN guidelines(R) insights: biliary tract cancers, Version 2.2023. J Natl Compr Canc Netw. 2023;21(7):694–704. doi:10.6004/jnccn.2023.0035
8. Bridgewater JA, Goodman KA, Kalyan A, Mulcahy MF. Biliary tract cancer: epidemiology, radiotherapy, and molecular profiling. Am Soc Clin Oncol Educ Book. 2016;35(36):e194–203. doi:10.1200/EDBK_160831
9. Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, Phase 2 study. Lancet Oncol. 2020;21(5):671–684. doi:10.1016/S1470-2045(20)30109-1
10. Javle M, Lowery M, Shroff RT, et al. Phase II study of bgj398 in patients with fgfr-altered advanced cholangiocarcinoma. J Clin Oncol. 2018;36(3):276–282. doi:10.1200/JCO.2017.75.5009
11. Wade M, Wang YV, Wahl GM. The p53 orchestra: mdm2 and Mdmx set the tone. Trends Cell Biol. 2010;20(5):299–309. doi:10.1016/j.tcb.2010.01.009
12. Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47(9):1003–1010. doi:10.1038/ng.3375
13. Bouattour M, Valle J, Vogel A, et al. Characterization of long-term survivors in the TOPAZ-1 study of durvalumab or placebo plus gemcitabine and cisplatin in advanced biliary tract cancer. J Clin Oncol. 2023;41(Suppl 4):531. doi:10.1200/JCO.2023.41.4_suppl.531
14. Pu X, Zhu L, Li F, et al. Target molecular treatment markers in intrahepatic cholangiocarcinoma based on Chinese population. Pathol Res Pract. 2020;216(9):153116. doi:10.1016/j.prp.2020.153116
15. Kendre G, Murugesan K, Brummer T, Segatto O, Saborowski A, Vogel A. Charting co-mutation patterns associated with actionable drivers in intrahepatic cholangiocarcinoma. J Hepatol. 2023;16(3):614–626. doi:10.1016/j.jhep.2022.11.030
16. Wong W, Lowery MA, Berger MF, et al. Ampullary cancer: evaluation of somatic and germline genetic alterations and association with clinical outcomes. Cancer. 2019;125(9):1441–1448. doi:10.1002/cncr.31951
17. Kim SJ, Akita M, Sung YN, et al. MDM2 amplification in intrahepatic cholangiocarcinomas: its relationship with large-duct type morphology and uncommon KRAS mutations. Am J Surg Pathol. 2018;42(4):512–521. doi:10.1097/PAS.0000000000001006
18. D’Afonseca V, Arencibia AD, Echeverría-Vega A, et al. Identification of altered genes in gallbladder cancer as potential driver mutations for diagnostic and prognostic purposes: a computational approach. Cancer Inform. 2020;19:1176935120922154. doi:10.1177/1176935120922154
19. Wu CE, Pan YR, Yeh CN, Lunec J. Targeting P53 as a future strategy to overcome gemcitabine resistance in biliary tract cancers. Biomolecules. 2020;10(11):1474. doi:10.3390/biom10111474
20. Rudolph D, Reschke M, Blake S, et al. BI 907828: a novel, potent MDM2 inhibitor that induces antitumor immunologic memory and acts synergistically with an anti-PD-1 antibody in syngeneic mouse models of cancer. Cancer. 2018;78(Suppl 13):Abstr4866. doi:10.1158/1538-7445.AM2018-4866
21. LoRusso P, Yamamoto N, Patel MR, et al. The MDM2-p53 antagonist brigimadlin (BI 907828) in patients with advanced or metastatic solid tumors: results of a phase ia, first-in-human, dose-escalation study. Cancer Discov. 2023;13(8):1802–1813. doi:10.1158/2159-8290.CD-23-0153
22. Yamamoto N, Hafez N, Tolcher AW, et al. A phase Ia/Ib, dose-escalation/expansion study of BI 907828 in combination with BI 754091 (ezabenlimab) and BI 754111 in patients (pts) with advanced solid tumors. J Clin Oncol. 2022;40(Suppl 16):Abstr3095. doi:10.1200/JCO.2022.40.16_suppl.3095
23. Schoeffski P, Yamamoto N, Bauer T, et al. Phase I dose-escalation and expansion study evaluating the safety and efficacy of the MDM2–p53 antagonist, BI 907828, in patients with solid tumours. Ann Oncol. 2022;33(Suppl 7):S197–S224. doi:10.1016/j.annonc.2022.07.581
24. Horie S, Endo K, Kawasaki H, Terada T. Overexpression of MDM2 protein in intrahepatic cholangiocarcinoma: relationship with p53 overexpression, Ki-67 labeling, and clinicopathological features. Virchows Arch. 2000;437(1):25–30. doi:10.1007/s004280000201
25. Wattanawongdon W, Simawaranon Bartpho T, Tongtawee T. Expression of CD44 and MDM2 in cholangiocarcinoma is correlated with poor clinicopathologic characteristics. Int J Clin Exp Pathol. 2019;12(10):3961–3967.
26. Lowery MA, Ptashkin R, Jordan E, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin Cancer Res. 2018;24(17):4154–4161. doi:10.1158/1078-0432.CCR-18-0078
27. Dembla V, Somaiah N, Barata P, et al. Prevalence of MDM2 amplification and coalterations in 523 advanced cancer patients in the MD Anderson Phase 1 clinic. Oncotarget. 2018;9(69):33232–33243. doi:10.18632/oncotarget.26075
28. Matsubara J, Mukai K, Kondo T, et al. First-line genomic profiling in previously untreated advanced solid tumors for identification of targeted therapy opportunities. JAMA Network Open. 2023;6(7):e2323336. doi:10.1001/jamanetworkopen.2023.23336
29. Konopleva M, Martinelli G, Daver N, et al. MDM2 inhibition: an important step forward in cancer therapy. Leukemia. 2020;34(11):2858–2874. doi:10.1038/s41375-020-0949-z
30. Stein EM, DeAngelo DJ, Chromik J, et al. Results from a first-in-human phase I study of siremadlin (HDM201) in patients with advanced wild-type TP53 solid tumors and acute leukemia. Clin Cancer Res. 2022;28(5):870–881. doi:10.1158/1078-0432.CCR-21-1295
31. McKean M, Tolcher AW, Reeves JA, et al. Newly updated activity results of alrizomadlin (APG-115), a novel MDM2/p53 inhibitor, plus pembrolizumab: phase 2 study in adults and children with various solid tumors. J Clin Oncol. 2022;40(Suppl 16):Abstr9517. doi:10.1200/JCO.2022.40.16_suppl.9517
32. ClinicalTrials.gov. Study of ASTX295 in patients with solid tumors with wild-type p53. 2022. Available FROM: https://clinicaltrials.gov/ct2/show/NCT03975387.
33. Rain Oncology Inc. Rain Oncology announces topline results from phase 3 MANTRA trial of milademetan for the treatment of dedifferentiated liposarcoma. Available from: https://www.rainoncology.com/news-press-releases/rain-oncology-announces-topline-results-from-phase-3-mantra-trial-of-milademetan-for-the-treatment-of-dedifferentiated-liposarcoma.
34. Rain Oncology Inc. Rain oncology provides company update and outlines strategic priorities of milademetan clinical programs. Press release available at https://www.rainoncology.com/news-press-releases/rain-oncology-provides-company-update-and-outlines-strategic-priorities-of-milademetan-clinical-programs.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.